SCIP/SIEA and PAP: The New Workhorse Flaps in Soft Tissue Reconstruction for All Body Regions
Abstract
1. Introduction
2. Materials and Methods
2.1. Retrospective Study
2.2. Statistical Study
3. Results
Retrospective Study Results
4. Statistical Study Results
4.1. Demographics and Clinical Characteristics
4.2. Flap Areas
4.3. Complication Rates
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Driel, A.A.; Mureau, M.A.M.; Goldstein, D.P.; Gilbert, R.W.; Irish, J.C.; Gullane, P.J.; Neligan, P.C.; Hofer, S.O.P. Aesthetic and oncologic outcome after microsurgical reconstruction of complex scalp and forehead defects after malignant tumor resection: An algorithm for treatment. Plast. Reconstr. Surg. 2010, 126, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Haddock, N.T.; Saadeh, P.B.; Siebert, J.W. Achieving Aesthetic Results in Facial Reconstructive Microsurgery. Plast. Reconstr. Surg. 2012, 130, 1236–1245. [Google Scholar] [CrossRef] [PubMed]
- Visser, N.J.; Damen, T.H.C.; Timman, R.; Hofer, S.O.P.; Mureau, M.A.M. Surgical results, aesthetic outcome, and patient satisfaction after microsurgical autologous breast reconstruction following failed implant reconstruction. Plast. Reconstr. Surg. 2010, 126, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Khansa, I.; Boehmler, J.H. Aesthetic outcomes in women undergoing breast-conserving therapy followed by mastectomy and microsurgical reconstruction. Microsurgery 2015, 35, 21–28. [Google Scholar] [CrossRef]
- Sinik, L.M.; Le, A.; Ehirim, H.B.; Collins, M.S.M. Optimizing Aesthetic Outcomes in Autologous Breast Reconstruction: 20 Pearls for Practice. Plast. Reconstr. Surg.-Glob. Open 2024, 12, e5750. [Google Scholar] [CrossRef]
- Zebolsky, A.L.; Patel, N.; Heaton, C.M.; Park, A.M.; Seth, R.; Knott, P.D. Patient-Reported Aesthetic and Psychosocial Outcomes After Microvascular Reconstruction for Head and Neck Cancer. Arch. Otolaryngol. Neck Surg. 2021, 147, 1035–1044. [Google Scholar] [CrossRef]
- Suh, J.M.; Chung, C.H.; Chang, Y.J. Head and neck reconstruction using free flaps: A 30-year medical record review. Arch. Craniofacial Surg. 2021, 22, 38–44. [Google Scholar] [CrossRef]
- Shen, A.Y.; Lonie, S.; Lim, K.; Farthing, H.; Hunter-Smith, D.J.; Rozen, W.M. Free Flap Monitoring, Salvage, and Failure Timing: A Systematic Review. J. Reconstr. Microsurg. 2021, 37, 300–308. [Google Scholar] [CrossRef]
- Jimenez, J.E.; Nilsen, M.L.; Gooding, W.E.; Anderson, J.L.; Khan, N.I.; Mady, L.J.; Wasserman-Wincko, T.; Duvvuri, U.; Kim, S.; Ferris, R.L.; et al. Surgical factors associated with patient-reported quality of life outcomes after free flap reconstruction of the oral cavity. Oral Oncol. 2021, 123, 105574. [Google Scholar] [CrossRef]
- Dolan, R.; Butler, J.; Murphy, S.; Cronin, K. Health-related quality of life, surgical and aesthetic outcomes following microvascular free flap reconstructions: An 8-year institutional review. Ind. Mark. Manag. 2011, 94, 43–51. [Google Scholar] [CrossRef]
- Kozusko, S.; Liu, X.; Riccio, C.; Chang, J.; Boyd, L.; Kokkalis, Z.; Konofaos, P. Selecting a free flap for soft tissue coverage in lower extremity reconstruction. Injury 2019, 50, S32–S39. [Google Scholar] [CrossRef] [PubMed]
- Lutz, B.S.; Wei, F.-C. Microsurgical Workhorse Flaps in Head and Neck Reconstruction. Clin. Plast. Surg. 2005, 32, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Benanti, E.; De Santis, G.; Acciaro, A.L.; Colzani, G.; Baccarani, A.; Starnoni, M. Soft tissue coverage of the upper limb: A flap reconstruction overview. Ann. Med. Surg. 2020, 60, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gazyakan, E.; Bigdeli, A.K.; Will-Marks, P.; Kneser, U.; Hirche, C. Soft tissue free flap for reconstruction of upper extremities: A meta-analysis on outcome and safety. Microsurgery 2019, 39, 463–475. [Google Scholar] [CrossRef]
- Al Omran, Y.; Evans, E.; Jordan, C.; Borg, T.-M.; AlOmran, S.; Sepehripour, S.; Akhavani, M.A. The Medial Sural Artery Perforator Flap versus Other Free Flaps in Head and Neck Reconstruction: A Systematic Review. Arch. Plast. Surg. 2023, 50, 264–273. [Google Scholar] [CrossRef]
- Altiparmak, M.; Cha, H.G.; Hong, J.P.; Suh, H.P. Superficial Circumflex Iliac Artery Perforator Flap as a Workhorse Flap: Systematic Review and Meta-analysis. J. Reconstr. Microsurg. 2020, 36, 600–605. [Google Scholar] [CrossRef]
- Ciudad, P.; Kaciulyte, J.; Torto, F.L.; Vargas, M.I.; Bustamante, A.; Chen, H.C.; Maruccia, M.; Zulueta, J.; Trignano, E.; Bolletta, A. The profunda artery perforator free flap for lower extremity reconstruction. Microsurgery 2022, 42, 13–21. [Google Scholar] [CrossRef]
- Wilson, R.; Cave, T.; Entezami, P.; Ware, E.; Chang, B.A. Systematic Review of the Profunda Artery Perforator Free Flap for Head and Neck Reconstruction. OTO Open 2024, 8, e70028. [Google Scholar] [CrossRef]
- Largo, R.D.; Bhadkamkar, M.A.; Asaad, M.; Chu, C.K.; Garvey, P.B.; Butler, C.E.; Yu, P.; Hanasono, M.M.; Chang, E.I. The Profunda Artery Perforator Flap: A Versatile Option for Head and Neck Reconstruction. Plast. Reconstr. Surg. 2021, 147, 1401–1412. [Google Scholar] [CrossRef]
- Chim, H. Perforator mapping and clinical experience with the superthin profunda artery perforator flap for reconstruction in the upper and lower extremities. J. Plast. Reconstr. Aesthetic Surg. JPRAS 2023, 81, 60–67. [Google Scholar] [CrossRef]
- Koshima, I.; Nanba, Y.; Tsutsui, T.; Takahashi, Y.; Urushibara, K.; Inagawa, K.; Hamasaki, T.; Moriguchi, T. Superficial circumflex iliac artery perforator flap for reconstruction of limb defects. Plast. Reconstr. Surg. 2004, 113, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Suh, H.S.P.; Jeong, H.H.; Choi, D.H.; Hong, J.P.J.P. Study of the Medial Superficial Perforator of the Superficial Circumflex Iliac Artery Perforator Flap Using Computed Tomographic Angiography and Surgical Anatomy in 142 Patients. Plast. Reconstr. Surg. 2017, 139, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Franchi, A.; Patanè, L.; Hummel, C.H.; Jung, F. Confusion Regarding the Anatomy of the Superficial Inferior Epigastric Artery and the Superficial Circumflex Iliac Artery Superficial Branch. Plast. Reconstr. Surg.-Glob. Open 2024, 12, e5714. [Google Scholar] [CrossRef] [PubMed]
- Blondeel, P.N.; Van Landuyt, K.H.I.; Monstrey, S.J.M.; Hamdi, M.; Matton, G.E.; Allen, R.J.; Dupin, C.; Feller, A.-M.; Koshima, I.; Kostakoglu, N.; et al. The “Gent” consensus on perforator flap terminology: Preliminary definitions. Plast. Reconstr. Surg. 2003, 112, 1378–1383; discussion 1384–1387. [Google Scholar] [CrossRef]
- Fernandez-Garrido, M.; Nunez-Villaveiran, T.; Zamora, P.; Masia, J.; Leon, X. The extended SCIP flap: An anatomical and clinical study of a new SCIP flap design. J. Plast. Reconstr. Aesthetic Surg. JPRAS 2022, 75, 3217–3225. [Google Scholar] [CrossRef]
- Hong, J.P. The Superficial Circumflex Iliac Artery Perforator Flap in Lower Extremity Reconstruction. Clin. Plast. Surg. 2021, 48, 225–233. [Google Scholar] [CrossRef]
- Yoshimatsu, H.; Steinbacher, J.; Meng, S.; Hamscha, U.M.; Weninger, W.J.; Tinhofer, I.E.; Harima, M.; Fuse, Y.; Yamamoto, T.; Tzou, C.H.J. Superficial Circumflex Iliac Artery Perforator Flap: An Anatomical Study of the Correlation of the Superficial and the Deep Branches of the Artery and Evaluation of Perfusion from the Deep Branch to the Sartorius Muscle and the Iliac Bone. Plast. Reconstr. Surg. 2019, 143, 589–602. [Google Scholar] [CrossRef]
- Haddock, N.T.; Teotia, S.S. Consecutive 265 Profunda Artery Perforator Flaps: Refinements, Satisfaction, and Functional Outcomes. Plast. Reconstr. Surg.-Glob. Open 2020, 8, e2682. [Google Scholar] [CrossRef]
- Cohen, Z.; Azoury, S.C.; Matros, E.; Nelson, J.A.; Allen, R.J. Modern Approaches to Alternative Flap-Based Breast Reconstruction: PAP Flap. Clin. Plast. Surg. 2023, 50, 289–299. [Google Scholar] [CrossRef]
- Hunter, J.; Lardi, A.; Dower, D.; Farhadi, J. Evolution from the TUG to PAP flap for breast reconstruction: Comparison and refinements of technique. J. Plast. Reconstr. Aesthetic Surg. 2015, 68, 960–965. [Google Scholar] [CrossRef]
- Atzeni, M.; Salzillo, R.; Haywood, R.; Persichetti, P.; Figus, A. Breast reconstruction using the profunda artery perforator (PAP) flap: Technical refinements and evolution, outcomes, and patient satisfaction based on 116 consecutive flaps. J. Plast. Reconstr. Aesthetic Surg. 2021, 75, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Tielemans, H.J.; van Kuppenveld, P.I.; Winters, H.; Hupkens, P.; Ulrich, D.J.; Hummelink, S. Breast reconstruction with the extended profunda artery perforator flap. J. Plast. Reconstr. Aesthetic Surg. JPRAS 2021, 74, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Haddock, N.T.; Lakatta, A.C.B.; Teotia, S.S. Categorizing Patient Selection, Outcomes, and Indications in a Decade of 405 PAP Flaps. Plast. Reconstr. Surg. 2024, 154, 632e–640e. [Google Scholar] [CrossRef]
- Marchi, F.; Iandelli, A.; Pace, G.M.; Bellini, E.; Tirrito, A.; Costantino, A.; Cerri, L.; Greco, A.; Polimeni, A.; Parrinello, G.; et al. Surgical outcomes of profunda artery perforator flap in head and neck reconstruction: A systematic review and meta-analysis. Head Neck 2024, 47, 98–111. [Google Scholar] [CrossRef]
- Choi, D.H.; Suh, H.; Mukarramah, D.A.; Tashti, T.; Lee, K.; Yoon, C.; Hong, J.P. A New Plane of Elevation: The superficial fascial plane for perforator flap elevation. J. Reconstr. Microsurg. 2014, 30, 491–496. [Google Scholar] [CrossRef]
- Park, B.Y. Flap thinning: Defatting after conventional elevation. Arch. Plast. Surg. 2018, 45, 314–318. [Google Scholar] [CrossRef]
- Park, S.O.; Chang, H.; Imanishi, N. Anatomic basis for flap thinning. Arch. Plast. Surg. 2018, 45, 298–303. [Google Scholar] [CrossRef]
- Agostini, T.; Lazzeri, D.; Spinelli, G. Anterolateral thigh flap thinning: Techniques and complications. Ann. Plast. Surg. 2014, 72, 246–252. [Google Scholar] [CrossRef]
- Daar, D.A.; Abdou, S.A.; Cohen, J.M.; Wilson, S.C.; Levine, J.P. Is the Medial Sural Artery Perforator Flap a New Workhorse Flap? A Systematic Review and Meta-Analysis. Plast. Reconstr. Surg. 2019, 143, 393e–403e. [Google Scholar] [CrossRef]
- Haddock, N.T.; Lakatta, A.C.B.; Steppe, C.B.; Teotia, S.S. DIEP Flap versus PAP Flap versus LAP Flap: A Propensity-Matched Analysis of Aesthetic Outcomes, Complications, and Satisfaction. Plast. Reconstr. Surg. 2024, 154, 41S–51S. [Google Scholar] [CrossRef]
- Nakatsuka, K.; Fuse, Y.; Karakawa, R.; Yano, T.; Yoshimatsu, H. Comparing seroma formation rate after harvest of the deep inferior epigastric artery perforator flap and the superficial abdominal perforator flaps in autologous breast reconstruction: A propensity-matched analysis. Microsurgery 2023, 43, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, Y.; Yamamoto, T.; Fujisawa, K.; Saito, T.; Ishiura, R.; Iida, T. Inguinal seroma prevention after superficial circumflex iliac artery perforator flap harvest using non-microsurgical lympho-venous shunt. J. Plast. Reconstr. Aesthetic Surg. 2015, 68, 1479–1481. [Google Scholar] [CrossRef] [PubMed]
- Scaglioni, M.F.; Meroni, M.; Fritsche, E. Inguinal seroma/lymphocele prevention after superficial circumflex iliac artery perforator (SCIP) flap harvest using the deep branch as donor vein for lymphovenous anastomosis (LVA). Microsurgery 2021, 41, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Domingo, M.J.; Bustos, V.P.; Akintayo, R.; Mahmoud, A.; Fanning, J.E.; Foppiani, J.A.; Miller, A.S.; Cauley, R.P.; Lin, S.J.; Lee, B.T. The versatility of the scapular free flap: A workhorse flap? A systematic review and meta-analysis. Microsurgery 2024, 44, e31203. [Google Scholar] [CrossRef]
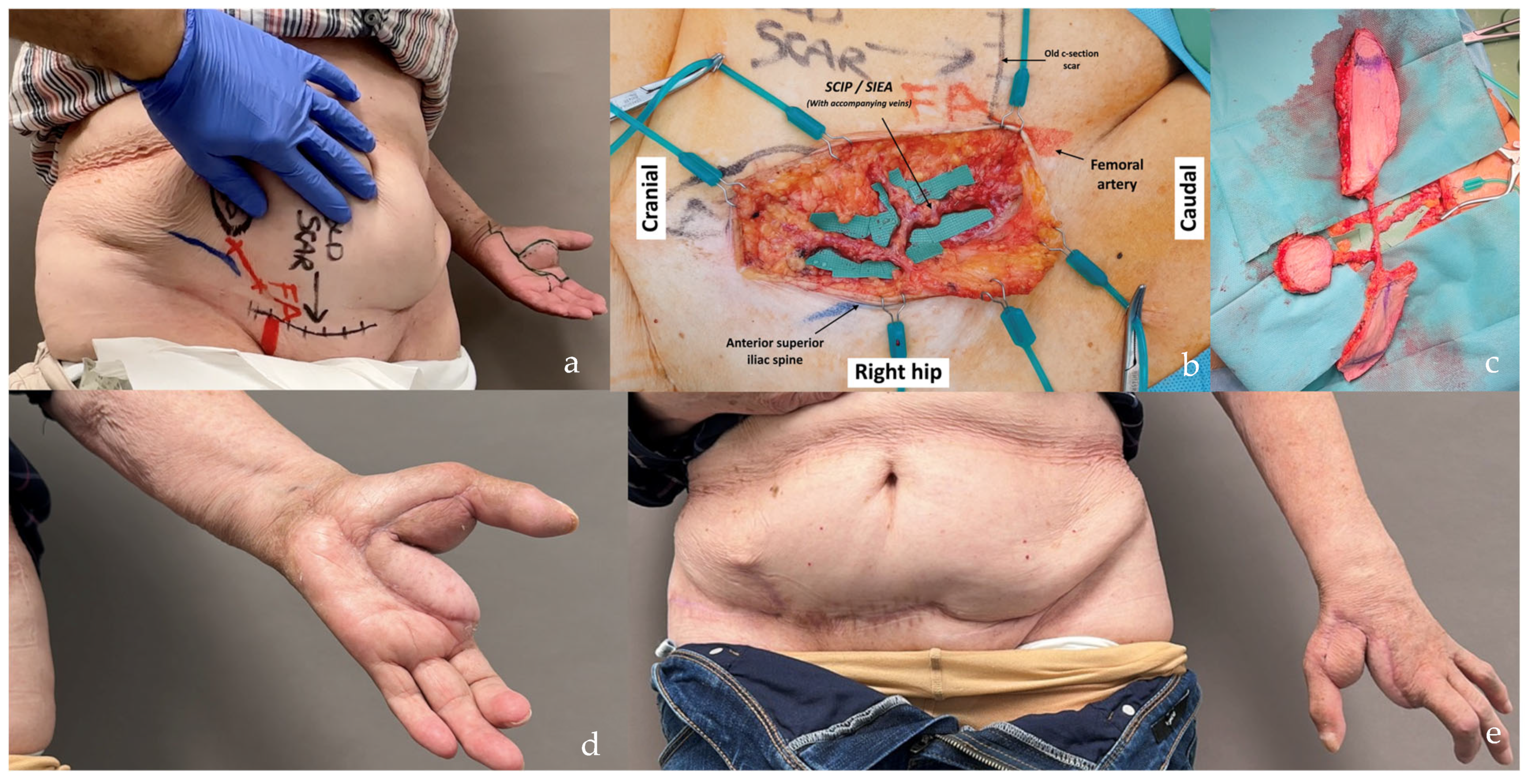
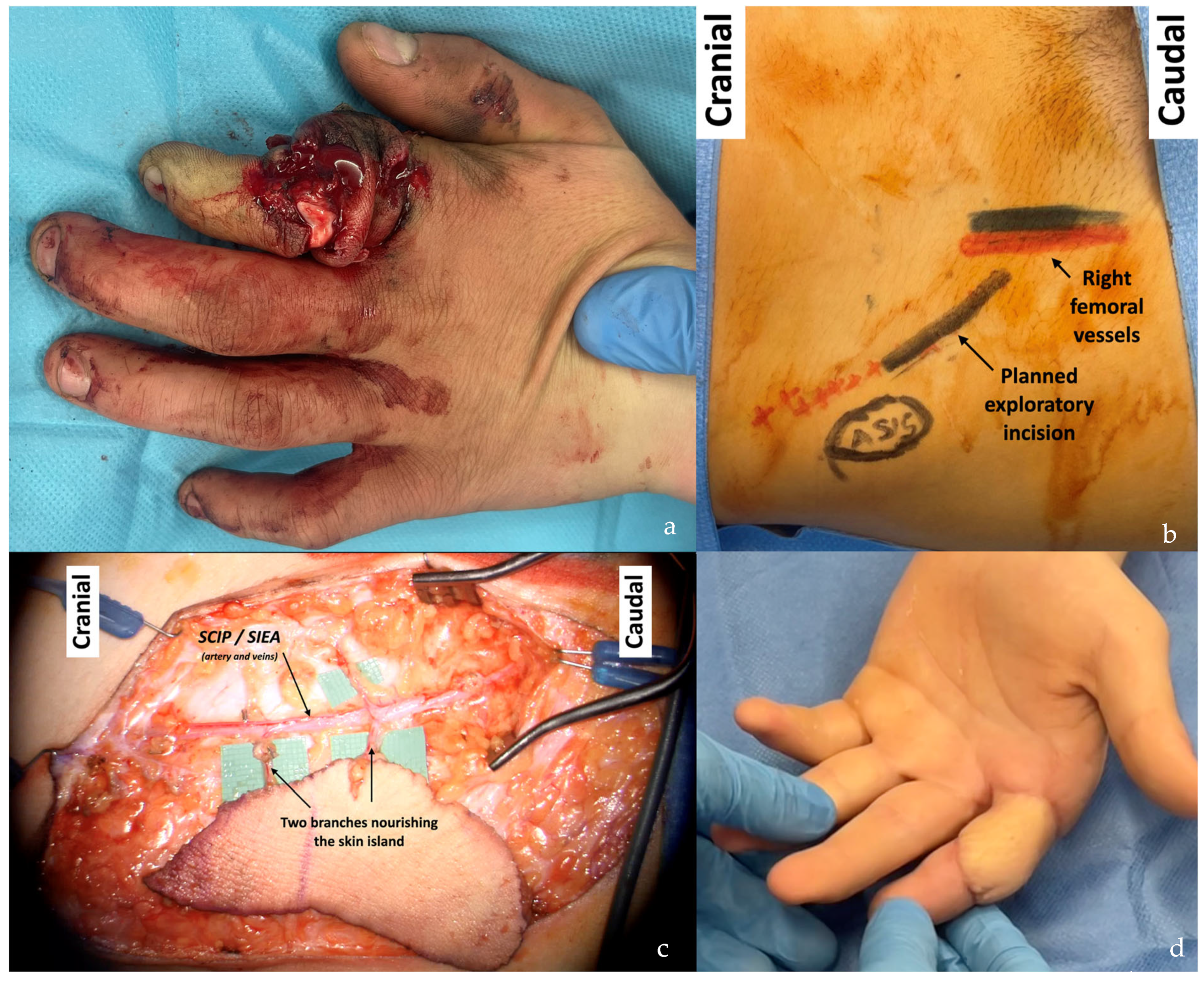
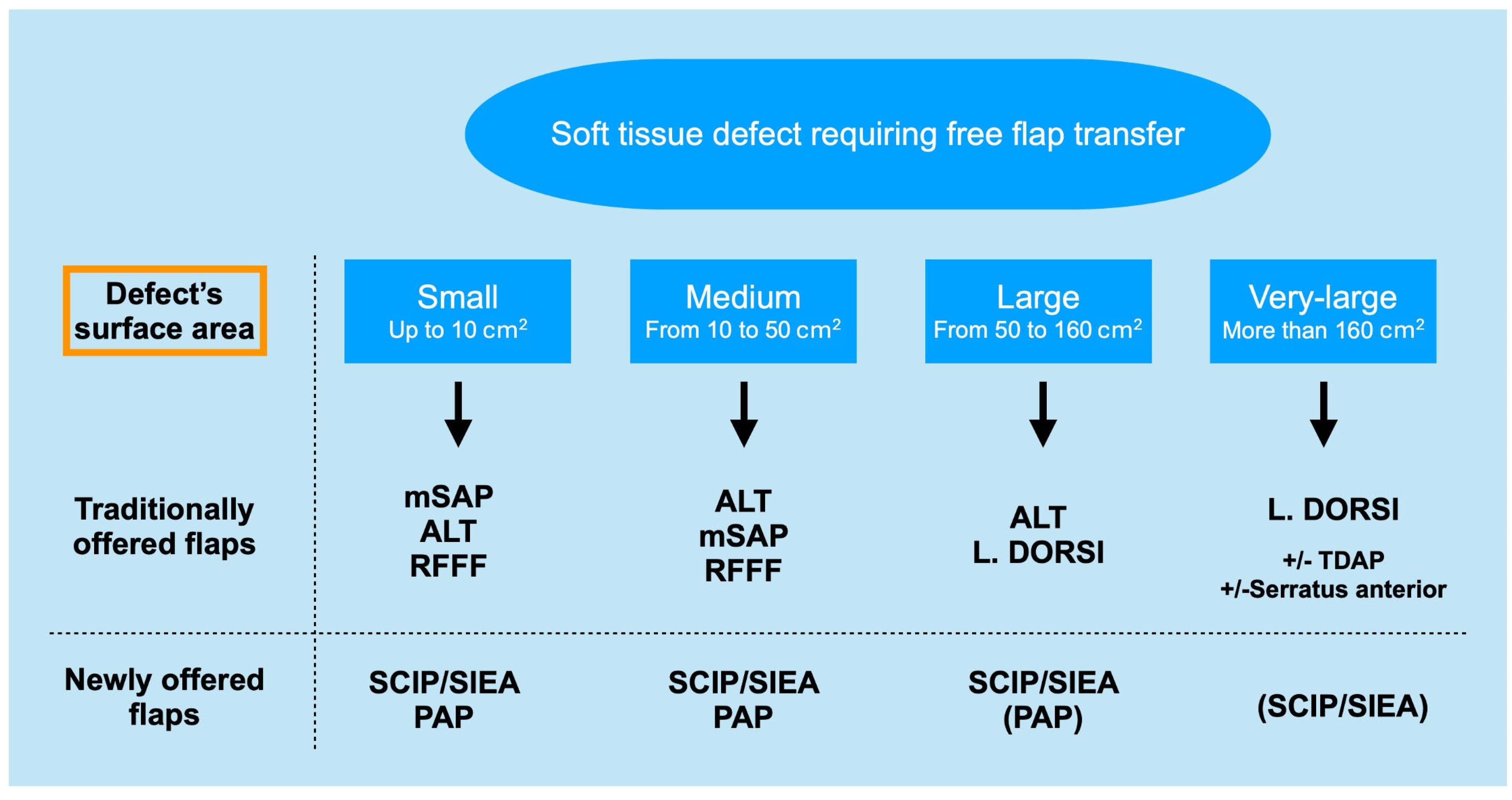
| Flap Type | Advantages | Disadvantages |
|---|---|---|
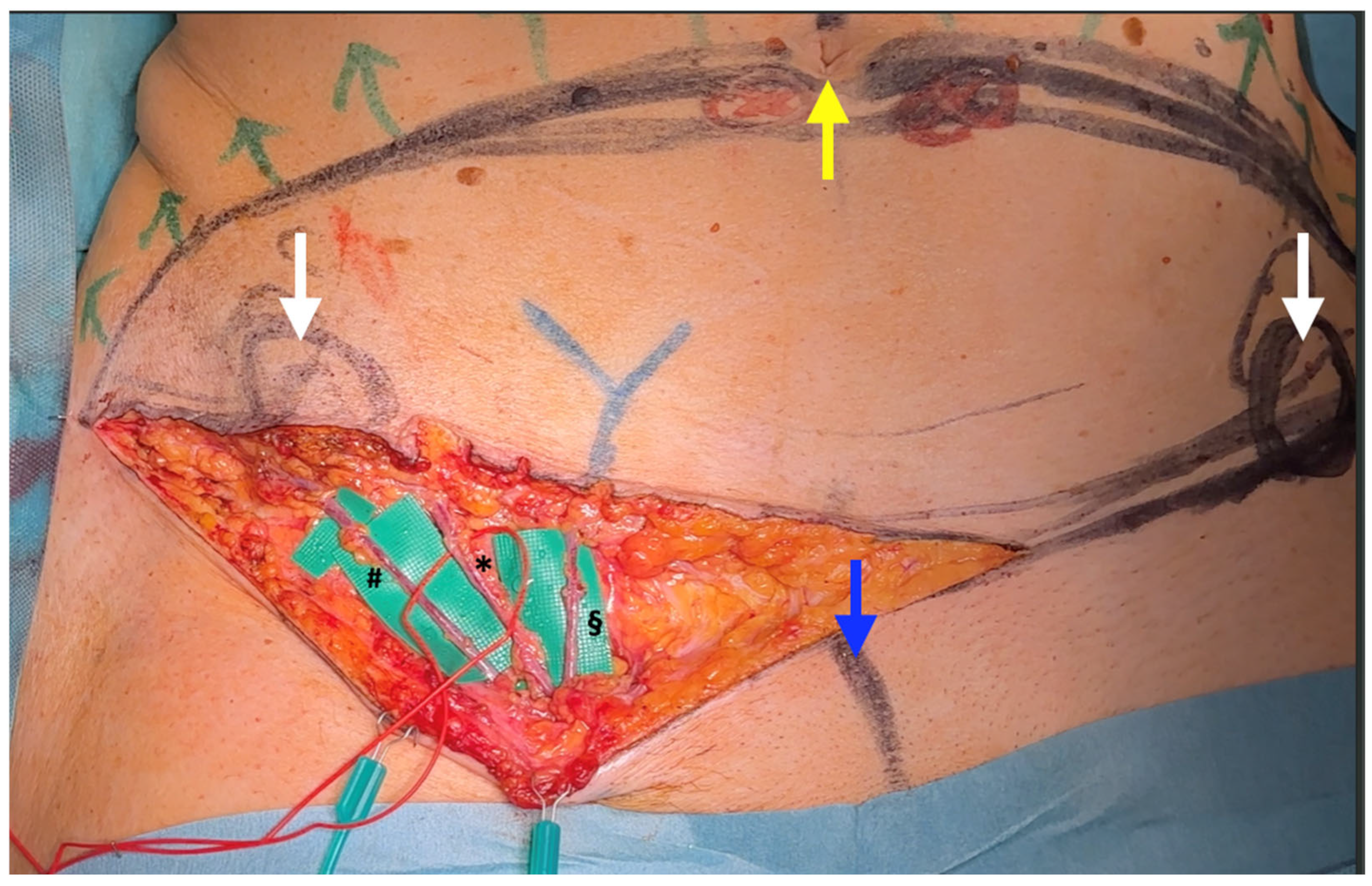
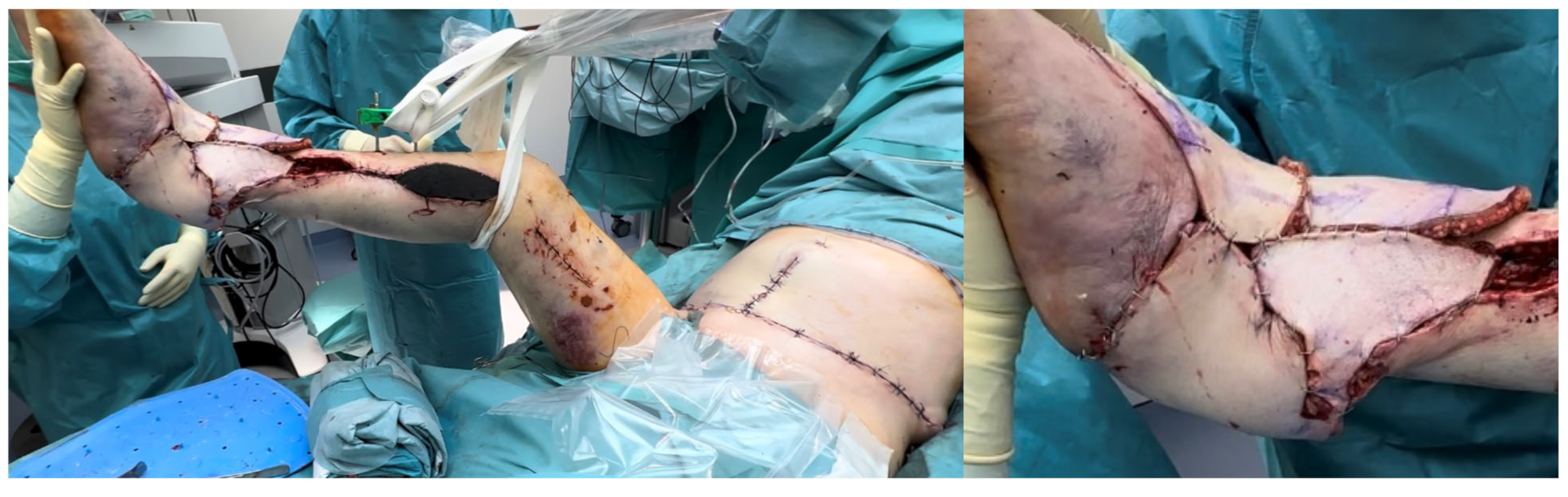
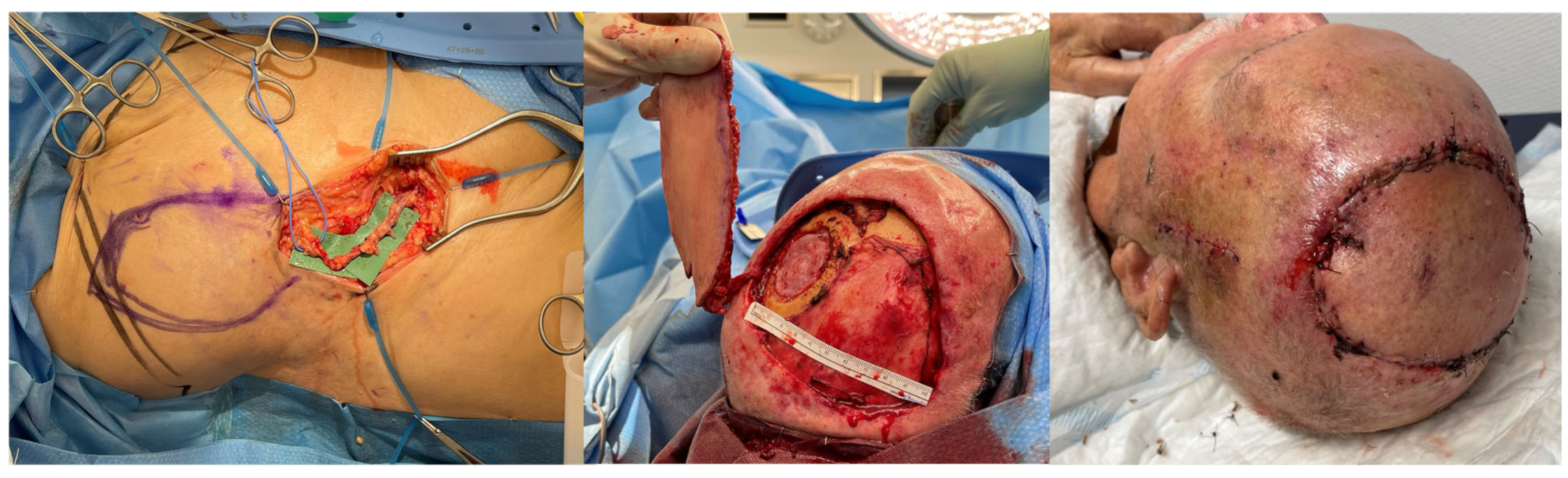
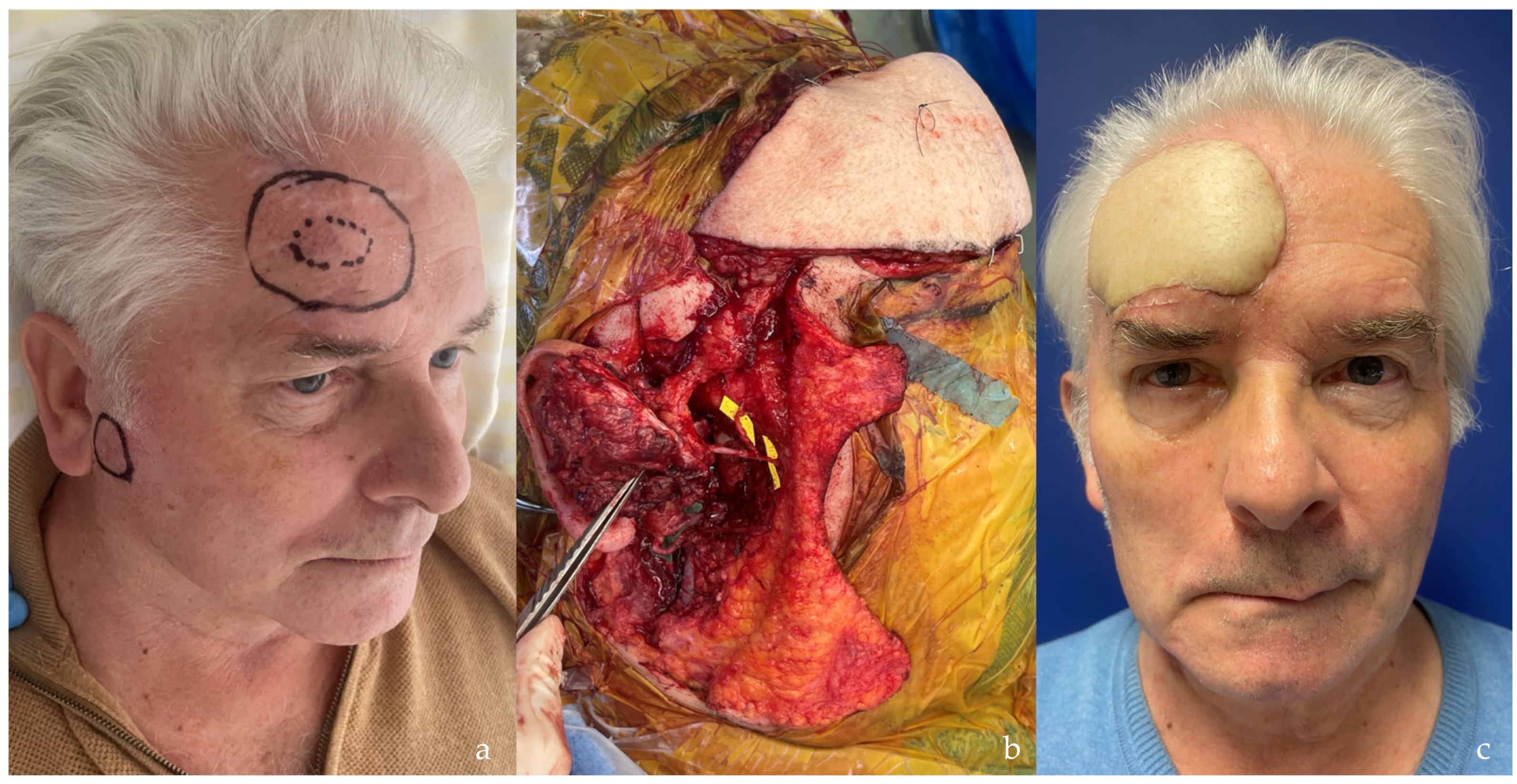
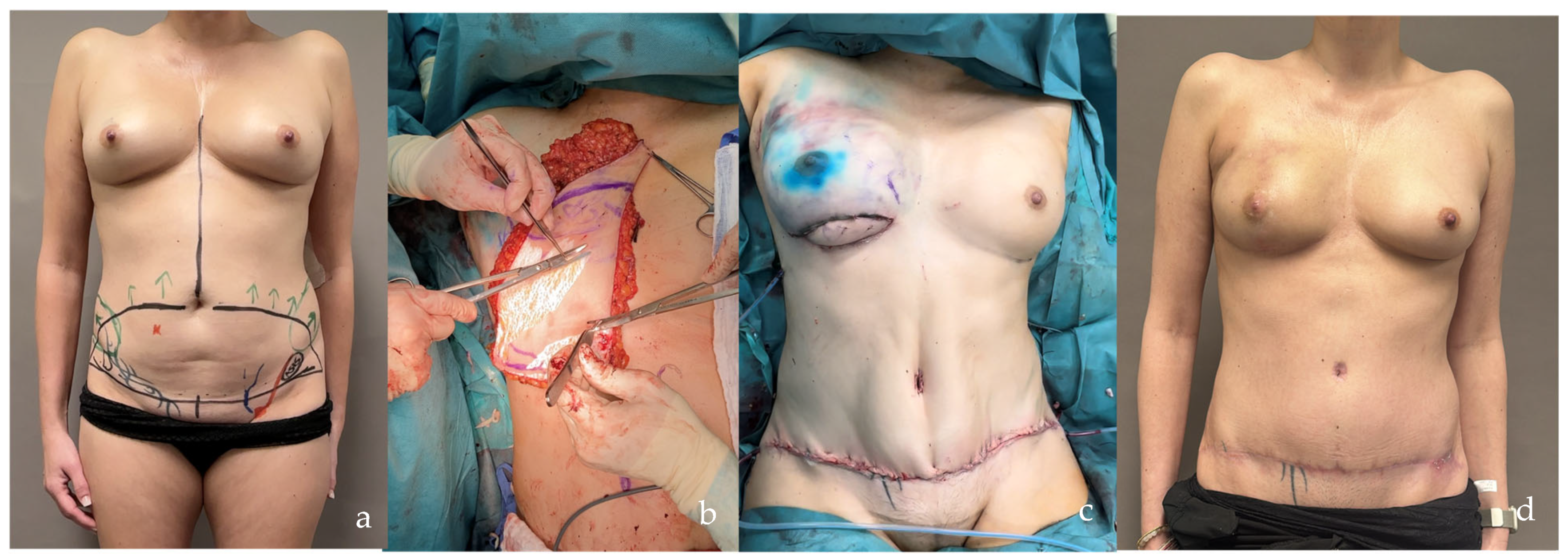
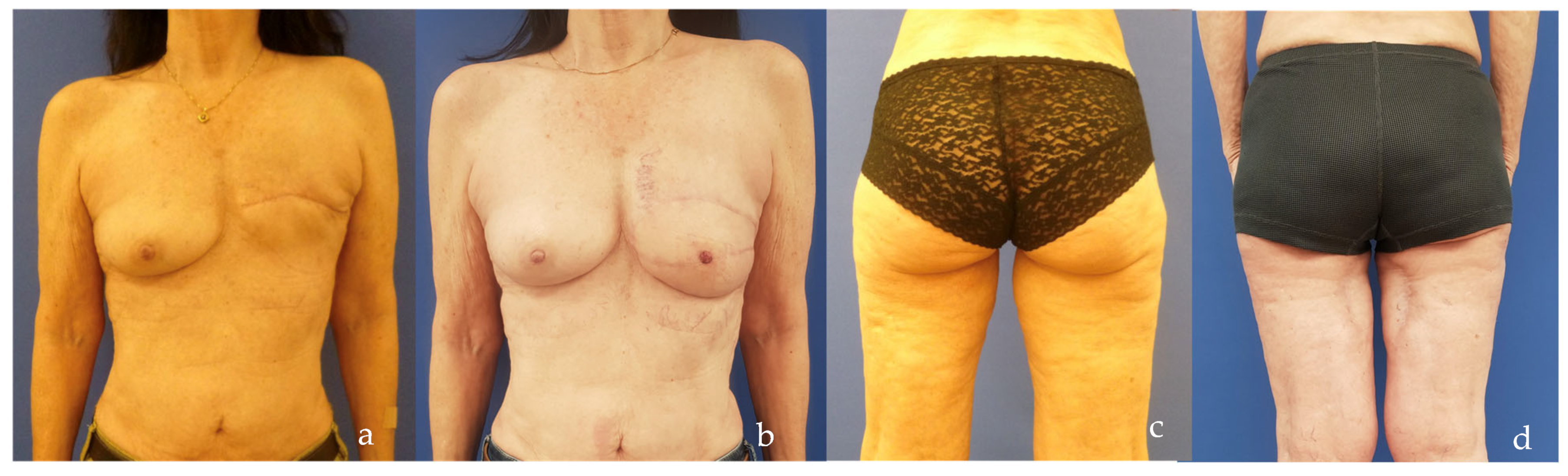
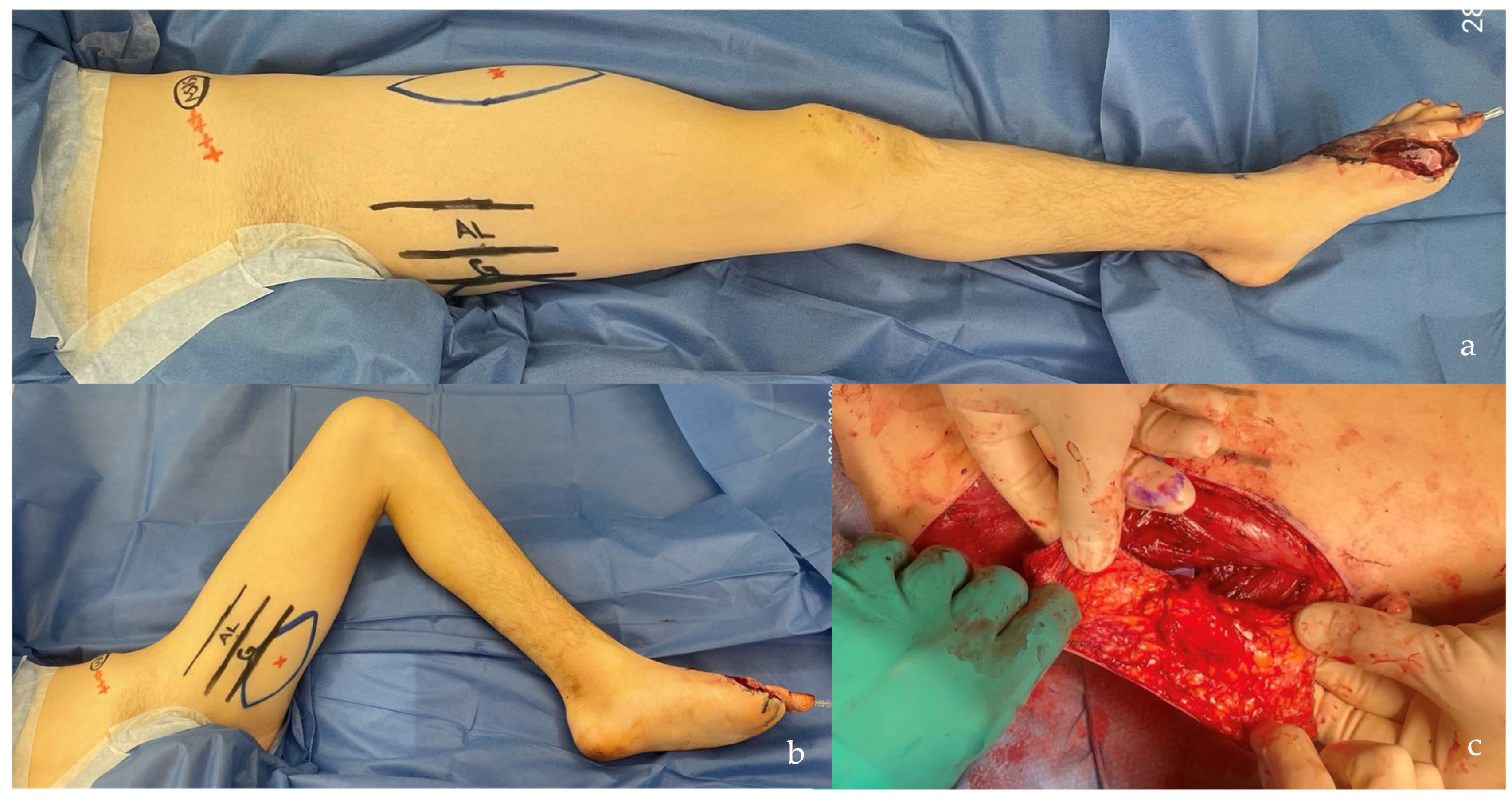
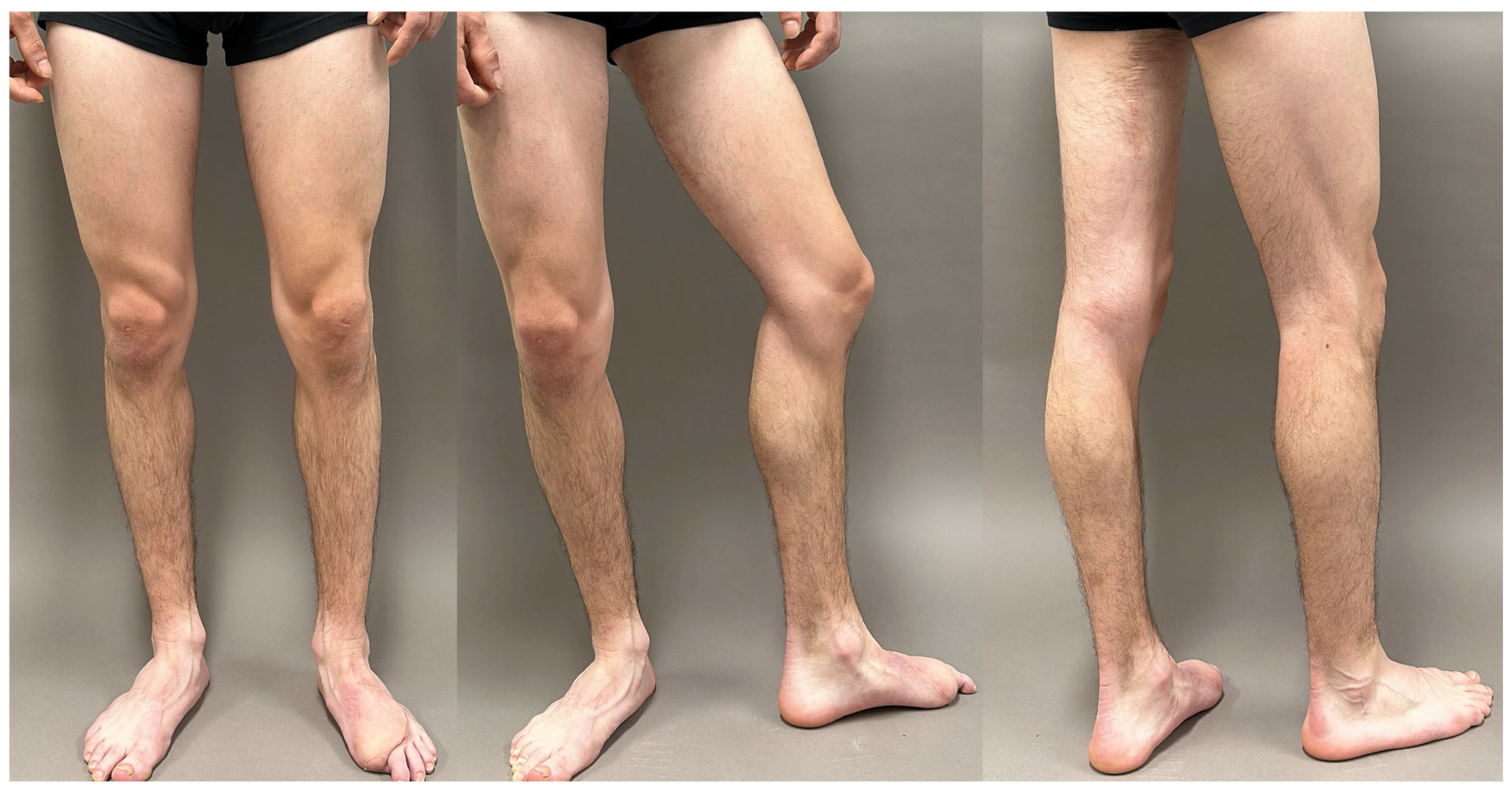
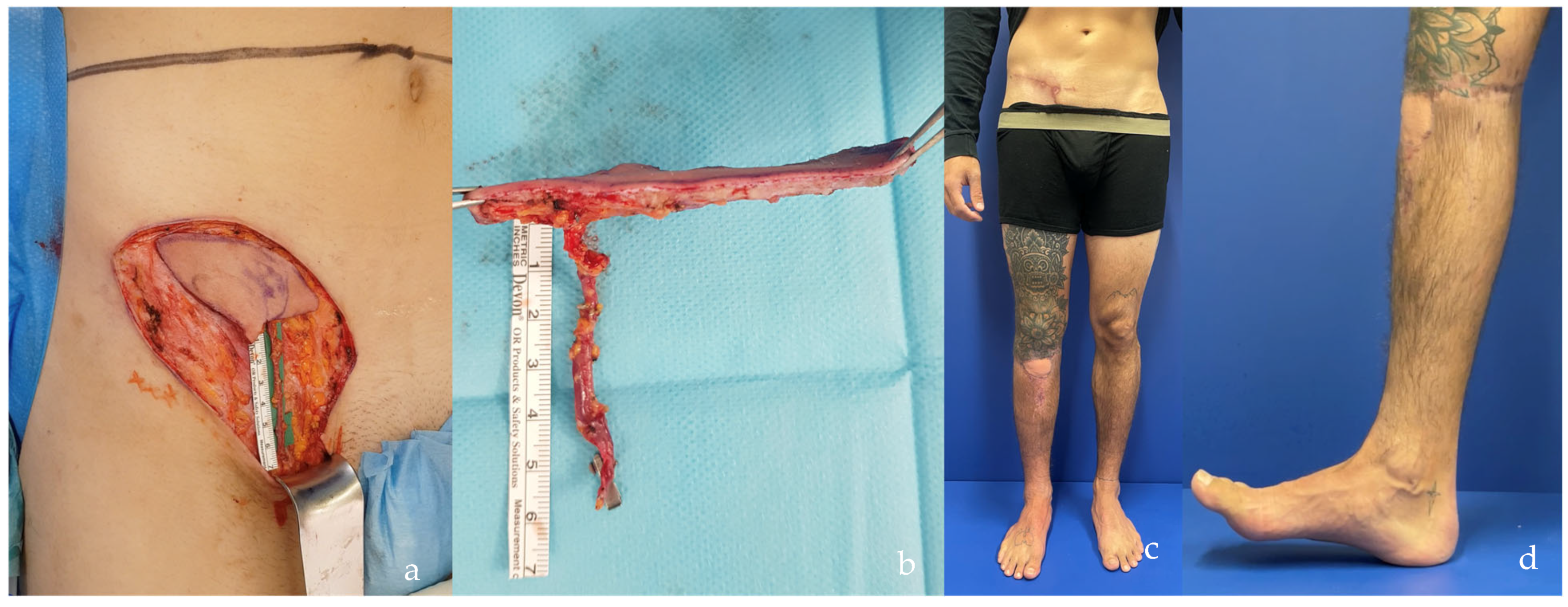
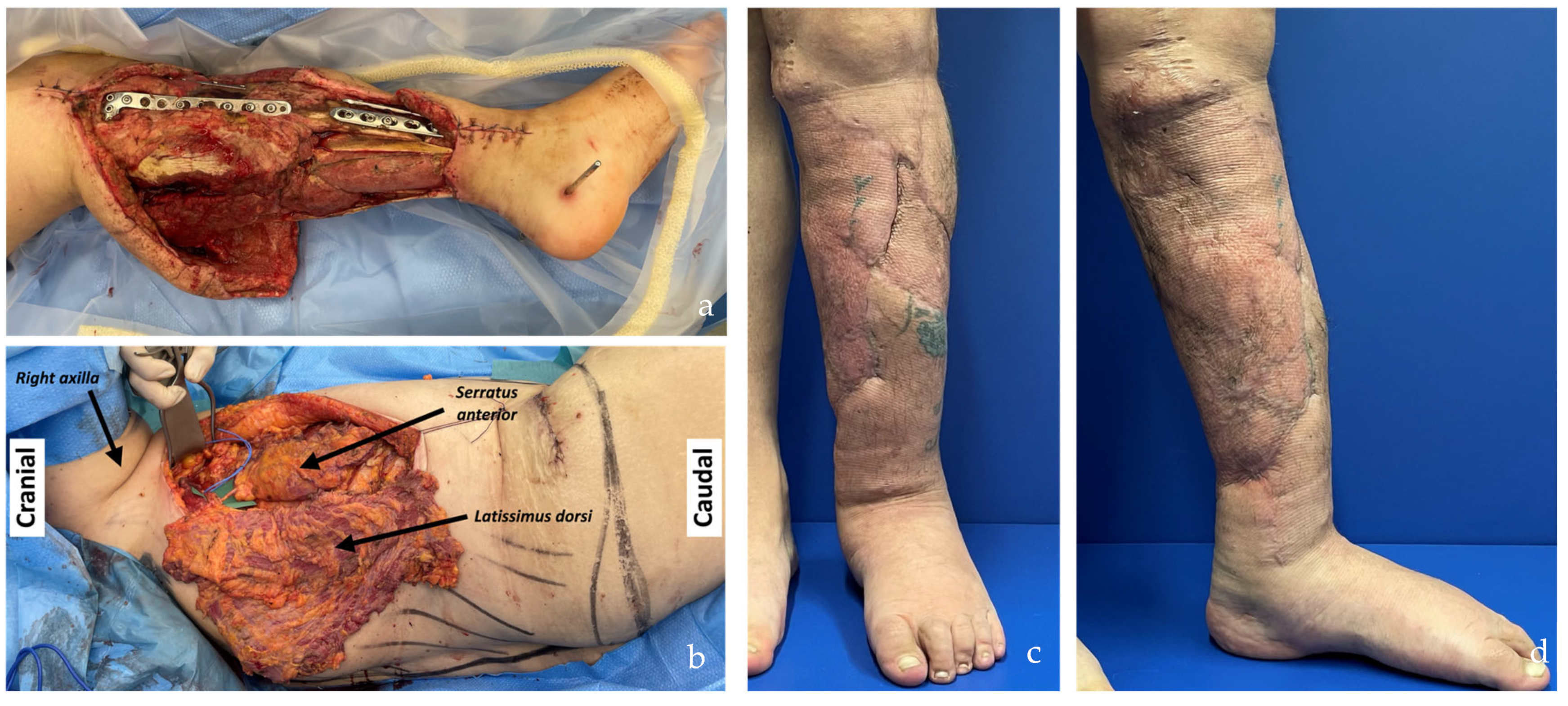
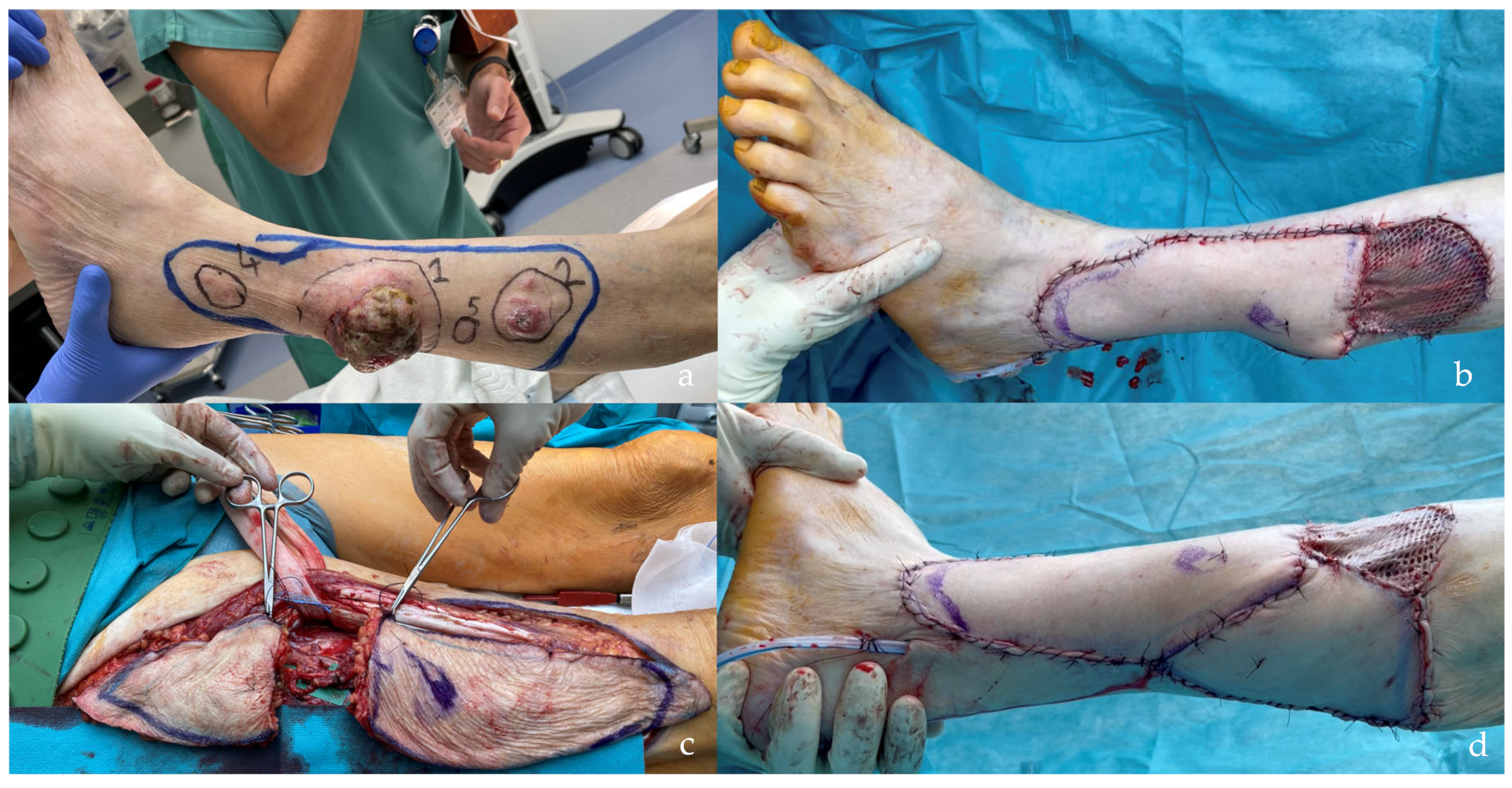
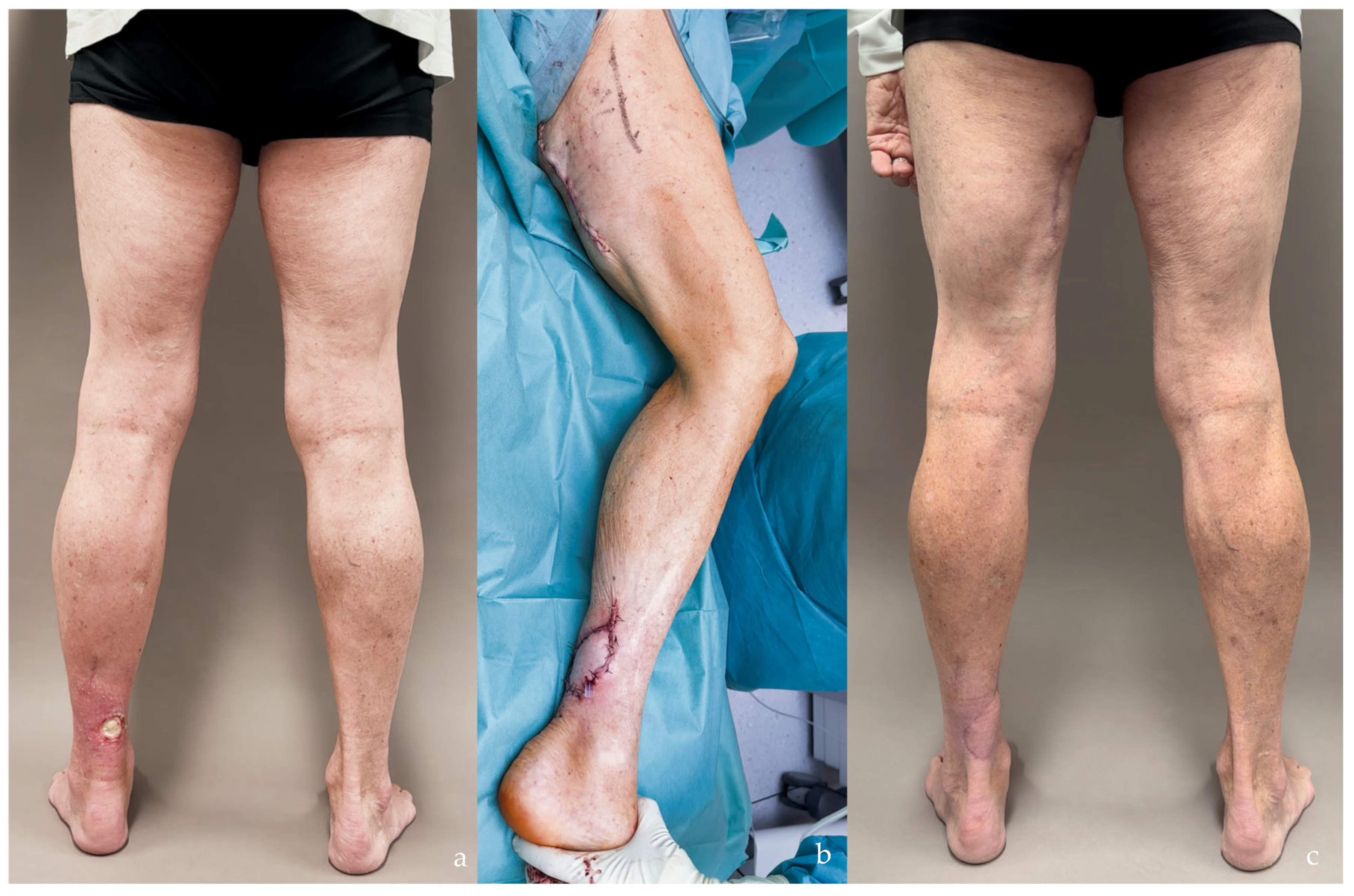
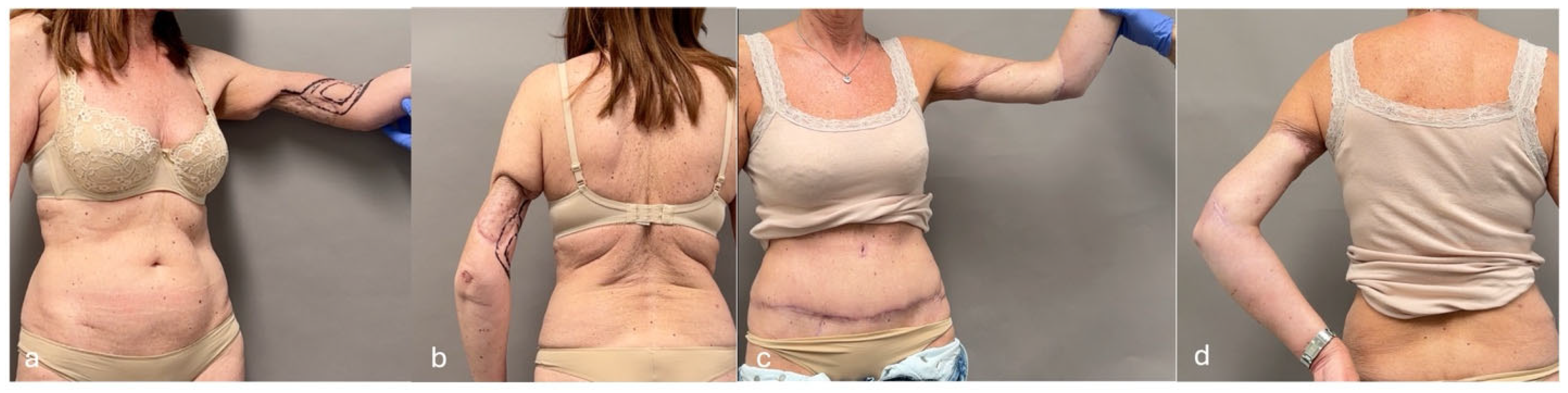
| SCIP/SIEA Flap | - Donor Site Scar: Mostly hidden by underwear/pants. If large flaps are harvested, the donor site can be closed in an abdominoplasty fashion to enhance aesthetics (Figure 1, Figure 8 and Figure 16). - Two-Team Approach: Ideal for limb cases, head and neck (H&N) cases, and suitable for breast cases. - Dissection Type: No intramuscular dissection or muscle sacrifice needed, and no muscle relaxation required. - Flap Dimensions and Shape Versatility: Ranges from small to very large with multiple pedicle branches for chimeric, multi-lobule flaps (Figure 17). Can be used in flow-through finger replantations (Figure 18). - Pedicle Length: Long pedicle possible with centrifugal pedicle dissection (Figure 1, Figure 5 and Figure 17). - Bone Chimerism: Can include the ASIS as a vascularized bone if using the deep branch of the SCIA. | - Vessel Variability: High anatomical variability in vessel size and branching. Small, delicate arteries pose higher complication risks (Figure 12 and Figure 17). Requires more than one vein anastomosis for adequate flap perfusion with larger flaps (Figure 1). - Donor Site Morbidity: Risk of lymphatic damage with poor dissection in the femoral triangle. Potential for sensory loss in the proximal thigh if the lateral femoral cutaneous or genitofemoral nerves are transected. - Skin Color and Texture: Mismatch with surrounding areas when used for head, neck, or limb reconstruction (Figure 6, Figure 12, Figure 16, Figure 17 and Figure 18). - Flap Thickness: Harvesting thin flaps can be challenging, especially in high-BMI patients. |
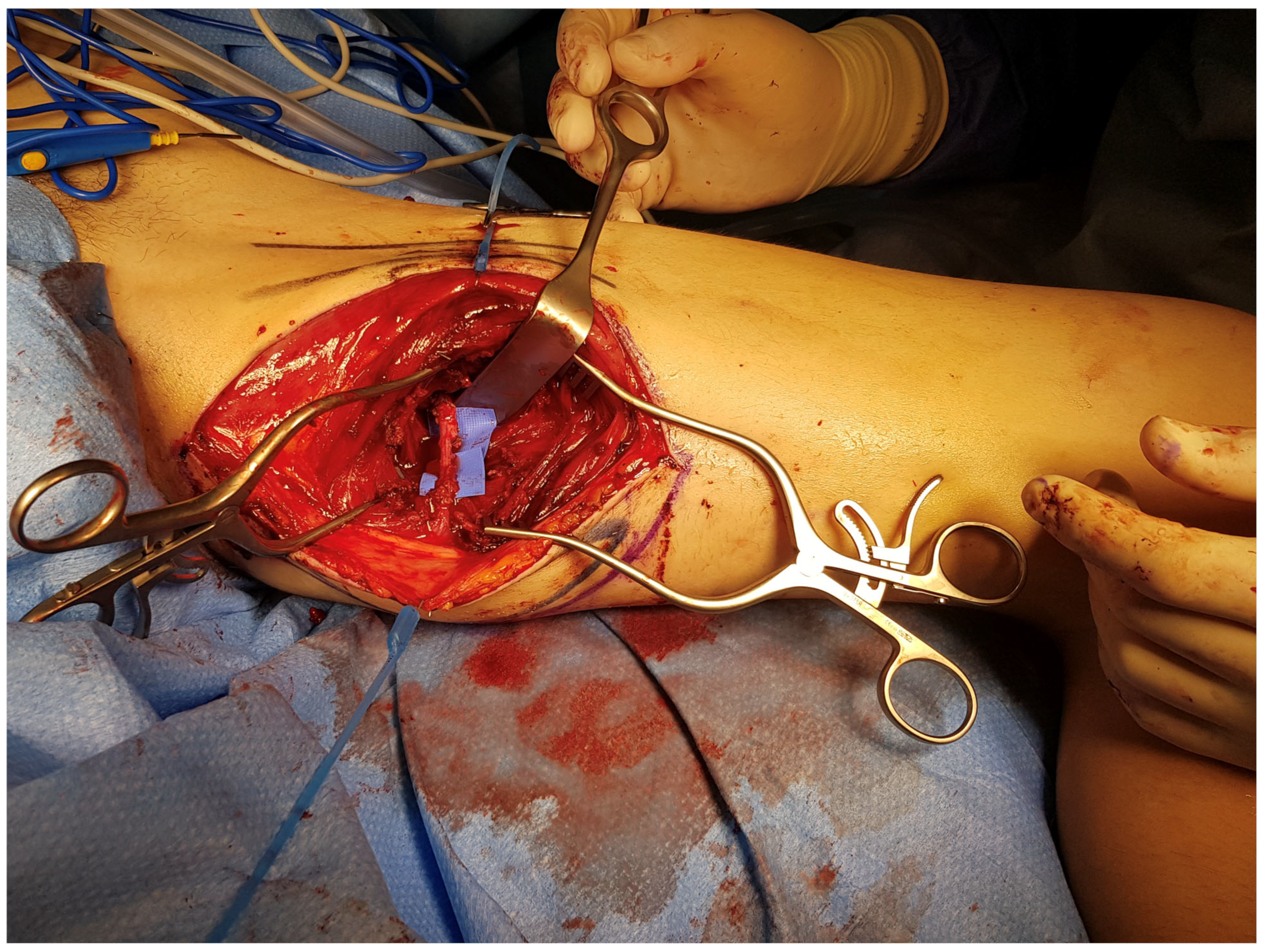
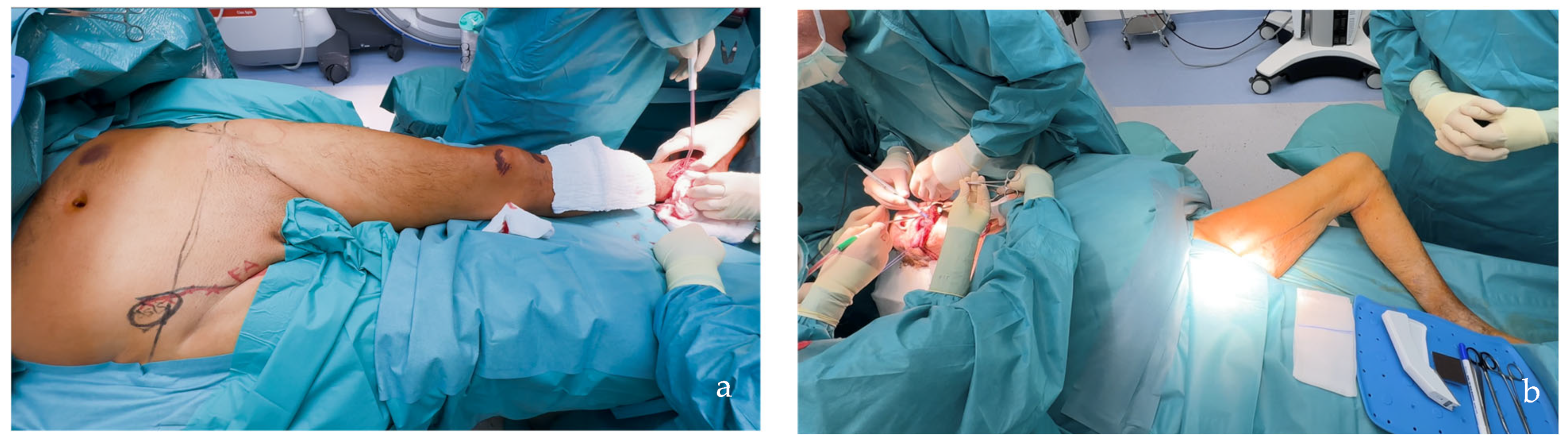
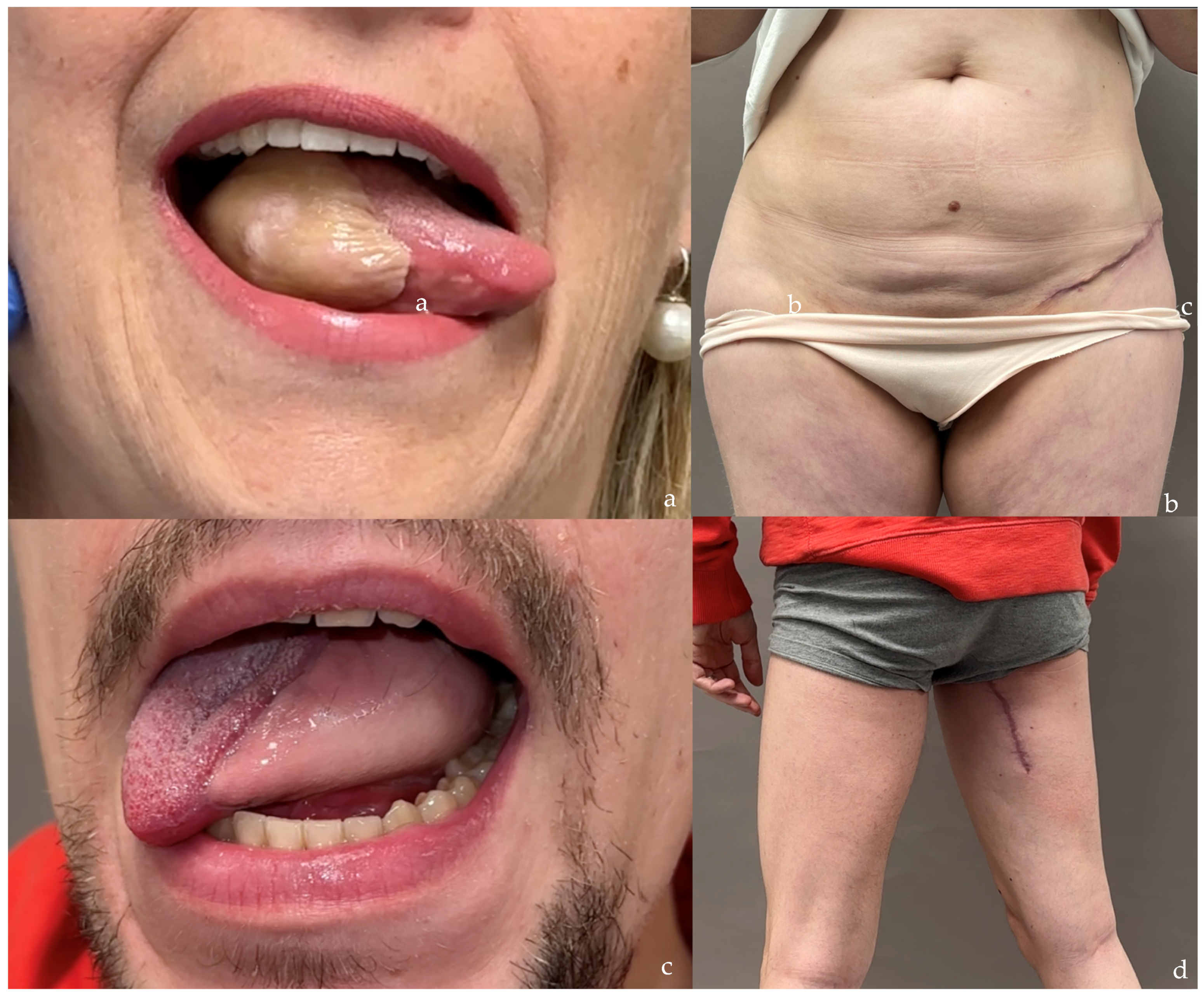
| PAP Flap | - Donor Site Scar: Positioned in the postero-medial thigh, hidden from patient view. - Pedicle: Reliable perforator, with a long pedicle and good vessel caliber. - Two-Team Approach: Optimal harvest position for two-team cases in H&N and breast procedures. - Muscle Chimerism: Muscular branches of the pedicle can include muscle as needed. | - Flap Thickness: Technically demanding to harvest thin flaps in high-BMI patients. - Surgeon Positioning: Harvesting requires a frog-leg position, which can be uncomfortable (Figure 4). - Dissection Type: Requires intramuscular dissection and some muscle sacrifice, and intraoperative muscle relaxation may not be possible if simultaneous facial nerve monitoring is necessary (Figure 2). - Pedicle Characteristics: Usually has only one perforator reaching the skin, making multi-lobule skin flaps difficult (see comparison with ALT flap, Figure 14). - Bone Chimerism: Not possible with this flap. - Nerve Characteristics: No accompanying motor nerve, and few reports exist on flap reinnervation with sensitive nerve inclusion. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franchi, A.; Perozzo, F.A.G.; Tiengo, C.; Walber, J.; Parisato, A.; Jandali, A.R.; Jung, F. SCIP/SIEA and PAP: The New Workhorse Flaps in Soft Tissue Reconstruction for All Body Regions. J. Clin. Med. 2025, 14, 921. https://doi.org/10.3390/jcm14030921
Franchi A, Perozzo FAG, Tiengo C, Walber J, Parisato A, Jandali AR, Jung F. SCIP/SIEA and PAP: The New Workhorse Flaps in Soft Tissue Reconstruction for All Body Regions. Journal of Clinical Medicine. 2025; 14(3):921. https://doi.org/10.3390/jcm14030921
Chicago/Turabian StyleFranchi, Alberto, Filippo Andrea Giovanni Perozzo, Cesare Tiengo, Jonas Walber, Alice Parisato, Abdul Rahman Jandali, and Florian Jung. 2025. "SCIP/SIEA and PAP: The New Workhorse Flaps in Soft Tissue Reconstruction for All Body Regions" Journal of Clinical Medicine 14, no. 3: 921. https://doi.org/10.3390/jcm14030921
APA StyleFranchi, A., Perozzo, F. A. G., Tiengo, C., Walber, J., Parisato, A., Jandali, A. R., & Jung, F. (2025). SCIP/SIEA and PAP: The New Workhorse Flaps in Soft Tissue Reconstruction for All Body Regions. Journal of Clinical Medicine, 14(3), 921. https://doi.org/10.3390/jcm14030921







