Adjunctive Treatment Effect of Non-Thermal Atmospheric Pressure Plasma in Periodontitis-Induced Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
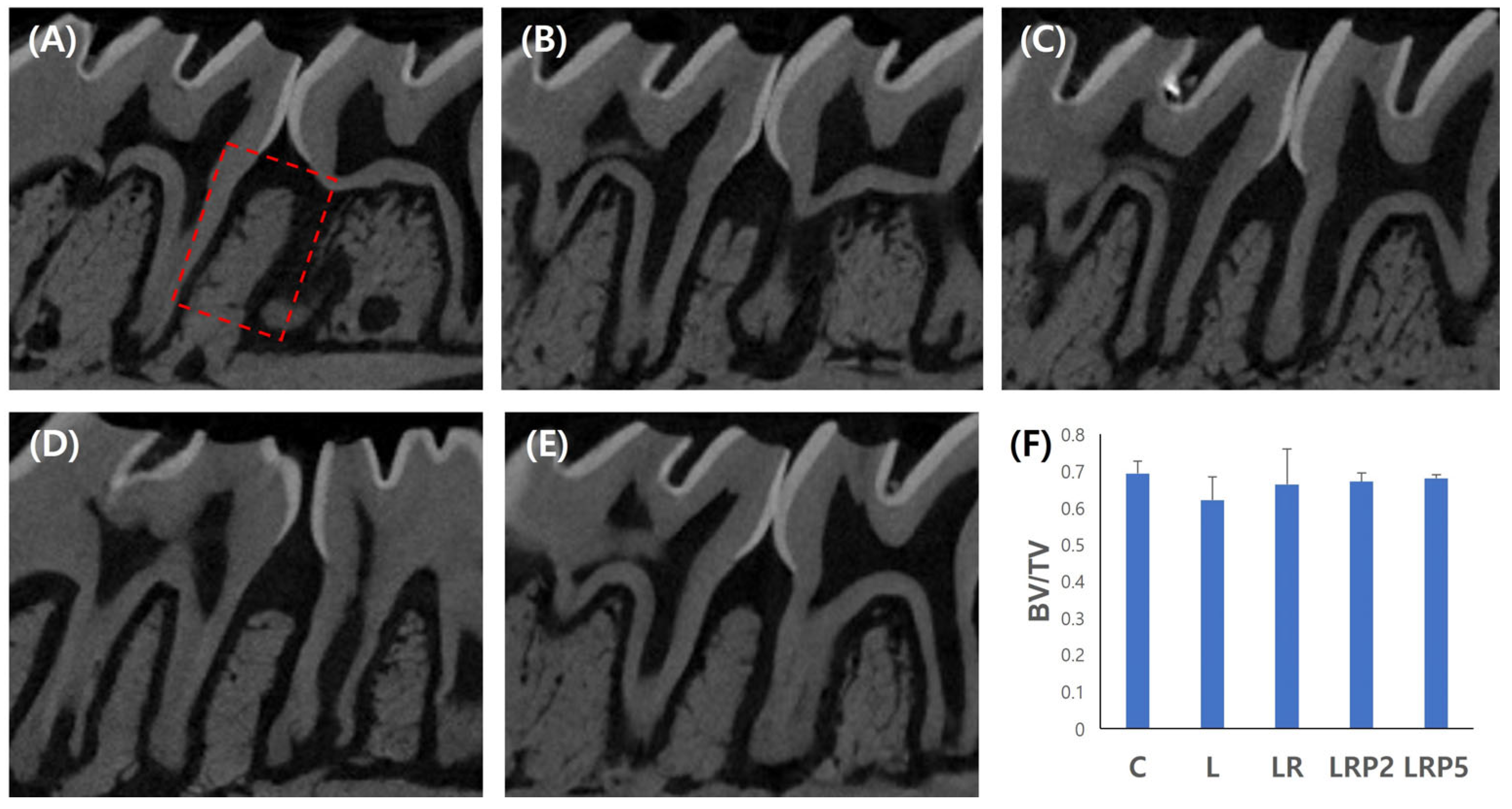
2.3. Micro-CT Analysis
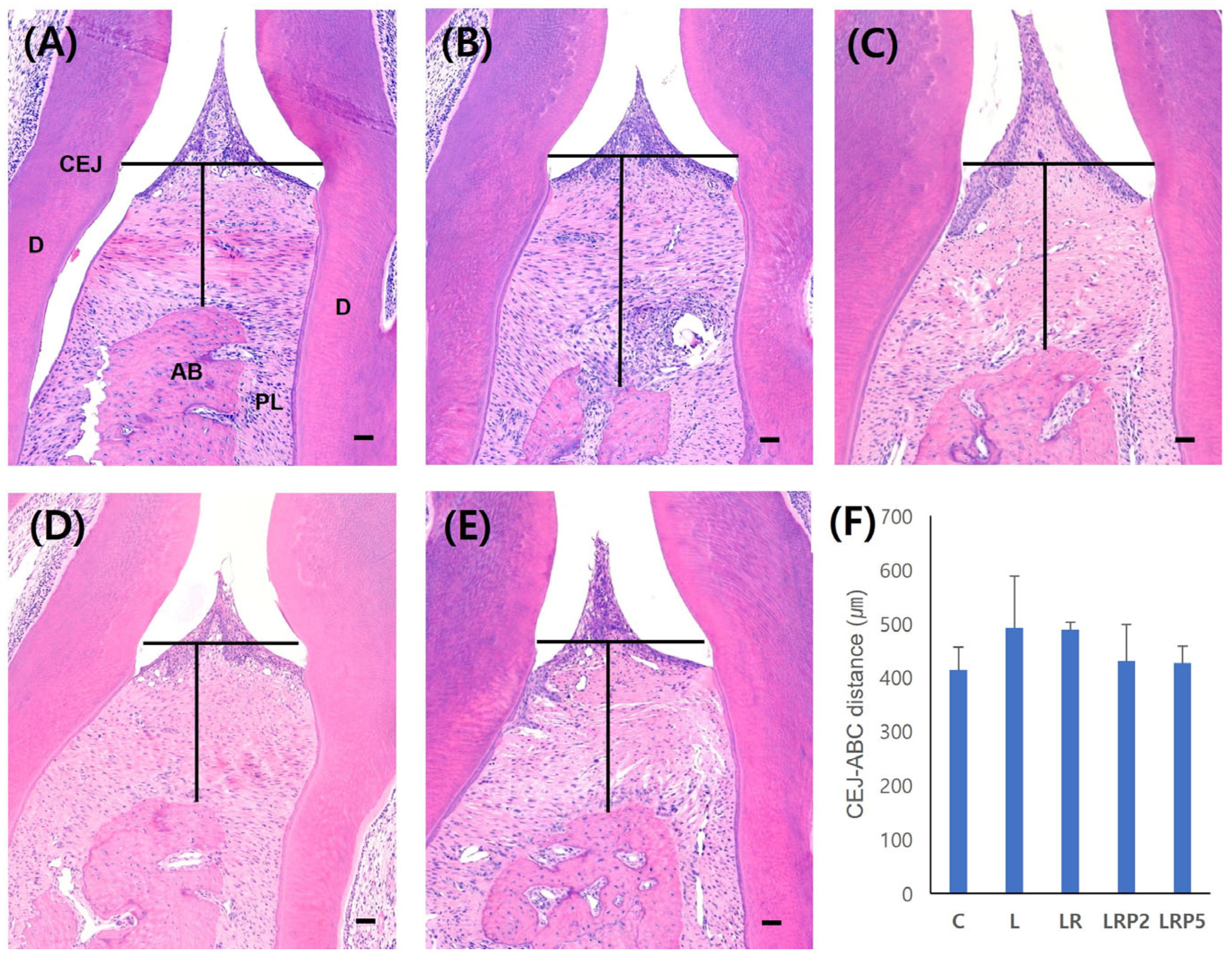
2.4. Histomorphometric Analysis
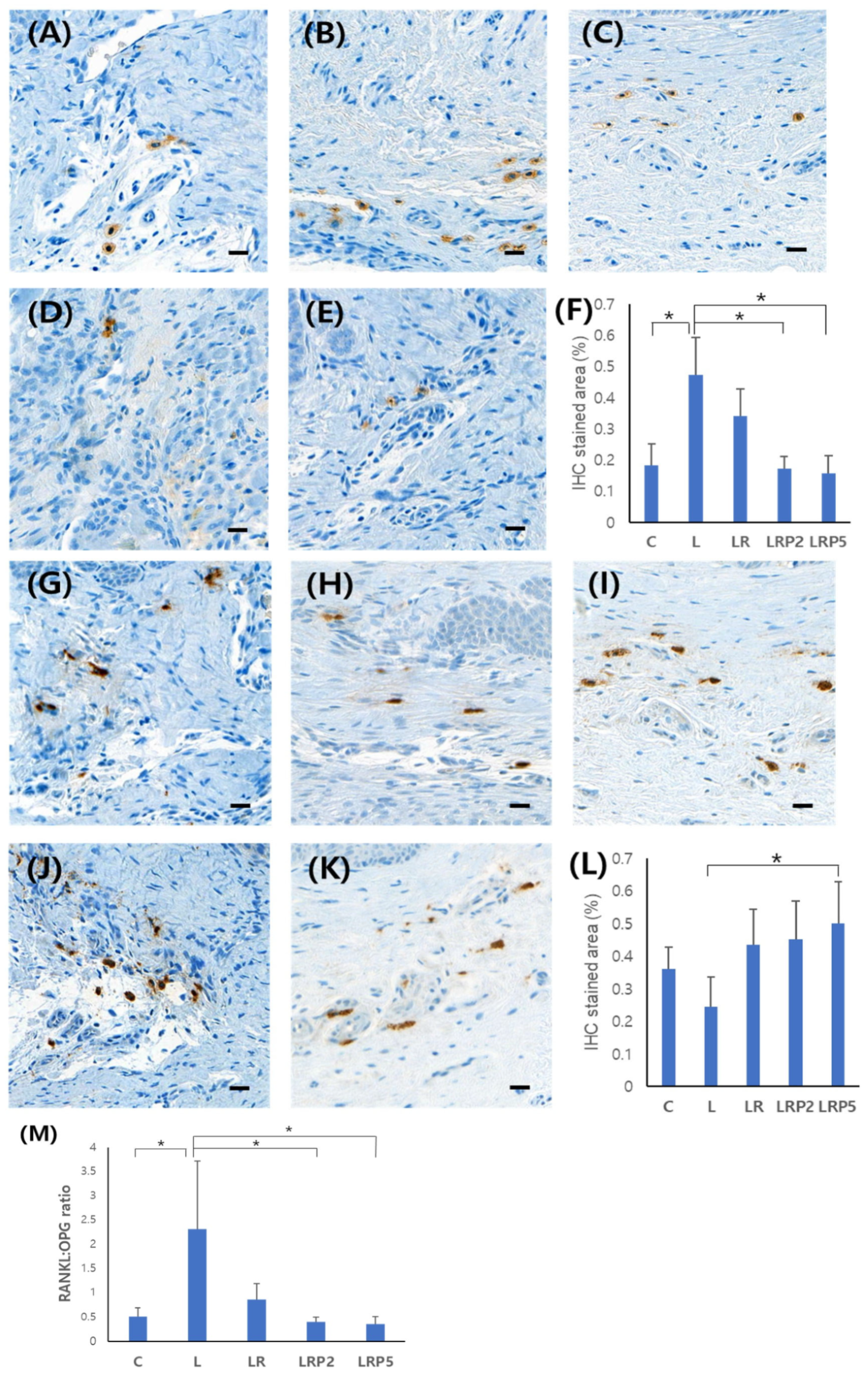
2.5. Immunohistochemistry Analysis of RANKL and OPG Expression
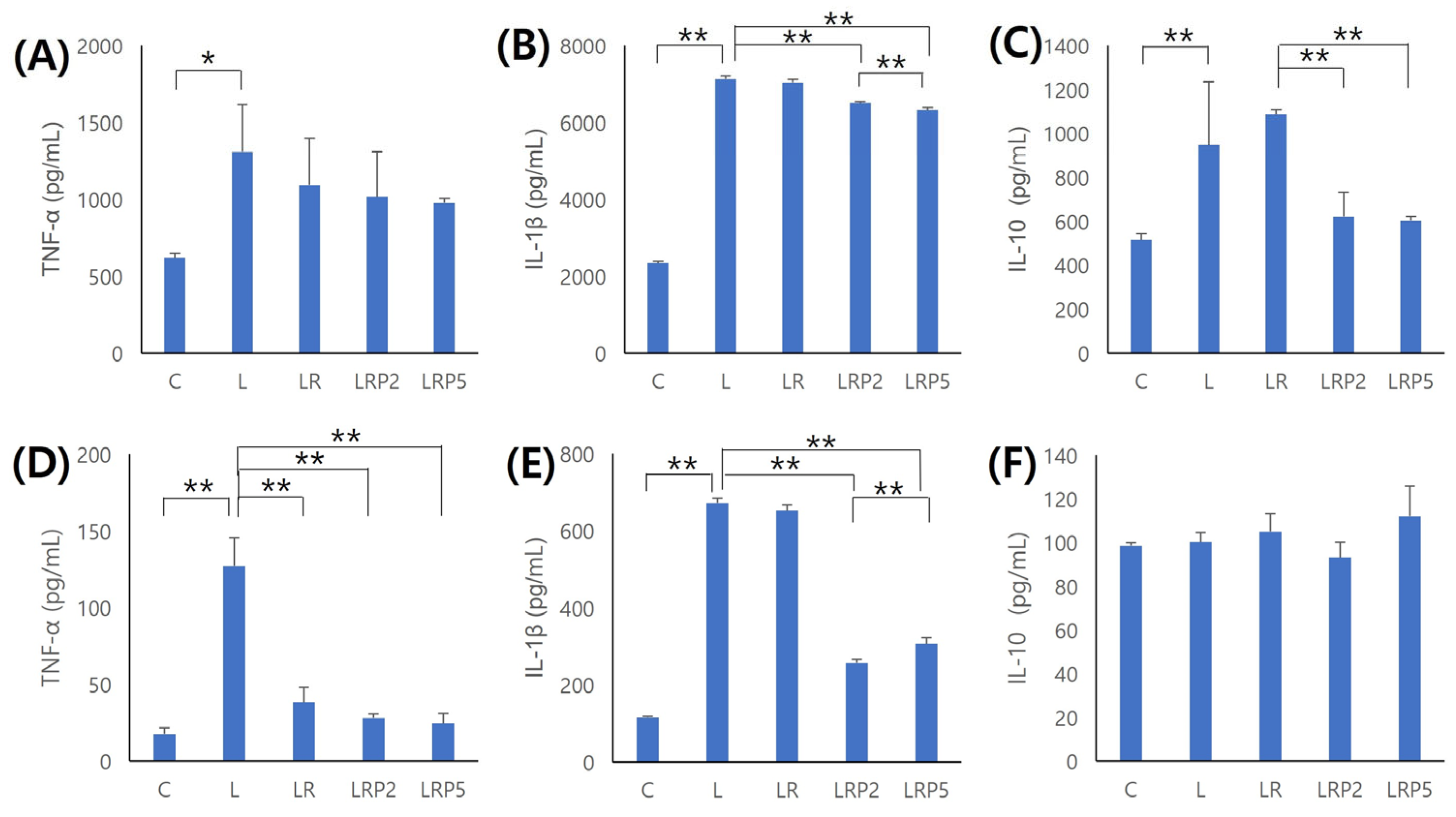
2.6. Inflammatory Cytokine Evaluation in Periodontal Tissue and Serum
2.7. Statistical Analysis
3. Results
3.1. Micro-CT Analysis for Alveolar Bone Resorption Evaluation
3.2. Histomorphometric Analysis and Quantification of Osteoclasts
3.3. Immunohistochemistry Analysis of RANKL and OPG Expression
3.4. Inflammatory Cytokine Evaluation in Periodontal Tissue and Serum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Darveau, R.P. Periodontitis: A polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 2010, 8, 481–490. [Google Scholar] [CrossRef]
- Ebersole, J.L.; Dawson, D.R., 3rd; Morford, L.A.; Peyyala, R.; Miller, C.S.; Gonzaléz, O.A. Periodontal disease immunology: ‘double indemnity’ in protecting the host. Periodontol. 2000 2013, 62, 163–202. [Google Scholar] [CrossRef] [PubMed]
- Bartold, P.M.; Van Dyke, T.E. Periodontitis: A host-mediated disruption of microbial homeostasis. Unlearning learned concepts. Periodontol. 2000 2013, 62, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Bartold, P.M.; Van Dyke, T.E. Host modulation: Controlling the inflammation to control the infection. Periodontol. 2000 2017, 75, 317–329. [Google Scholar] [CrossRef]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef]
- Darveau, R.P.; Hajishengallis, G.; Curtis, M.A. Porphyromonas gingivalis as a potential community activist for disease. J. Dent. Res. 2012, 91, 816–820. [Google Scholar] [CrossRef]
- Roi, C.; Gaje, P.N.; Ceausu, R.A.; Roi, A.; Rusu, L.C.; Boia, E.R.; Boia, S.; Luca, R.E.; Rivis, M. Heterogeneity of blood vessels and assessment of microvessel density-MVD in gingivitis. J. Clin. Med. 2022, 11, 2758. [Google Scholar] [CrossRef]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Berglundh, T.; Sculean, A.; Tonetti, M.S. EFP Workshop Participants and Methodological Consultants. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47 (Suppl. S22), 4–60. [Google Scholar] [CrossRef]
- Tomasi, C.; Abrahamsson, K.H.; Apatzidou, D. Subgingival instrumentation. Periodontol. 2000 2023. online ahead of print. [Google Scholar] [CrossRef]
- Salvi, G.E.; Stähli, A.; Schmidt, J.C.; Ramseier, C.A.; Sculean, A.; Walter, C. Adjunctive laser or antimicrobial photodynamic therapy to non-surgical mechanical instrumentation in patients with untreated periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47, 176–198. [Google Scholar] [CrossRef]
- Slots, J. Periodontitis: Facts, fallacies and the future. Periodontol. 2000 2017, 1, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; McGrath, C.; Jin, L.; Zhang, C.; Yang, Y. The effectiveness of low-level laser therapy as an adjunct to non-surgical periodontal treatment: A meta-analysis. J. Periodontal Res. 2017, 52, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Teughels, W.; Feres, M.; Oud, V.; Martín, C.; Matesanz, P.; Herrera, D. Adjunctive effect of systemic antimicrobials in periodontitis therapy: A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47, 257–281. [Google Scholar] [CrossRef]
- Sheridan, R.A.; Wang, H.L.; Eber, R.; Oh, T.J. Systemic chemotherapeutic agents as adjunctive periodontal therapy: A narrative review and suggested clinical recommendations. J. Int. Acad. Periodontol. 2015, 17, 123–134. [Google Scholar]
- Weltmann, K.D.; Von Woedtke, T. Plasma medicine-current state of research and medical application. Plasma Phys. Control. Fusion 2017, 59, 014031. [Google Scholar] [CrossRef]
- Park, S.R.; Hong, J.W.; Lee, H.J.; Kim, G.C. Plasma medicine; How can nonthermal atmospheric plasma be applied to medicine? J. Life Sci. 2013, 23, 838–846. [Google Scholar] [CrossRef]
- Haertel, B.; Von Woedtke, T.h.; Weltmann, K.D.; Lindequist, U. Non-thermal atmospheric-pressure plasma possible application in wound healing. Biomol. Ther. 2014, 22, 477–490. [Google Scholar] [CrossRef]
- Daeschlein, G.; Scholz, S.; Arnold, A.; von Podewils, S.; Haase, H.; Emmert, S.; Woedtke, T.V.; Weltmann, K.D.; Junger, M. In Vitro Susceptibility of Important Skin and Wound Pathogens Against Low Temperature Atmospheric Pressure Plasma Jet (APPJ) and Dielectric Barrier Discharge Plasma (DBD). Plasma Processes Polym. 2012, 9, 380–389. [Google Scholar] [CrossRef]
- Napp, M.; Daeschlein, G.; Podewils, S.V.; Hinz, P.; Emmert, S.; Haase, H.; Spitzmueller, R.; Gumbel, D.; Kasch, R.; Junger, M. In vitro susceptibility of methicillin-resistant and methicillin-susceptible strains of Staphylococcus aureus to two different cold atmospheric plasma sources. Infection 2016, 44, 531–537. [Google Scholar] [CrossRef]
- Barton, A.; Wende, K.; Bundscherer, L.; Hasse, S.; Schmidt, A.; Bekeschus, S.; Weltmann, K.D.; Lindequist, U.; Masur, K. Nonthermal plasma increases expression of wound healing related genes in a keratinocyte cell line. Plasma Med. 2013, 3, 125–136. [Google Scholar] [CrossRef]
- Kim, D.; Gweon, B.; Kim, D.B.; Choe, W.; Shin, J.H. A feasibility study for the cancer therapy using cold plasma. IFMBE Proc. 2008, 23, 355–357. [Google Scholar]
- Kim, G.C.; Lee, H.J.; Shon, H. The effects of a micro plasma on melanoma (G361) cancer cells. J. Korean Phys. Soc. 2009, 54, 628–632. [Google Scholar] [CrossRef]
- Perrotti, V.; Caponio, V.C.A.; Muzio, L.L.; Choi, E.H.; Di Marcantonio, M.C.; Mazzone, M.; Kaushik, N.K.; Mincione, G. Open Questions in Cold Atmospheric Plasma Treatment in Head and Neck Cancer: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 10238. [Google Scholar] [CrossRef]
- Sanesi, L.; Puca, V.; Caponio, V.C.A.; Pinti., M.; Balice, G.; Femminella, B.; Paolantonio, M.; Cela, I.; Kaushik, N.K.; Choi, E.H.; et al. Disinfection of dental root canals by cold atmospheric plasma: A systematic review and meta-analysis of dental biofilm. Front. Oral Health 2024, 5, 1483078. [Google Scholar] [CrossRef]
- Park, J.K.; Nam, S.H.; Kwon, H.C.; Mohamed, A.A.H.; Lee, J.K.; Kim, G.C. Feasibility of nonthermal atmospheric pressure plasma for intracoronal bleaching. Int. Endod. J. 2011, 44, 170–175. [Google Scholar] [CrossRef]
- Kim, Y.M.; Lee, H.Y.; Lee, H.J.; Kim, J.B.; Joo, J.Y.; Kim, G.C. Retention Improvement in Fluoride Application with Cold Atmospheric Plasma. J. Dent. Res. 2018, 97, 179–183. [Google Scholar] [CrossRef]
- Mahasneh, A.; Darby, M.; Tolle, S.L.; Hynes, W.; Laroussi, M.; Karakas, E. Inactivation of Porphyromonas gingivalis by low-temperature atmospheric pressure plasma. Plasma Med. 2011, 1, 191–204. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Lee, H.G.; Choi, J.H.; Jang, Y.S.; Kim, U.K.; Kim, G.C.; Hwang, D.S. Non-thermal plasma accelerates the healing process of peripheral nerve crush injury in rats. Int. J. Med. Sci. 2020, 17, 1112–1120. [Google Scholar] [CrossRef]
- Munteanu, I.R.; Luca, R.E.; Mateas, M.; Darawasha, L.D.; Boia, S.; Boia, E.R.; Todea, C.D. The efficiency of photodynamic therapy in the bacterial decontamination of periodontal pockets and its impact on the patient. Diagnostics 2022, 12, 3026. [Google Scholar] [CrossRef]
- Cobb, C.M.; Sottosanti, J.S. A re-evaluation of scaling and root planning. J. Periodontol. 2021, 10, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Aimetti, M. Nonsurgical periodontal treatment. Int. J. Esthet. Dent. 2014, 2, 251–267. [Google Scholar]
- Wielento, A.; Lagosz-Cwik, K.B.; Potempa, J.; Grabiec, A.M. The role of gingival fibroblasts in the pathogenesis of periodontitis. J. Dent. Res. 2023, 102, 489–496. [Google Scholar] [CrossRef]
- Cobb, C.M. Lasers and the treatment of periodontitis: The essence and the noise. Periodontol. 2000 2017, 75, 205–295. [Google Scholar] [CrossRef]
- De Oliveira, R.R.; Schwartz-Filho, H.O.; Novaes, A.B., Jr.; Taba, M., Jr. Antimicrobial photodynamic therapy in the non-surgical treatment of aggressive periodontitis: A preliminary randomized controlled clinical study. J. Periodontol. 2007, 78, 965–973. [Google Scholar] [CrossRef]
- Zandbergen, D.; Slot, D.E.; Niederman, R.; Weijden, F.A.V. The concomitant administration of systemic amoxicillin and metronidazole compared to scaling and root planning alone in treating periodontitis: =a systematic review=. BMC Oral Health 2016, 29, 27. [Google Scholar]
- Feres, M.; Figueiredo, L.C.; Soares, G.M.; Faveri, M. Systemic antibiotics in the treatment of periodontitis. Periodontol. 2000 2015, 67, 131–186. [Google Scholar] [CrossRef]
- Hoffmann, C.; Berganza, C.; Zhang, J. Cold atmospheric plasma: Methods of production and application in dentistry and oncology. Med. Gas. Res. 2013, 3, 21. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, Y.; Xie, P.; Ao, X.; Zheng, Z.; Dong, X.; Li, H.; Yu, Q.; Zhu, Z.; Chen, M.; et al. Non-thermal plasma reduces periodontitis-induced alveolar bone loss in rats. Biochem. Biophys. Res. Commun. 2018, 503, 2040–2046. [Google Scholar] [CrossRef]
- Crotti, T.; Smith, M.D.; Hirsch, R.; Soukoulis, S.; Weedon, H.; Capone, M.; Ahern, M.J.; Haynes, D. Receptor activator NF kappaB ligand (RANKL) and osteoprotegerin (OPG) protein expression in periodontitis. J. Periodontal Res. 2003, 38, 380–387. [Google Scholar] [CrossRef]
- Bostanci, N.; Ilgenli, T.; Emingil, G.; Afacan, B.; Han, B.; Töz, H.; Berdeli, A.; Atilla, G.; McKay, I.J.; Hughes, F.J.; et al. Differential expression of receptor activator of nuclear factor-kappaB ligand and osteoprotegerin mRNA in periodontal diseases. J. Periodontal Res. 2007, 42, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Belibasakis, G.N.; Bostanci, N. The RANKL-OPG system in clinical periodontology. J. Clin. Periodontol. 2012, 39, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Cochran, D.L. Inflammation and bone loss in periodontal disease. J. Periodontol. 2008, 79, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Górska, R.; Gregorek, H.; Kowalski, J.; Laskus-Perendyk, A.; Syczewska, M.; Madaliński, K. Relationship between clinical parameters and cytokine profiles in inflamed gingival tissue and serum samples from patients with chronic periodontitis. J. Clin. Periodontol. 2003, 30, 1046–1052. [Google Scholar] [CrossRef]
- Hienz, S.A.; Paliwal, S.; Ivanovski, S. Mechanisms of Bone Resorption in Periodontitis. J. Immunol. Res. 2015, 2015, 615486. [Google Scholar] [CrossRef]
- Andrukhov, O.; Ulm, C.; Reischl, H.; Nguyen, P.Q.; Matejka, M.; Rausch-Fan, X. Serum cytokine levels in periodontitis patients in relation to the bacterial load. J. Periodontol. 2011, 82, 885–892. [Google Scholar] [CrossRef]
- Graves, D.T.; Cochran, D. The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J. Periodontol. 2003, 74, 391–401. [Google Scholar] [CrossRef]
- Duarte, P.M.; da Rocha, M.; Sampaio, E.; Mestnik, M.J.; Feres, M.; Figueiredo, L.C.; Bastos, M.F.; Faveri, M. Serum levels of cytokines in subjects with generalized chronic and aggressive periodontitis before and after non-surgical periodontal therapy: A pilot study. J. Periodontol. 2010, 81, 1056–1063. [Google Scholar] [CrossRef]
- Reis, C.; DA Costa, A.V.; Guimarães, J.T.; Tuna, D.; Braga, A.C.; Pacheco, J.J.; Arosa, F.A.; Salazar, F.; Cardoso, E.M. Clinical improvement following therapy for periodontitis: Association with a decrease in IL1 and IL6. Exp. Ther. Med. 2014, 8, 323–327. [Google Scholar] [CrossRef]
- Pan, W.; Wang, Q.; Chen, Q. The cytokine network involved in the host immune response to periodontitis. Int. J. Oral Sci. 2019, 11, 30. [Google Scholar] [CrossRef]
- Kinane, D.F.; Preshaw, P.M.; Loos, B.G. Host-response: Understanding the cellular and molecular mechanisms of host-microbial interactions—Consensus of the Seventh European Workshop on Periodontology. J. Clin. Periodontol. 2011, 38, 44–48. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.-Y.; Kim, H.-J.; Lee, J.-Y.; Joo, J.-Y. Adjunctive Treatment Effect of Non-Thermal Atmospheric Pressure Plasma in Periodontitis-Induced Rats. J. Clin. Med. 2025, 14, 896. https://doi.org/10.3390/jcm14030896
Choi H-Y, Kim H-J, Lee J-Y, Joo J-Y. Adjunctive Treatment Effect of Non-Thermal Atmospheric Pressure Plasma in Periodontitis-Induced Rats. Journal of Clinical Medicine. 2025; 14(3):896. https://doi.org/10.3390/jcm14030896
Chicago/Turabian StyleChoi, Hee-Young, Hyun-Joo Kim, Ju-Youn Lee, and Ji-Young Joo. 2025. "Adjunctive Treatment Effect of Non-Thermal Atmospheric Pressure Plasma in Periodontitis-Induced Rats" Journal of Clinical Medicine 14, no. 3: 896. https://doi.org/10.3390/jcm14030896
APA StyleChoi, H.-Y., Kim, H.-J., Lee, J.-Y., & Joo, J.-Y. (2025). Adjunctive Treatment Effect of Non-Thermal Atmospheric Pressure Plasma in Periodontitis-Induced Rats. Journal of Clinical Medicine, 14(3), 896. https://doi.org/10.3390/jcm14030896





