Predictors of Severe Coughing and Its Impact on Bronchoalveolar Lavage and Transbronchial Lung Biopsy in Patients with Diffuse Lung Disease: Evaluation of Bronchoscopy Safety
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.1.1. Bronchoscopy Procedure
2.1.2. Method of Anesthesia
2.1.3. Score of Cough Severity on Bronchoscopy
2.1.4. Diagnostic Criteria in Bronchoscopy
2.1.5. Collection of Associated Date
2.1.6. Statistical Analysis
3. Results
3.1. Patient Characteristics and Clinical Background
3.2. Comparison Between Mild and Severe Cough Groups
3.3. Multivariate Analysis of the Factors Associated with Severe Cough
3.4. Relationship Between Cough Severity and Bleeding Severity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Hirose, T.; Okuda, K.; Ishida, H.; Sugiyama, T.; Kusumoto, S.; Nakashima, M.; Yamaoka, T.; Adachi, M. Patient satisfaction with sedation for flexible bronchoscopy. Respirology 2008, 13, 722–727. [Google Scholar] [CrossRef]
- Du Rand, I.A.; Blaikley, J.; Booton, R.; Chaudhuri, N.; Gupta, V.; Khalid, S.; Mandal, S.; Martin, J.; Mills, J.; Navani, N.; et al. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults: Accredited by NICE. Thorax 2013, 68 (Suppl. S1), i1–i44. [Google Scholar] [CrossRef] [PubMed]
- Wahidi, M.M.; Jain, P.; Jantz, M.; Lee, P.; Mackensen, G.B.; Barbour, S.Y.; Lamb, C.; Silvestri, G.A. American College of Chest Physicians consensus statement on the use of topical anesthesia, analgesia, and sedation during flexible bronchoscopy in adult patients. Chest 2011, 140, 1342–1350. [Google Scholar] [CrossRef]
- American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. Practice Guidelines for Sedation and Analgesia by Non-Anesthesiologists. Anesthesiology 2002, 96, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, F.; Saraya, T.; Oda, M.; Sakuma, S.; Watanabe, M.; Takata, S.; Tamura, M.; Takakura, H.; Nakamoto, K.; Honda, K.; et al. Novel predictive factors for patient discomfort and severe cough during bronchoscopy: A prospective questionnaire analysis. PLoS ONE 2020, 15, e0240485. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, F.; Saraya, T.; Akizawa, T.; Abe, T.; Takagi, R.; Ieki, E.; Ishikawa, N.; Kurokawa, N.; Aso, J.; Nunokawa, H.; et al. Impact of Cough Severity on the Diagnostic Yield of Endobronchial Ultrasonography Transbronchial Biopsy with Guide Sheath: A Retrospective Observational Study. J. Clin. Med. 2024, 13, 347. [Google Scholar] [CrossRef] [PubMed]
- Kheir, F.; Uribe Becerra, J.P.; Bissell, B.; Ghazipura, M.; Herman, D.; Hon, S.M.; Hossain, T.; Khor, Y.H.; Knight, S.L.; Kreuter, M.; et al. Transbronchial Lung Cryobiopsy in Patients with Interstitial Lung Disease: A Systematic Review. Ann. Am. Thorac. Soc. 2022, 19, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Bulpa, P.A.; Dive, A.M.; Mertens, L.; Delos, M.A.; Jamart, J.; Evrard, P.A.; Gonzalez, M.R.; Installé, E.J. Combined bronchoalveolar lavage and transbronchial lung biopsy: Safety and yield in ventilated patients. Eur. Respir. J. 2003, 21, 489–494. [Google Scholar] [CrossRef]
- Fend, F.; Mikuz, G.; Ott, G.; Rothmund, J. Diagnostic value of combined bronchoalveolar lavage and transbronchial lung biopsy. Pathol. Res. Pract. 1989, 184, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.H.; Um, S.W.; Koh, W.J.; Suh, G.Y.; Chung, M.P.; Kim, H.; Kwon, O.J.; Jeon, K. Safety and Yield of Combined Bronchoalveolar Lavage and Transbronchial Lung Biopsy in Mechanically Ventilated Patients. Am. J. Respir. Crit. Care Med. 2011, 183, A5845. [Google Scholar]
- Bernasconi, M.; Koegelenberg, C.F.N.; Koutsokera, A.; Ogna, A.; Casutt, A.; Nicod, L.; Lovis, A. Iatrogenic bleeding during flexible bronchoscopy: Risk factors, prophylactic measures and management. ERJ Open Res. 2017, 3, 00084-2016. [Google Scholar] [CrossRef]
- Meyer, K.C.; Raghu, G.; Baughman, R.P.; Brown, K.K.; Costabel, U.; du Bois, R.M.; Drent, M.; Haslam, P.L.; Kim, D.S.; Nagai, S.; et al. An official American Thoracic Society clinical practice guideline: The clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am. J. Respir. Crit. Care Med. 2012, 185, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Venkatnarayan, K.; Devaraj, U.; Krishnaswamy, U.M.; Ramachandran, P.; Thomas, T.; D’Souza, G. Comparison of spray catheter with “spray-as-you-go” technique for airway anesthesia during flexible bronchoscopy—A randomized trial. Lung 2020, 37, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Minami, D.; Takigawa, N.; Watanabe, H.; Ninomiya, T.; Kubo, T.; Ohashi, K.; Sato, A.; Hotta, K.; Tabata, M.; Tanimoto, M.; et al. Safety and discomfort during bronchoscopy performed under sedation with fentanyl and midazolam: A prospective study. Jpn. J. Clin. Oncol. 2016, 46, 871–874. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; King, T.E.; Bateman, E.D.; Lynch, D.A.; Capron, F.; Center, D.; Colby, T.V.; Cordier, J.F.; DuBois, R.M.; Galvin, J.; et al. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. Am. J. Respir. Crit. Care Med. 2002, 165, 277–304. [Google Scholar]
- Travis, W.D.; Costabel, U.; Hansell, D.M.; King, T.E., Jr.; Lynch, D.A.; Nicholson, A.G.; Ryerson, C.J.; Ryu, J.H.; Selman, M.; Wells, A.U.; et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013, 188, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Ryerson, C.J.; Myers, J.L.; Kreuter, M.; Vasakova, M.; Bargagli, E.; Chung, J.H.; Collins, B.F.; Bendstrup, E.; et al. Diagnosis of Hypersensitivity Pneumonitis in Adults. An Official ATS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2020, 202, e36–e69. [Google Scholar] [CrossRef]
- Fischer, A.; du Bois, R. Interstitial lung disease in connective tissue disorders. Lancet 2012, 380, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, M.; Bigliazzi, C.; Casoni, G.; Chilosi, M.; Carloni, A.; Dubini, A.; Gurioli, C.; Tomassetti, S.; Gurioli, C.; Poletti, V. The role of transbronchial lung biopsy for the diagnosis of diffuse drug-induced lung disease: A case series of 44 patients. Sarcoidosis Vasc. Diffuse Lung Dis. 2008, 25, 26–45. [Google Scholar]
- Han, Q.; Luo, Q.; Chen, X.; Xie, J.; Wu, L.; Chen, R. The evaluation of clinical usefulness of transbrochoscopic lung biopsy in undefined interstitial lung diseases: A retrospective study. The Clin. Respir. J. 2017, 11, 168–175. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, F.; Xu, X.; Xu, L.; Wang, Z.; Tong, Z. Is conventional transbronchial lung biopsy out: The evaluation of clinical value in diffuse parenchymal lung disease. Clin. Respir. J. 2022, 16, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Folch, E.E.; Mahajan, A.K.; Oberg, C.L.; Maldonado, F.; Toloza, E.; Krimsky, W.S.; Oh, S.; Bowling, M.R.; Benzaquen, S.; Kinsey, C.M.; et al. Standardized Definitions of Bleeding After Transbronchial Lung Biopsy: A Delphi Consensus Statement From the Nashville Working Group. Chest 2020, 158, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ’EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Fujimoto, K.; Ishiwata, T.; Kasai, H.; Terada, J.; Shionoya, Y.; Ikari, J.; Kawata, N.; Tada, Y.; Tsushima, K.; Tatsumi, K. Identification of factors during bronchoscopy that affect patient reluctance to undergo repeat examination: Questionnaire analysis after initial bronchoscopy. PLoS ONE 2018, 13, e0208495. [Google Scholar] [CrossRef]
- Kashkash, F.; Khorri, A. Observational findings of transbronchial lung biopsy in patients with interstitial lung disease: A retrospective study in Aleppo University Hospital. Ann. Med. Surg. 2023, 85, 146–152. [Google Scholar] [CrossRef]
- Descombes, E.; Gardiol, D.; Leuenberger, P. Transbronchial lung biopsy: An analysis of 530 cases with reference to the number of samples. Monaldi Arch. Chest Dis. 1997, 52, 324–329. [Google Scholar] [PubMed]
- Suzuki, Y.; Suda, T. Eosinophilic pneumonia: A review of the previous literature, causes, diagnosis, and management. Allergol Int. 2019, 68, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Carbone, R.G.; Puppo, F.; Mattar, E.; Roden, A.C.; Hirani, N. Acute and chronic eosinophilic pneumonia: An overview. Front. Med. 2024, 11, 1355247. [Google Scholar]
| Cough Score | Description | Impact on Procedure |
|---|---|---|
| 0 | None | No cough present |
| 1 | Mild | Cough present but does not interfere with the procedure |
| 2 | Moderate | Cough necessitates temporary interruption within the trachea |
| 3 | Severe | Cough requires scope removal from the trachea |
| Category | Description |
|---|---|
| Specific | Findings consistent with guidelines or similar standards |
| Probable | Nonspecific findings that align with the final diagnosis. |
| Not Specific | Findings that do not contribute to the diagnosis. |
| Insufficient Sample | The sample quantity was insufficient for diagnosis |
| n | Diagnostic Utility | Cough Severity | |||
|---|---|---|---|---|---|
| Diagnosed Group | Non-Diagnosed Group | Mild Cough Group | Severe Cough Group | ||
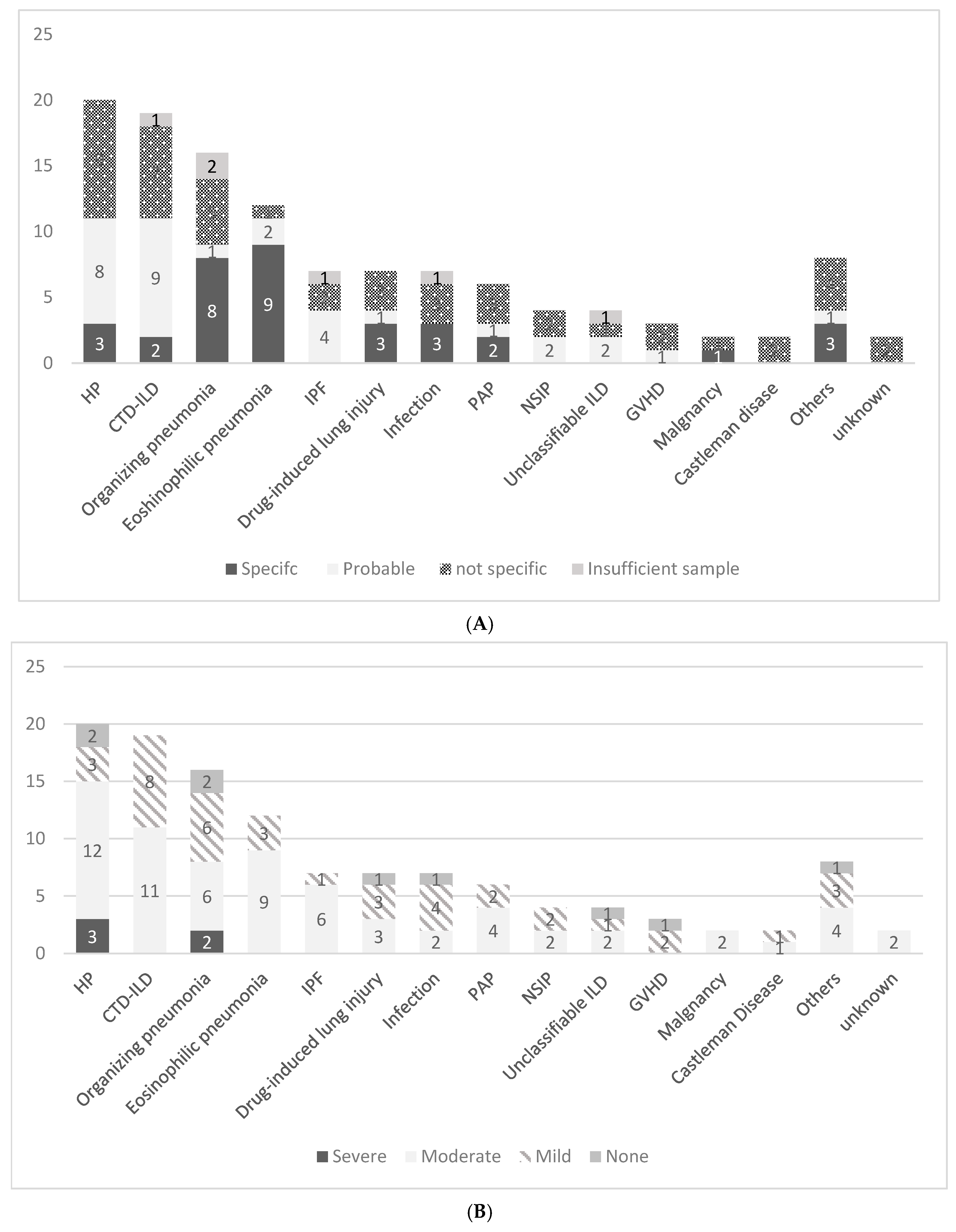
| HP | 20 | 11 (55.0%) | 9 (45.0%) | 5 (25.0%) | 15 (75.0%) |
| CTD-ILD | 19 | 11 (57.9%) | 8 (42.1%) | 8 (42.1%) | 11 (57.9%) |
| OP | 16 | 9 (56.3%) | 7 (43.7%) | 8 (50.0%) | 8 (50.0%) |
| EP | 12 | 11 (91.7%) * | 1 (8.3%) | 3 (25.0%) | 9 (75.0%) |
| Total | 67 | 42 (62.7%) | 25 (37.3%) | 24 (35.8%) | 43 (64.2%) |
| Variable | Mild Cough Group (n = 48) | Severe Cough Group (n = 71) | p Value |
|---|---|---|---|
| Age, Median [IQR], years | 70.5 [58.5–75.0] | 65.0 [50.5–75.0] | 0.294 |
| Sex | |||
| Male | 30 (62.5%) | 38 (53.5%) | 0.352 |
| Female | 18 (37.5%) | 33 (46.5%) | |
| Smoking History | |||
| Yes | 25 (52.1%) | 33 (46.5%) | 0.579 |
| No | 23 (47.9%) | 38 (53.5%) | |
| Smoking index, Median [IQR], pack year | 40.0 [24.0–50.0] (n = 25) | 22.75 [11.4–38.8] (n = 33) | 0.032 |
| BMI, Median [IQR], kg/m2 | 21.24 [19.05–23.13] | 23.51 [20.30–25.55] | 0.016 |
| Asthma History | |||
| Yes | 6 (12.5%) | 10 (14.1%) | 1.000 |
| No | 42 (87.5%) | 61 (85.9%) | |
| Requirement for antithrombotic withdrawal | |||
| Yes | 10 (20.8%) | 6 (8.5%) | 0.061 |
| No | 38 (79.2%) | 65 (91.5%) | |
| WBC, Median [IQR],/μL | 7400 [5900–9625] | 7500 [6000–9000] | 1.000 |
| Blood Neutrophils, Median [IQR], % | 72.8 [62.3–79.0] | 67.9 [59.1–72.2] | 0.015 |
| Blood Eosinophils, Median [IQR], % | 1.6 [0.8–4.4] | 2.80 [1.70–4.85] | 0.015 |
| CRP, Median [IQR], mg/dL | 2.4 [0.2–5.4] | 1.0 [0.2–4.6] | 0.431 |
| KL-6, Median [IQR], U/mL | 475 [284–835] | 790 [352–1657] | 0.008 |
| Lung Abnormalities on chest X-ray | |||
| Visible | 38 (79.2%) | 63 (88.7%) | 0.194 |
| Invisible | 10 (20.8%) | 8 (11.3%) | |
| Ground-glass Opacity on Thoracic CT | |||
| Yes | 39 (81.2%) | 68 (95.8%) | 0.013 |
| No | 9 (18.8%) | 3 (4.2%) | |
| Infiltrative Shadow on Thoracic CT | |||
| Yes | 29 (60.4%) | 40 (56.3%) | 0.708 |
| No | 19 (39.6%) | 31 (43.7%) | |
| Emphysematous Change on Thoracic CT | |||
| Yes | 10 (20.8%) | 14 (19.7%) | 1.000 |
| No | 38 (79.2%) | 57 (80.3%) | |
| Honeycomb Lung on Thoracic CT | |||
| Yes | 3 (6.2%) | 6 (8.5%) | 0.738 |
| No | 45 (93.8%) | 65 (91.5%) | |
| Operator Experience | |||
| ≥5 years | 15 (31.2%) | 24 (33.8%) | 0.844 |
| <5 years | 33 (68.8%) | 47 (66.2%) | |
| pharyngolaryngeal anesthesia | |||
| Jackson spray | 8 (16.7%) | 19 (26.8%) | 0.265 |
| Spray catheter | 40 (83.3%) | 52 (73.2%) | |
| BAL Site | |||
| Middle Lobe or Lingular | 32 (66.7%) | 54 (76.1%) | 0.300 |
| Other | 16 (33.3%) | 17 (23.9%) | |
| Number of TBLB Specimens, Median [IQR] | 5.00 [4.00–6.00] | 4.00 [3.00–5.00] | 0.012 |
| Procedure Duration, Median [IQR], min | 34.00 [29.00–37.25] | 33.00 [29.00–38.00] | 0.888 |
| Intra-procedural Bleeding | |||
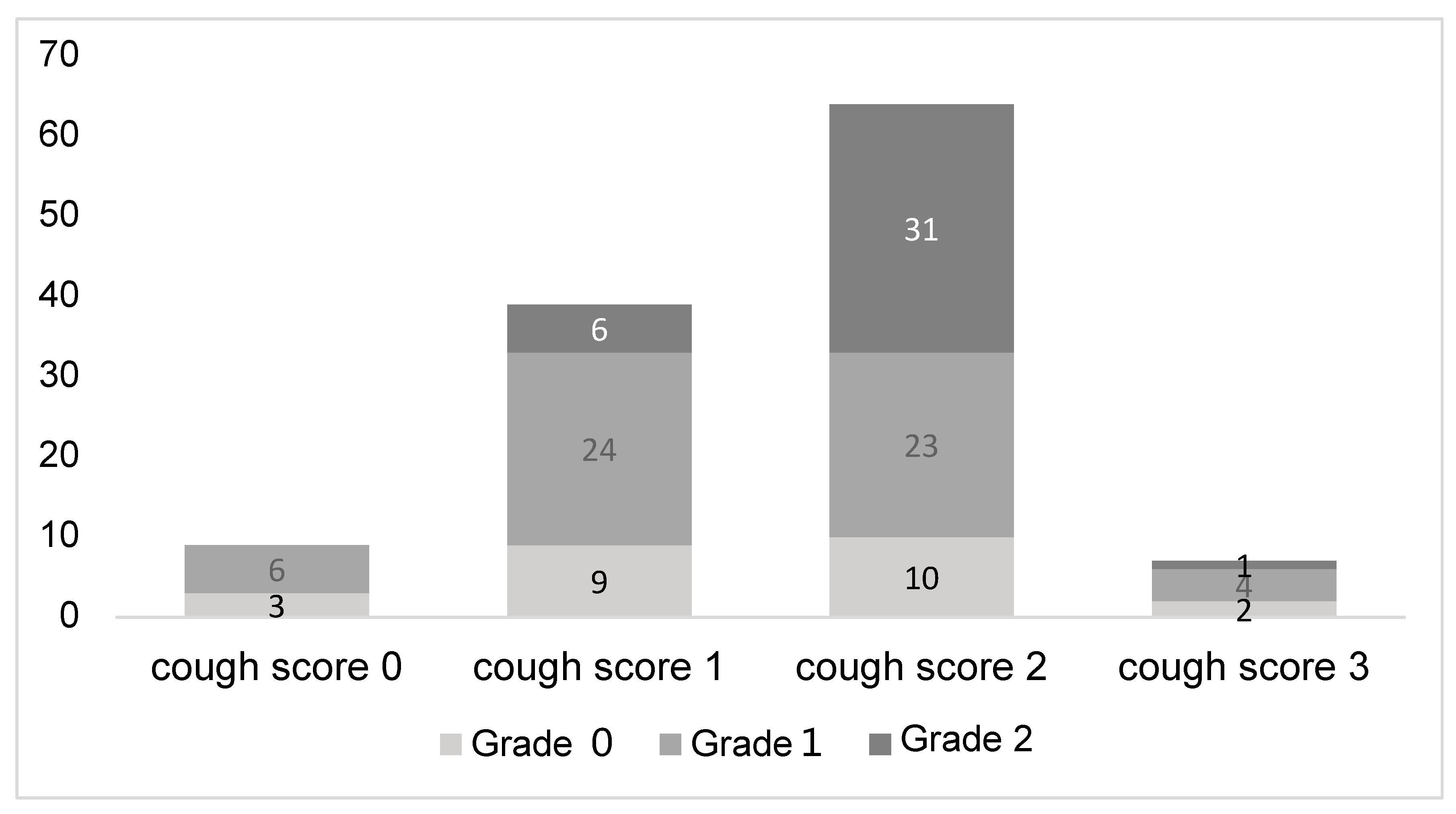
| Grade 2 or higher | 6 (12.5%) | 32 (45.1%) | <0.001 |
| Grade 1 or lower | 42 (87.5%) | 39 (54.9%) | |
| Oxygen supplementation | |||
| ≥10 L/min | 3 (6.2%) | 11 (15.5%) | 0.155 |
| <10 L/min | 45 (93.8%) | 60 (84.5%) | |
| TBLB diagnostic success | |||
| Yes | 30 (62.5%) | 34 (47.9%) | 0.136 |
| No | 18 (37.5%) | 37 (52.1%) | |
| BALF Recovery Rate, Median [IQR], % | 54.3 [48.0–63.3] | 53.3 [47.0–60.9] | 0.749 |
| Post-procedural Pneumothorax | |||
| Yes | 1 (2.1%) | 1 (1.4%) | |
| No | 47 (97.9%) | 70 (98.6%) | 1.000 |
| Post-procedural acute exacerbation of interstitial pneumonia | |||
| Yes | 1 (2.1%) | 0 (0%) | |
| No | 47 (97.9%) | 71 (100%) | 0.403 |
| Post-procedural bacterial pneumonia | |||
| Yes | 1 (2.1%) | 1 (1.4%) | |
| No | 47 (97.9%) | 70 (98.6%) | 1.000 |
| Post-procedural fever | |||
| Yes | 2 (4.2%) | 2 (2.8%) | 1.000 |
| No | 46 (95.8%) | 69 (97.2%) |
| Variable | Odds Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Intra-procedure Bleeding (Grade 2 or higher) | 6.23 | 2.220–17.400 | <0.001 |
| Number of TBLB Specimens | 0.708 | 0.530–0.945 | 0.019 |
| Pre-procedure Dyspnea | 2.56 | 1.110–5.870 | 0.027 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, F.; Saraya, T.; Kurokawa, N.; Aso, J.; Yamada, S.; Nakajima, K.; Doi, K.; Akizawa, T.; Takagi, R.; Ishikawa, N.; et al. Predictors of Severe Coughing and Its Impact on Bronchoalveolar Lavage and Transbronchial Lung Biopsy in Patients with Diffuse Lung Disease: Evaluation of Bronchoscopy Safety. J. Clin. Med. 2025, 14, 893. https://doi.org/10.3390/jcm14030893
Kobayashi F, Saraya T, Kurokawa N, Aso J, Yamada S, Nakajima K, Doi K, Akizawa T, Takagi R, Ishikawa N, et al. Predictors of Severe Coughing and Its Impact on Bronchoalveolar Lavage and Transbronchial Lung Biopsy in Patients with Diffuse Lung Disease: Evaluation of Bronchoscopy Safety. Journal of Clinical Medicine. 2025; 14(3):893. https://doi.org/10.3390/jcm14030893
Chicago/Turabian StyleKobayashi, Fumi, Takeshi Saraya, Nozomi Kurokawa, Jumpei Aso, Sho Yamada, Kei Nakajima, Kazuyuki Doi, Takatora Akizawa, Ryo Takagi, Narishige Ishikawa, and et al. 2025. "Predictors of Severe Coughing and Its Impact on Bronchoalveolar Lavage and Transbronchial Lung Biopsy in Patients with Diffuse Lung Disease: Evaluation of Bronchoscopy Safety" Journal of Clinical Medicine 14, no. 3: 893. https://doi.org/10.3390/jcm14030893
APA StyleKobayashi, F., Saraya, T., Kurokawa, N., Aso, J., Yamada, S., Nakajima, K., Doi, K., Akizawa, T., Takagi, R., Ishikawa, N., Kasuga, K., Saito, M., Yamaguchi, C., Nunokawa, H., Nakamoto, Y., Ishida, M., Sada, M., Nakamoto, K., Takata, S., & Ishii, H. (2025). Predictors of Severe Coughing and Its Impact on Bronchoalveolar Lavage and Transbronchial Lung Biopsy in Patients with Diffuse Lung Disease: Evaluation of Bronchoscopy Safety. Journal of Clinical Medicine, 14(3), 893. https://doi.org/10.3390/jcm14030893







