Quantitative Analysis of Propofol Dosage in Cannabis Users: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Data Analysis
2.5. Risk of Bias Assessment
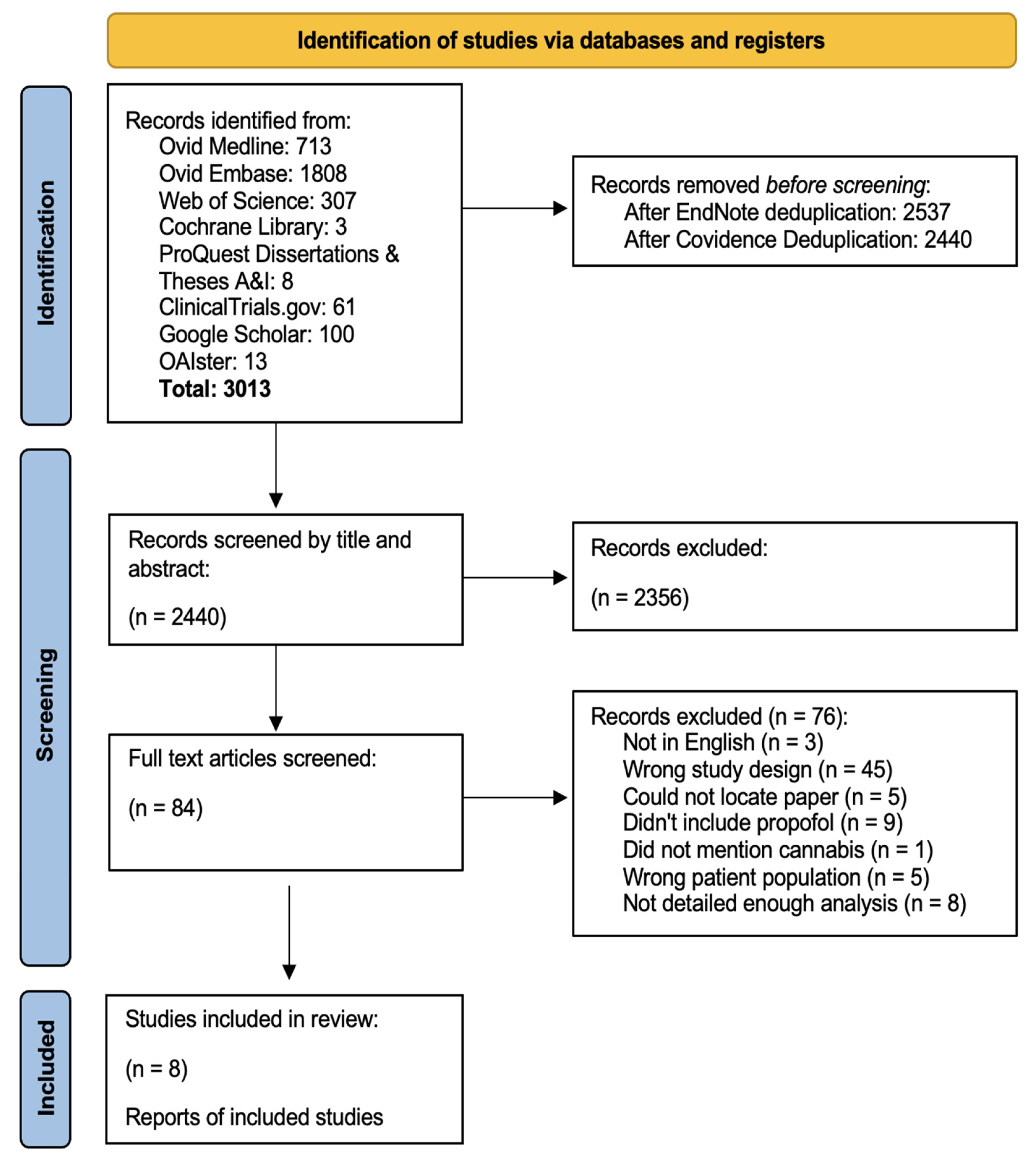
3. Results
3.1. Study Characteristics
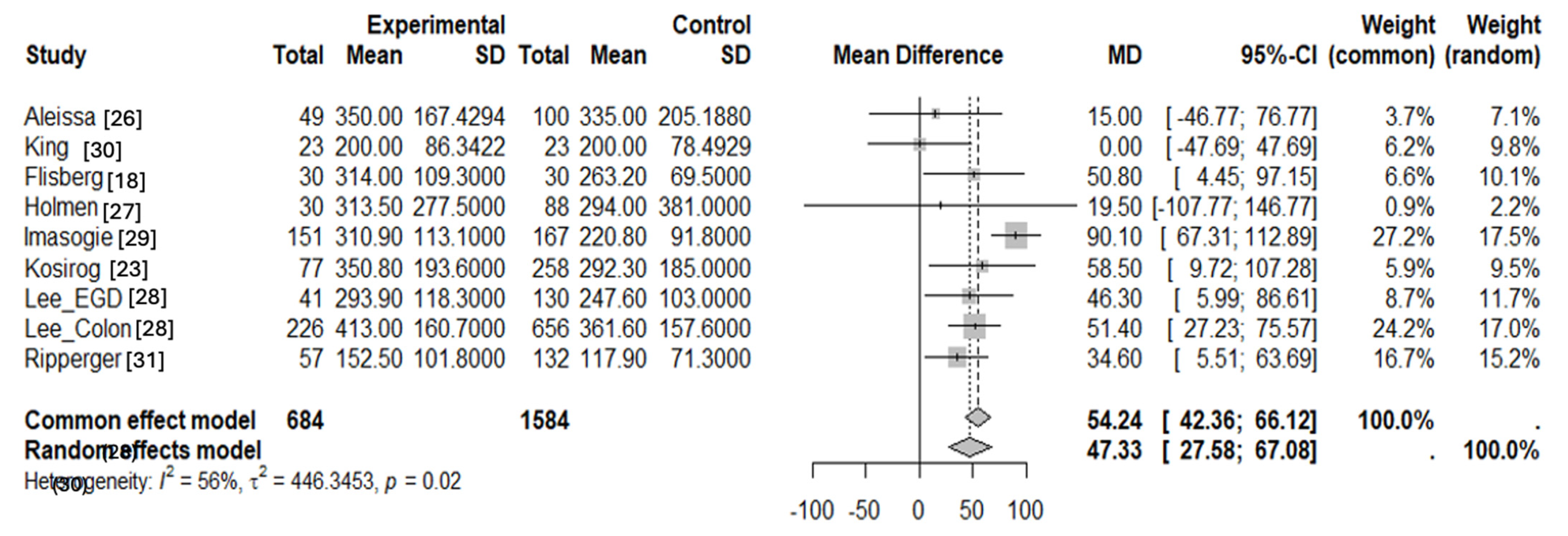
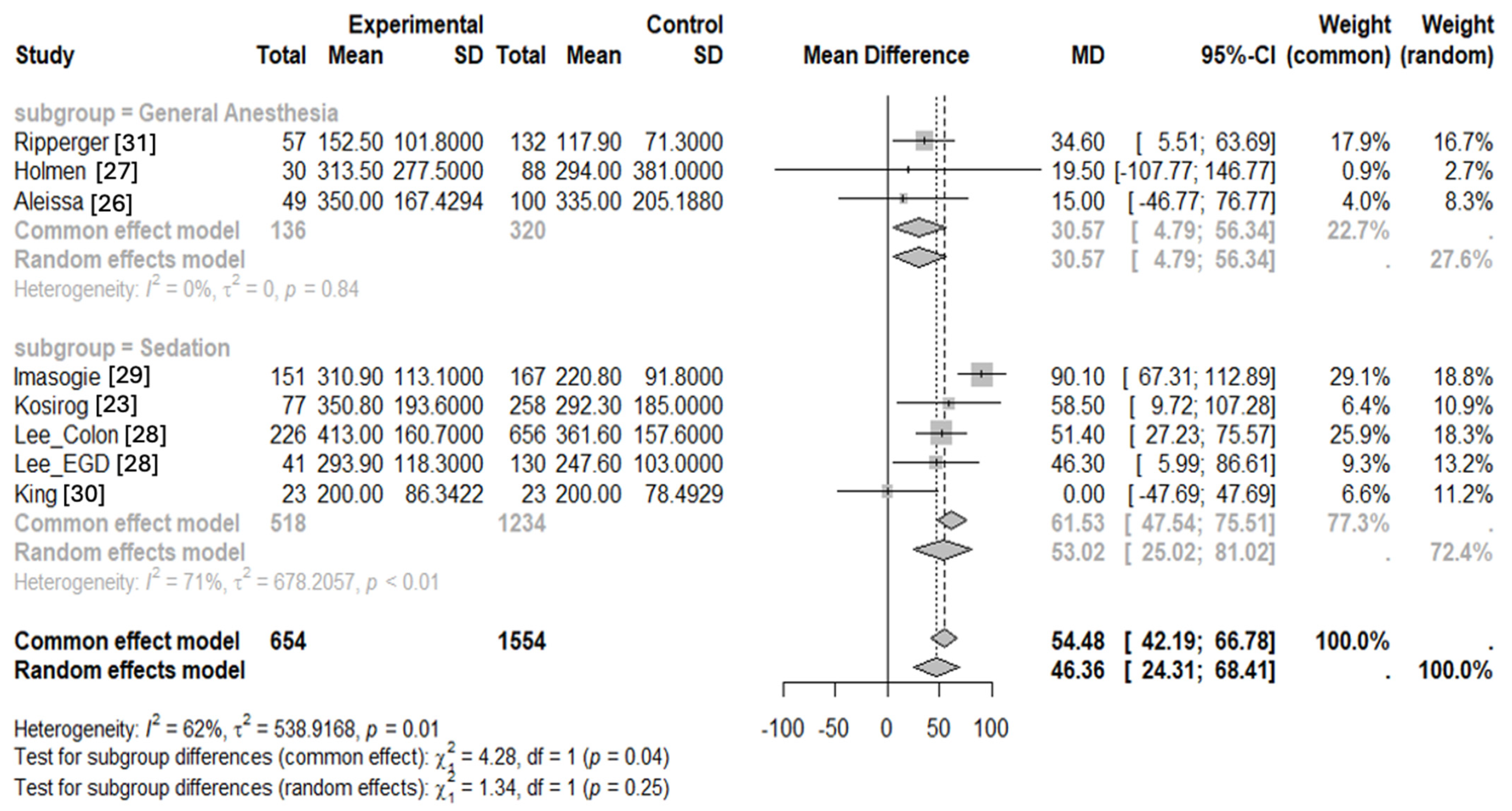
3.2. Statistical Analysis
3.3. Risk of Bias Findings
4. Discussion
4.1. Physiological Mechanisms
4.2. Clinical Implications
4.3. Public Health, Multidisciplinary Collaboration, and Economic Implications
4.4. Limitations of This Review
4.5. Directions for Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Glossary of Terms
| THC | Tetrahydrocannabinol |
| CB | Cannabinoid |
| U.S. | United States |
| GA | General anesthesia |
| TRPV1 | Transient receptor potential cation channel subfamily V member 1 |
| NMDA | N-Methyl-D-Aspartate |
| GABA | Gamma amino butyric acid |
| CYP | Cytochrome P450 |
| MAC | Minimum alveolar concentration |
| BIS | Bispectral index |
| LMA | Laryngeal mask airway |
| MeSH | Medical subject heading |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| FAAH | Fatty acid amide hydrolase |
| ASRA Pain Medicine | American Society of Regional Anesthesia and Pain Medicine |
References
- Chiu, R.G.; Patel, S.; Siddiqui, N.; Nunna, R.S.; Mehta, A.I. Cannabis Abuse and Perioperative Complications Following Inpatient Spine Surgery in the United States. Spine 2021, 46, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Lipari, R.N. Key Substance Use and Mental Health Indicators in the United States: Results from the 2018 National Survey on Drug Use and Health; SAMHSA: Rockville, MD, USA, 2018.
- 2021 NSDUH Annual National Report|CBHSQ Data. Available online: https://www.samhsa.gov/data/report/2021-nsduh-annual-national-report (accessed on 6 December 2024).
- National Institute on Drug Abuse. Marijuana and Hallucinogen Use, Binge Drinking Reached Historic Highs Among Adults 35 to 50|National Institute on Drug Abuse (NIDA). Available online: https://nida.nih.gov/news-events/news-releases/2023/08/marijuana-and-hallucinogen-use-binge-drinking-reached-historic-highs-among-adults-35-to-50 (accessed on 6 December 2024).
- Khelemsky, Y.; Goldberg, A.T.; Hurd, Y.L.; Winkel, G.; Ninh, A.; Qian, L.; Oprescu, A.; Ciccone, J.; Katz, D.J. Perioperative Patient Beliefs Regarding Potential Effectiveness of Marijuana (Cannabinoids) for Treatment of Pain: A Prospective Population Survey. Reg. Anesth. Pain Med. 2017, 42, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Trapani, G.M.; Altomare, C.; Sanna, E.; Biggio, G.; Liso, G. Propofol in Anesthesia. Mechanism of Action, Structure-Activity Relationships, and Drug Delivery. Curr. Med. Chem. 2000, 7, 249–271. [Google Scholar] [CrossRef] [PubMed]
- Habchi, K.M.; Li, M.T.; Mallard, C.A.; Baker, M.; Ortega, R. The Anesthesiologist’s Armamentarium: From Recreation to Medication and Back. J. Anesth. Hist. 2020, 6, 17–26. [Google Scholar] [CrossRef]
- Lee, B.H.; Sideris, A.; Ladha, K.S.; Johnson, R.L.; Wu, C.L. Cannabis and Cannabinoids in the Perioperative Period. Anesthesia Analg. 2024, 138, 16–30. [Google Scholar] [CrossRef]
- Brand, P.-A.; Paris, A.; Bein, B.; Meybohm, P.; Scholz, J.; Ohnesorge, H.; Tonner, P.H. Propofol sedation is reduced by delta9-tetrahydrocannabinol in mice. Anesth. Analg. 2008, 107, 102–106. [Google Scholar] [CrossRef]
- Maslonka, M.A.; Schertz, A.R.; Markowski, L.M.; Miller, P.J. Sedation challenges in patients with E-cigarette, or vaping, product use-associated lung injury (EVALI). BMJ Case Rep. 2020, 13, e233866. [Google Scholar] [CrossRef]
- Farasatinasab, M.; Nasiripour, S.; Aghabiklooei, A. Effect of cannabis use on propofol requirement for ICU sedation. Anaesthesiol. Intensive Ther. 2022, 54, 344–345. [Google Scholar] [CrossRef]
- Richtig, G.; Bosse, G.; Arlt, F.; von Heymann, C. Cannabis consumption before surgery may be associated with increased tolerance of anesthetic drugs: A case report. Int. J. Case Rep. Images 2015, 6, 436–439. [Google Scholar] [CrossRef]
- Symons, I.E. Cannabis smoking and anaesthesia. Anaesthesia 2002, 57, 1142–1143. [Google Scholar] [CrossRef]
- Walker, F.; Baric, A.; Sekharan, L.; James, E.; Farook, F.; Itrat, Q.; Jagadheesan, K. Anaesthetic safety with undisclosed substance misuse during electroconvulsive therapy. Australas. Psychiatry 2022, 30, 278–279. [Google Scholar] [CrossRef] [PubMed]
- Twardowski, M.A.; Link, M.M.; Twardowski, N.M. Effects of Cannabis Use on Sedation Requirements for Endoscopic Procedures. J. Am. Osteopat. Assoc. 2019, 119, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Gangwani, P.; Lillian, D.; Dobbins, J.; Feng, C.; Vorrasi, J.; Kolokythas, A. Is Recreational Marijuana Use Associated With Changes in the Vital Signs or Anesthetic Requirements During Intravenous Sedation? J. Oral Maxillofac. Surg. 2023, 81, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Yeung, B.G.; Ma, M.W.; Scolaro, J.A.; Nelson, A.M. Cannabis Exposure Decreases Need for Blood Pressure Support During General Anesthesia in Orthopedic Trauma Surgery. Cannabis Cannabinoid Res. 2022, 7, 328–335. [Google Scholar] [CrossRef]
- Flisberg, P.; Paech, M.; Shah, T.; Ledowski, T.; Kurowski, I.; Parsons, R. Induction dose of propofol in patients using cannabis. Eur. J. Anaesthesiol. 2009, 26, 192–195. [Google Scholar] [CrossRef]
- Zhang, H.-X.B.; Bean, B.P. Cannabidiol Inhibition of Murine Primary Nociceptors: Tight Binding to Slow Inactivated States of Nav1.8 Channels. J. Neurosci. 2021, 41, 6371–6387. [Google Scholar] [CrossRef]
- Laudanski, K.; Wain, J. Considerations for Cannabinoids in Perioperative Care by Anesthesiologists. J. Clin. Med. 2022, 11, 558. [Google Scholar] [CrossRef]
- Alexander, J.C.; Joshi, G.P. A review of the anesthetic implications of marijuana use. Proc. Bayl. Univ. Med. Cent. 2019, 32, 364–371. [Google Scholar] [CrossRef]
- Ladha, K.S.; Manoo, V.; Virji, A.-F.; Hanlon, J.G.; Mclaren-Blades, A.; Goel, A.; Wijeysundera, D.N.; Kotra, L.P.; Ibarra, C.; Englesakis, M.; et al. The Impact of Perioperative Cannabis Use: A Narrative Scoping Review. Cannabis Cannabinoid Res. 2019, 4, 219–230. [Google Scholar] [CrossRef]
- Kosirog, J.; Bouvette, C.; Pannu, J.; Gondal, J.; Madhoun, M. Marijuana and endoscopy: The effects of marijuana on sedation. Gastrointest. Endosc. 2024, 100, 177–182. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.; Bossuyt, P.; Boutron, I.; Hoffmann, T.; Mulrow, C.; Shamseer, L.; Tetzlaff, J.; Aki, E.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 29 March 2024).
- Aleissa, M.M.; Ahern, K.L.; Stern, G.M. Peri-operative opioid and sedation requirements in patients who use marijuana and are undergoing total knee or total hip arthroplasty: A retrospective study. J. Clin. Anesth. 2020, 66, 109953. [Google Scholar] [CrossRef] [PubMed]
- Holmen, I.C.; Beach, J.P.; Kaizer, A.M.; Gumidyala, R. The association between preoperative cannabis use and intraoperative inhaled anesthetic consumption: A retrospective study. J. Clin. Anesth. 2020, 67, 109980. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Nagra, N.; La Selva, D.; Kozarek, R.A.; Ross, A.; Weigel, W.; Beecher, R.; Chiorean, M.; Gluck, M.; Boden, E.; et al. Nurse-Administered Propofol Continuous Infusion Sedation for Gastrointestinal Endoscopy in Patients Who Are Difficult to Sedate. Clin. Gastroenterol. Hepatol. 2021, 19, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Imasogie, N.; Rose, R.V.; Wilson, A. High quantities: Evaluating the association between cannabis use and propofol anesthesia during endoscopy. PLoS ONE 2021, 16, e0248062. [Google Scholar] [CrossRef] [PubMed]
- King, D.D.; Stewart, S.A.; Collins-Yoder, A.; Fleckner, T.; Price, L.L. Anesthesia for Patients Who Self-Report Cannabis (Marijuana) Use Before Esophagogastroduodenoscopy: A Retrospective Review. AANA J. 2021, 89, 205–212. [Google Scholar]
- Ripperger, D.; Atte, A.; Ritto, F. Cannabis Users Require More Anesthetic Agents for General Anesthesia in Ambulatory Oral and Maxillofacial Surgery Procedures. J. Oral Maxillofac. Surg. 2023, 81, 1460–1465. [Google Scholar] [CrossRef]
- Huson, H.B.; Granados, T.M.; Rasko, Y. Surgical considerations of marijuana use in elective procedures. Heliyon 2018, 4, e00779. [Google Scholar] [CrossRef]
- Uhing, M.R.; Beno, D.W.A.; Jiyamapa-Serna, V.A.; Chen, Y.; Galinsky, R.E.; Hall, S.D.; Kimura, R.E. the effect of anesthesia and surgery on CYP3A activity in rats. Drug Metab. Dispos. 2004, 32, 1325–1330. [Google Scholar] [CrossRef]
- Schelling, G.; Hauer, D.; Azad, S.C.; Schmoelz, M.; Chouker, A.; Schmidt, M.; Hornuss, C.; Rippberger, M.; Briegel, J.; Thiel, M.; et al. Effects of General Anesthesia on Anandamide Blood Levels in Humans. Anesthesiology 2006, 104, 273–277. [Google Scholar] [CrossRef]
- Goyal, H.; Awad, H.H.; Ghali, J.K. Role of cannabis in cardiovascular disorders. J. Thorac. Dis. 2017, 9, 2079–2092. [Google Scholar] [CrossRef]
- Beaulieu, P.; Boulanger, A.; Desroches, J.; Clark, A.J. Medical cannabis: Considerations for the anesthesiologist and pain physician. Can. J. Anaesth. 2016, 63, 608–624. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Schwenk, E.S.; Sondekoppam, R.V.; Clarke, H.; Zakowski, M.; Rzasa-Lynn, R.S.; Yeung, B.; Nicholson, K.; Schwartz, G.; Hooten, W.M.; et al. ASRA Pain Medicine consensus guidelines on the management of the perioperative patient on cannabis and cannabinoids. Reg. Anesth. Pain Med. 2023, 48, 97–117. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.J.; Galettis, P.; Schneider, J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br. J. Clin. Pharmacol. 2018, 84, 2477–2482. [Google Scholar] [CrossRef] [PubMed]
- Tetrault, J.M.; Crothers, K.; Moore, B.A.; Mehra, R.; Concato, J.; Fiellin, D.A. Effects of Marijuana Smoking on Pulmonary Function and Respiratory Complications: A Systematic Review. Arch. Intern. Med. 2007, 167, 221–228. [Google Scholar] [CrossRef]
- Wong, G.T.C.; Irwin, M.G. Poisoning with illicit substances: Toxicology for the anaesthetist. Anaesthesia 2013, 68 (Suppl. S1), 117–124. [Google Scholar] [CrossRef]
- Combined Beta-Agonists and Anticholinergics Compared to Beta-Agonists Alone for Adults with Asthma Treated in Emergency Departments. Available online: https://www.cochrane.org/CD001284/AIRWAYS_combined-beta-agonists-and-anticholinergics-compared-beta-agonists-alone-adults-asthma-treated (accessed on 6 December 2024).
- Echeverria-Villalobos, M.; Todeschini, A.B.; Stoicea, N.; Fiorda-Diaz, J.; Weaver, T.; Bergese, S.D. Perioperative care of cannabis users: A comprehensive review of pharmacological and anesthetic considerations. J. Clin. Anesth. 2019, 57, 41–49. [Google Scholar] [CrossRef]
- Gan, T.J.; Belani, K.G.; Bergese, S.; Chung, F.; Diemunsch, P.; Habib, A.S.; Jin, Z.; Kovac, A.L.; Meyer, T.A.; Urman, R.D.; et al. Fourth Consensus Guidelines for the Management of Postoperative Nausea and Vomiting. Anesth. Analg. 2020, 131, 411–448. [Google Scholar] [CrossRef]
- Brenne, J.; Burney, E.; Mauer, K.; Orina, J.; Philipp, T.; Yoo, J. Risks associated with chronic cannabis use on opioid use, length of stay, and revision rate for patients undergoing posterior lumbar interbody fusion. Spine J. 2024, 24, 851–857. [Google Scholar] [CrossRef]
- Ekrami, E.; Sari, S.; Kopac, O.; Wang, D.; Mascha, E.J.; Stamper, S.; Esa, W.A.S.; Nair, H.; Ruetzler, K.; Turan, A. Association Between Cannabis Use and Opioid Consumption, Pain, and Respiratory Complications After Surgery: A Retrospective Cohort Analysis. Anesth. Analg. 2024, 139, 724–733. [Google Scholar] [CrossRef]
- Sumrak, K. In the Weeds: Nation’s First Guidelines for Management of Perioperative Patients on Cannabis. ASA Monit. 2024, 88, 30–33. [Google Scholar] [CrossRef]
- Potnuru, P.P.; Jonna, S.; Williams, G.W. Cannabis Use Disorder and Perioperative Complications. JAMA Surg. 2023, 158, 935–944. [Google Scholar] [CrossRef] [PubMed]
- King, D.D.; Gill, C.J.; Cadieux, C.S.; Singh, N. The role of stigma in cannabis use disclosure: An exploratory study. Harm Reduct. J. 2024, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Bicket, M.C.; Stone, E.M.; McGinty, E.E. Use of Cannabis and Other Pain Treatments Among Adults With Chronic Pain in US States With Medical Cannabis Programs. JAMA Netw. Open 2023, 6, e2249797. [Google Scholar] [CrossRef]
- Urbanoski, K.; Pauly, B.; Inglis, D.; Cameron, F.; Haddad, T.; Phillips, J.; Phillips, P.; Rosen, C.; Schlotter, G.; Hartney, E.; et al. Defining culturally safe primary care for people who use substances: A participatory concept mapping study. BMC Health Serv. Res. 2020, 20, 1060. [Google Scholar] [CrossRef]
- Baker, M.B.; Liu, E.C.; Bully, M.A.; Hsieh, A.; Nozari, A.; Tuler, M.; Binda, D.D. Overcoming Barriers: A Comprehensive Review of Chronic Pain Management and Accessibility Challenges in Rural America. Healthcare 2024, 12, 1765. [Google Scholar] [CrossRef]
- Greco, C.M.; Gaylord, S.A.; Faurot, K.; Weinberg, J.M.; Gardiner, P.; Roth, I.; Barnhill, J.L.; Thomas, H.N.; Dhamne, S.C.; Lathren, C.; et al. The design and methods of the OPTIMUM study: A multisite pragmatic randomized clinical trial of a telehealth group mindfulness program for persons with chronic low back pain. Contemp. Clin. Trials 2021, 109, 106545. [Google Scholar] [CrossRef]
- Baker, M.B.; Hsieh, A.; Gupta, V.; Kim, Y.; Merriel, M.; Nozari, A.; Binda, D. The Color of Climate Change: Can Choice of Anesthetic Be Institutionally Racist? Anesth. Analg. 2024, 138, 1154–1158. [Google Scholar] [CrossRef]
- Suhre, W.; O’Reilly-Shah, V.; Van Cleve, W. Cannabis use is associated with a small increase in the risk of postoperative nausea and vomiting: A retrospective machine-learning causal analysis. BMC Anesthesiol. 2020, 20, 115. [Google Scholar] [CrossRef]
- Dahan, A.; Hooten, W.M.; Furnish, T. Cannabis Use and Anesthesia. Adv. Anesth. 2024, 42, 85–96. [Google Scholar] [CrossRef]
- Goel, A.; McGuinness, B.; Jivraj, N.K.; Wijeysundera, D.N.; Mittleman, M.A.; Bateman, B.T.; Clarke, H.; Kotra, L.P.; Ladha, K.S. Cannabis Use Disorder and Perioperative Outcomes in Major Elective Surgeries: A Retrospective Cohort Analysis. Anesthesiology 2020, 132, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chung, T.; Dey, A.; Bae, S.W. Exploring Algorithmic Explainability: Generating Explainable AI Insights for Personalized Clinical Decision Support Focused on Cannabis Intoxication in Young Adults. In Proceedings of the 2024 International Conference on Activity and Behavior Computing (ABC), Oita, Japan; Kitakyushu, Japan, 29–31 May 2024. [Google Scholar] [CrossRef]
- Dalton, J.E.; Bolen, S.D.; Mascha, E.J. Publication Bias: The Elephant in the Review. Anesth. Analg. 2016, 123, 812–813. [Google Scholar] [CrossRef]
- Öztürk, E.; Aydoğan, M.S.; Karaaslan, K.; Doğan, Z.; Topuz, U. Does smoking increase the anesthetic requirement? Turk. J. Med Sci. 2019, 49, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Sajdeya, R.; Rouhizadeh, M.; Cook, R.L.; Ison, R.L.; Bai, C.; Jugl, S.; Gao, H.; Mardini, M.T.; Zandbiglari, K.; Adiba, F.I. Cannabis Use and Inhalational Anesthesia Administration in Older Adults: A Propensity-matched Retrospective Cohort Study. Anesthesiology 2024, 141, 870–880. [Google Scholar] [CrossRef] [PubMed]
| Study | Design | Sample Size | Procedure | Procedure Length (Min) | Cannabis Use Criteria | Frequency of Cannabis Use | Other Significant Outcomes |
|---|---|---|---|---|---|---|---|
| Flisberg, 2009 [18] | Prospective observational single center | 60 | Day-case general anesthesia with laryngeal mask | N/A | Regular use at least once per week for at least the past 6 months | 1 day/week × 6 months | N/A |
| Aleissa, 2020 [26] | Retrospective single center | 149 | Total knee or total hip Arthroplasty | N/A | Social history of marijuana use within 6 months or + THC screen on admission | N/A | Higher postop opioid usage and higher postop pain scores in the cannabis group, and higher NSAID use in the controls. |
| Holmen, 2020 [27] | Retrospective single center | 118 | Isolated tibia open reduction and internal fixation | N/A | Self-reported in the month prior to surgery | N/A | The average total volume of sevoflurane administered was significantly higher among the cannabis-user group. |
| Lee, 2020 [28] | Retrospective single center | 882 | Colonoscopy | 21.1–22.9 | Daily use for more than 3 months | Daily × 3 months | N/A |
| Esophagogastroduodenoscopy | 7–9 | ||||||
| King, 2021 [30] | Retrospective single center | 46 | Esophagogastroduodenoscopy | N/A | Three primary documents for verbal self-report of cannabis use: (1) the preprocedural history and physical examination findings, (2) the nursing intake form, and (3) the pre-anesthesia assessment | N/A | N/A |
| Imasogie, 2021 [29] | Retrospective single center | 318 | Colonoscopy and/or esophagogastroduodenoscopy | N/A | Any duration of self-reported inhaled cannabis exposure | Daily (4/7 days × 1 week), weekly (1–2 days/week × weeks), monthly (1–2 times/month × 9 months), or occasional (<1 × 2 months) | N/A |
| Ripperger, 2023 [31] | Retrospective single center | 189 | Extraction of at least 2 teeth requiring general anesthesia | 15–40 | Any self-reported regular use of cannabis to provider | N/A | Cannabis users received significantly more midazolam, ketamine, and fentanyl than non-users. |
| Kosirog, 2024 [23] | Prospective observational single center | 976 | Colonoscopy and/or esophagogastroduodenoscopy | N/A | Patient survey prior to the endoscopy which addressed marijuana usage and frequency | N/A | N/A |
| Cannabis Users | Non-Cannabis Patients | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Propofol (mg) Required (Mean ± SD) | Mean Age (Mean ± SD) | Procedure Length | Sample Size (n) | Female Sex (%) | Mean Weight (Mean ± SD) | BMI | Largest ASA Groups | Prior Smoking History (%) | Alcohol Use (%) | Narcotic Use (%) | Respiratory Disease (%) | Propofol (mg) Required (Mean ± SD) | Mean Age (Mean ± SD) | Procedure Length | Sample Size | Female Sex (%) | Mean Weight (Mean ± SD) | BMI | Largest ASA Groups | Smoking History (%) | Narcotic Use (%) | Respiratory Disease (%) |
| Flisberg, 2009 [18] | Total induction dose for LMA insertion 314.0 ± 109.3 | 28.0 ± 8.0 | N/A | 30 | 0 | 80.7 ± 12.4 | N/A | ASA I and ASA II | N/A | N/A | Excluded | N/A | Total induction dose for LMA insertion 263.2 ± 69.5 | 22.0 ± 9.0 | N/A | 30 | 0 | 78.9 ± 12.5 | N/A | ASA I and ASA II | N/A | Excluded | N/A |
| Aleissa, 2020 [26] | Intraoperative propofol used 350.0 ± 167.4 | 56.7 ± 12.4 | N/A | 49 | 38.7 | N/A | 29.5 ± 6.3 | N/A | N/A | N/A | Excluded | N/A | Intraoperative propofol used 335.0 ± 205.2 | 66.8 ± 10.2 | N/A | 100 | 62 | N/A | 31.1 ± 5.9 | N/A | N/A | Excluded | N/A |
| Holmen, 2020 [27] | Intraoperative propofol used 313.5 ± 277.5 | N/A | 166 | 30 | N/A | N/A | N/A | N/A | N/A | No significant difference between groups | Excluded | N/A | Intraoperative propofol used 294.0 ± 381.0 | N/A | 165 | 88 | N/A | N/A | N/A | N/A | N/A | Excluded | N/A |
| Lee, 2020 Colon [28] | Total propofol used 413.0 ± 160.7 | 53.5 ± 13.9 | 22.9 ± 10.8 | 226 | 39.0 | 84.8 ± 21.4 | 28.0 ± 6.0 | ASA II | 14.6 | N/A | N/A | N/A | Total propofol used 361.6 ± 157.6 | 60.1 ± 12.5 | 8.5 ± 5.5 | 656 | 51.4 | 79.9 ± 19.6 | 27.2 ± 5.7 | ASA I and ASA II | 3.5 | Excluded | N/A |
| Lee, 2020 EGD [28] | Total propofol used 293.9 ± 118.3 | 47.3 ± 16.5 | 7.9 ± 3.8 | 41 | 61.1 | 80.7 ± 18.5 | 27.3 ± 5.2 | ASA II | 14.6 | N/A | N/A | N/A | Total propofol used 247.6 ± 103.0 | 67.7 ± 11.3 | 22.8 ± 10.0 | 130 | 58.5 | 79.3 ± 18.2 | 29.1 ± 15.2 | ASA II | 5.4 | Excluded | N/A |
| King, 2021 [30] | Total propofol used 200.0 ± 86.3 | 41.1 ± 14.2 | 5.7 ± 2.2 | 23 | 78.3 | 81.3 ± 17.6 | 28.4 ± 6.0 | ASA II and ASA III | N/A | N/A | N/A | N/A | Total propofol used 200.0 ± 78.5 | 41.5 ± 14.7 | 5.7 ± 2.2 | 23 | 78.3 | 79.8 ± 17.5 | 29.7 ± 5.5 | ASA II and ASA III | N/A | N/A | N/A |
| Imasogie, 2021 [29] | Total propofol used 310.9 ± 113.1 | 43.7 (18–71) | 16.7 ± 10.9 | 151 | 30.4 | 82.9 ± 23.4 | N/A | ASA II | 68.9 | N/A | 17.9 | COPD, asthma, or OSA: 25.8 | Total propofol used 220.8 ± 91.8 | 53.8 (23–88) | 18.3 ± 9.6 | 167 | 60.4 | 77.4 ± 17.2 | N/A | ASA II | 41.3 | 9.6 | COPD, asthma or OSA: 16.8 |
| Ripperger, 2023 [31] | Intraoperative propofol used 152.5 ± 101.8 | 26.6 ± 6.4 | N/A | 57 | 71.9 | N/A | N/A | ASA I and ASA II | N/A | N/A | N/A | N/A | Intraoperative propofol used 117.9 ± 71.3 | 28.2 ± 7.8 | N/A | 132 | 72.7 | N/A | N/A | ASA I and ASA II | N/A | N/A | N/A |
| Kosirog, 2024 [23] | Total propofol used 350.8 ± 193.6 | 57.7 ± 13.7 | 25.2 ± 17.1 | 210 | 71.9 | N/A | 28.2 ± 5.9 | N/A | Smoking tobacco: 35.7; vape: 13.5 | N/A | 5.7 | COPD: 10.9; OSA: 26.2 | Total propofol used 292.3 ± 185.0 | 61.4 ± 12.7 | 24.1 ± 18.3 | 766 | 72.7 | N/A | 31.2 ± 6.4 | N/A | Smoking tobacco: 19.0; vape: 3.7 | 4.9 | COPD: 13.5; OSA: 40.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baker, M.B.; Binda, D.D.; Nozari, A.; Kennedy, J.M.; Dienes, E.; Baker, W.E. Quantitative Analysis of Propofol Dosage in Cannabis Users: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 858. https://doi.org/10.3390/jcm14030858
Baker MB, Binda DD, Nozari A, Kennedy JM, Dienes E, Baker WE. Quantitative Analysis of Propofol Dosage in Cannabis Users: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(3):858. https://doi.org/10.3390/jcm14030858
Chicago/Turabian StyleBaker, Maxwell B., Dhanesh D. Binda, Ala Nozari, Joseph M. Kennedy, Erin Dienes, and William E. Baker. 2025. "Quantitative Analysis of Propofol Dosage in Cannabis Users: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 3: 858. https://doi.org/10.3390/jcm14030858
APA StyleBaker, M. B., Binda, D. D., Nozari, A., Kennedy, J. M., Dienes, E., & Baker, W. E. (2025). Quantitative Analysis of Propofol Dosage in Cannabis Users: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(3), 858. https://doi.org/10.3390/jcm14030858








