Alterations in Implantation Genes and Dendritic Cells in Endometrial Samples After Antibiotic Treatment
Abstract
1. Introduction
2. Materials and Methods
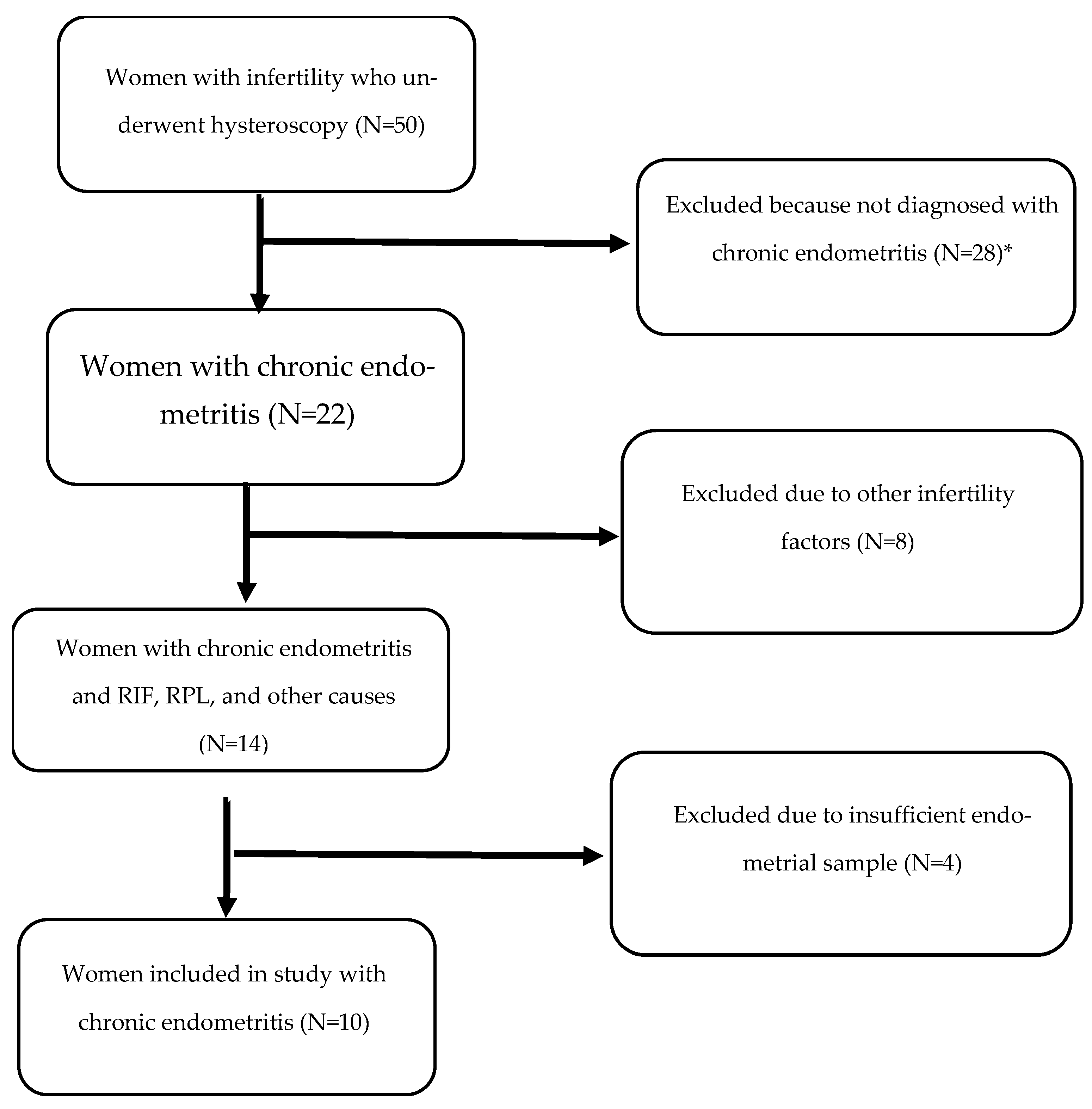
2.1. Patients
2.2. Exclusion Criteria
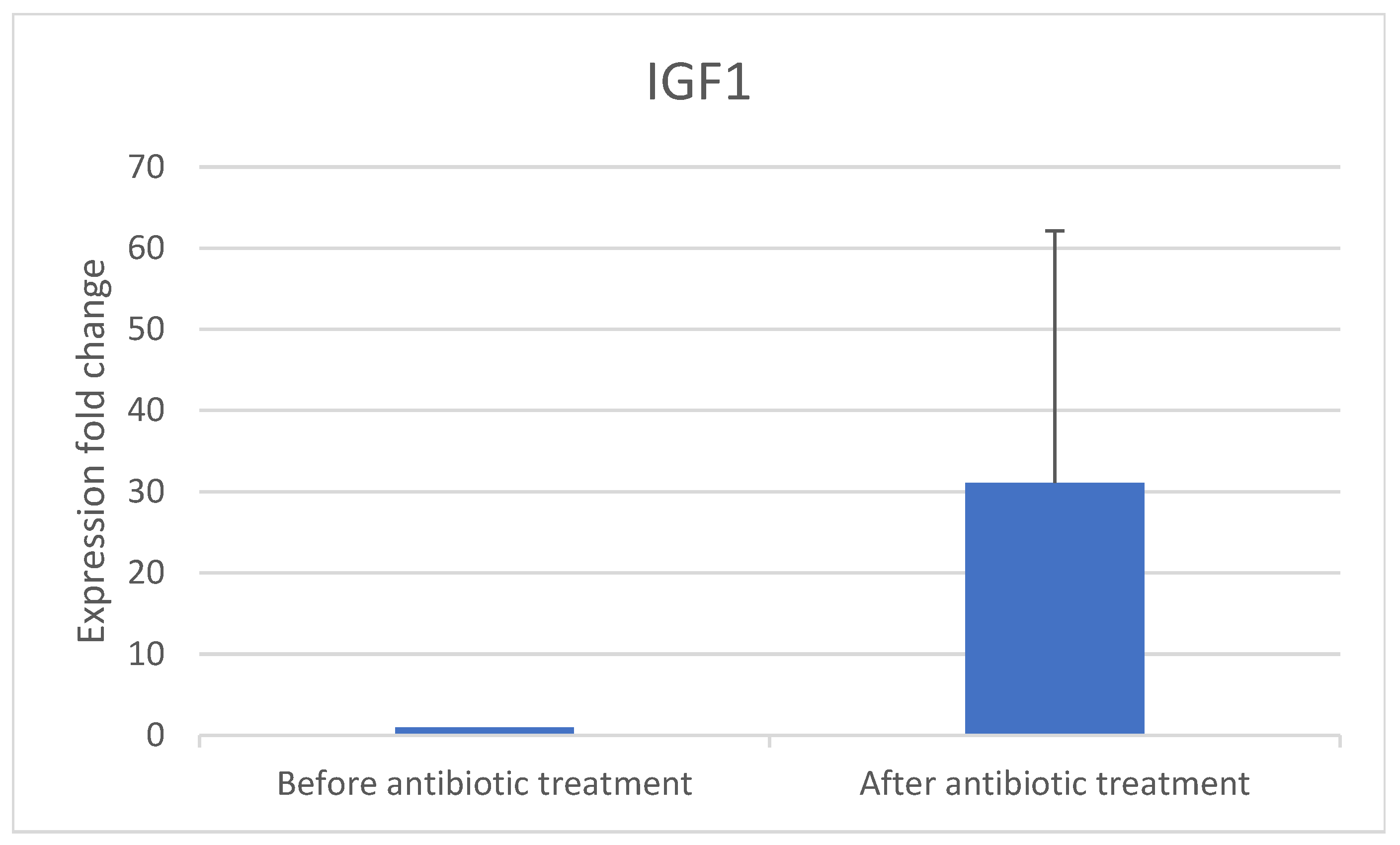
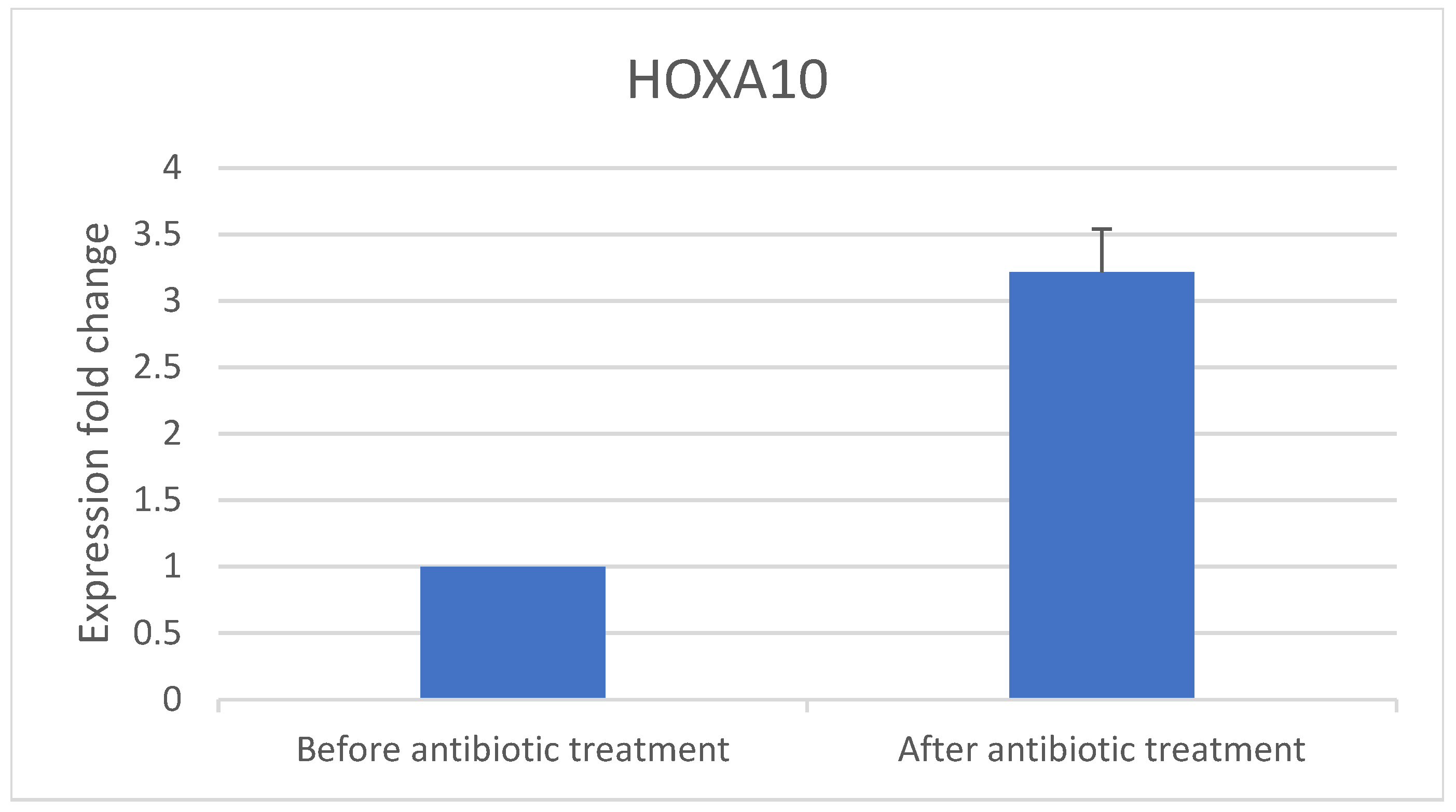
- The implantation genes HOXA10 and IGF-1 were quantified using RT-PCR in samples obtained before and after antibiotic treatment.
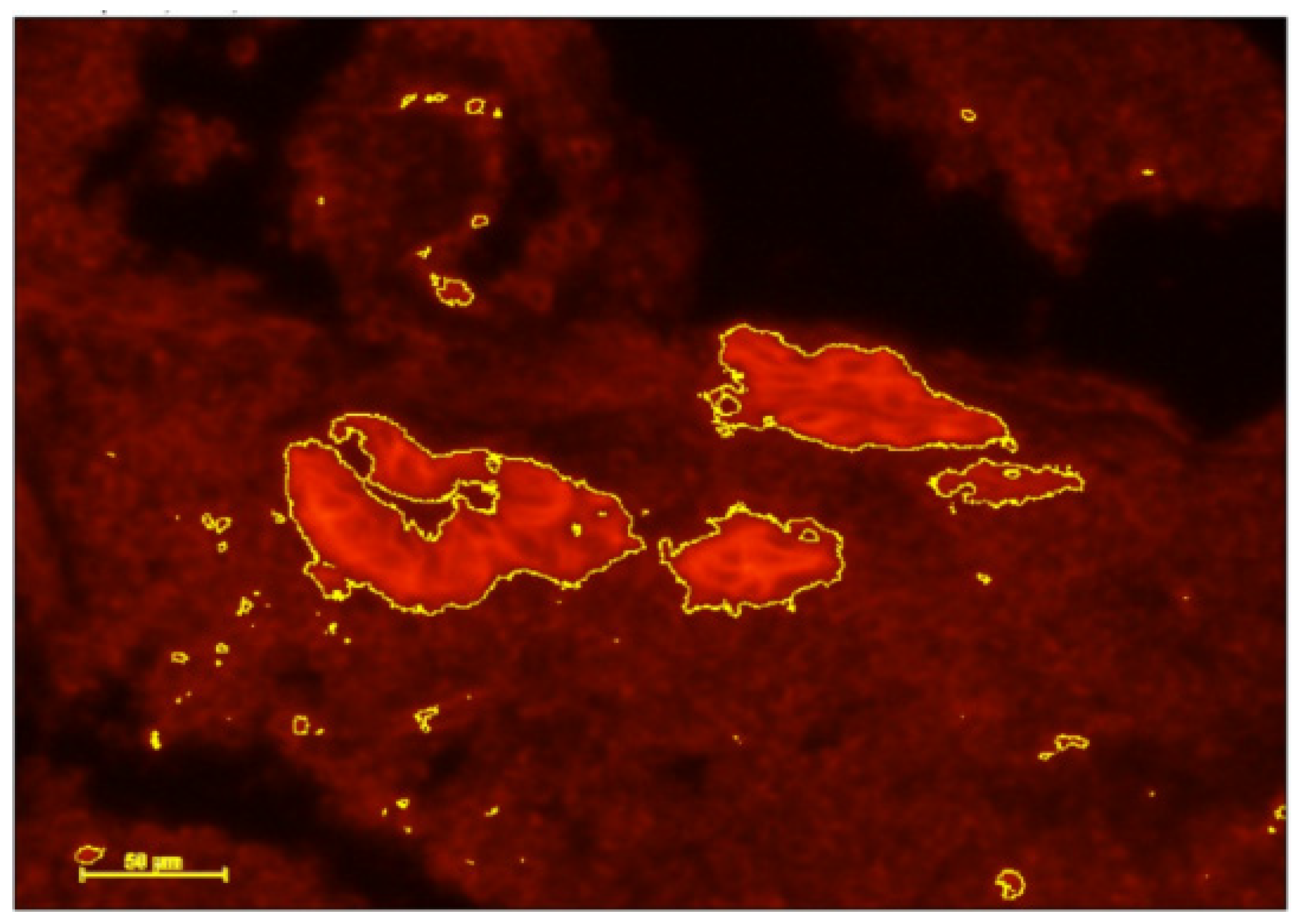
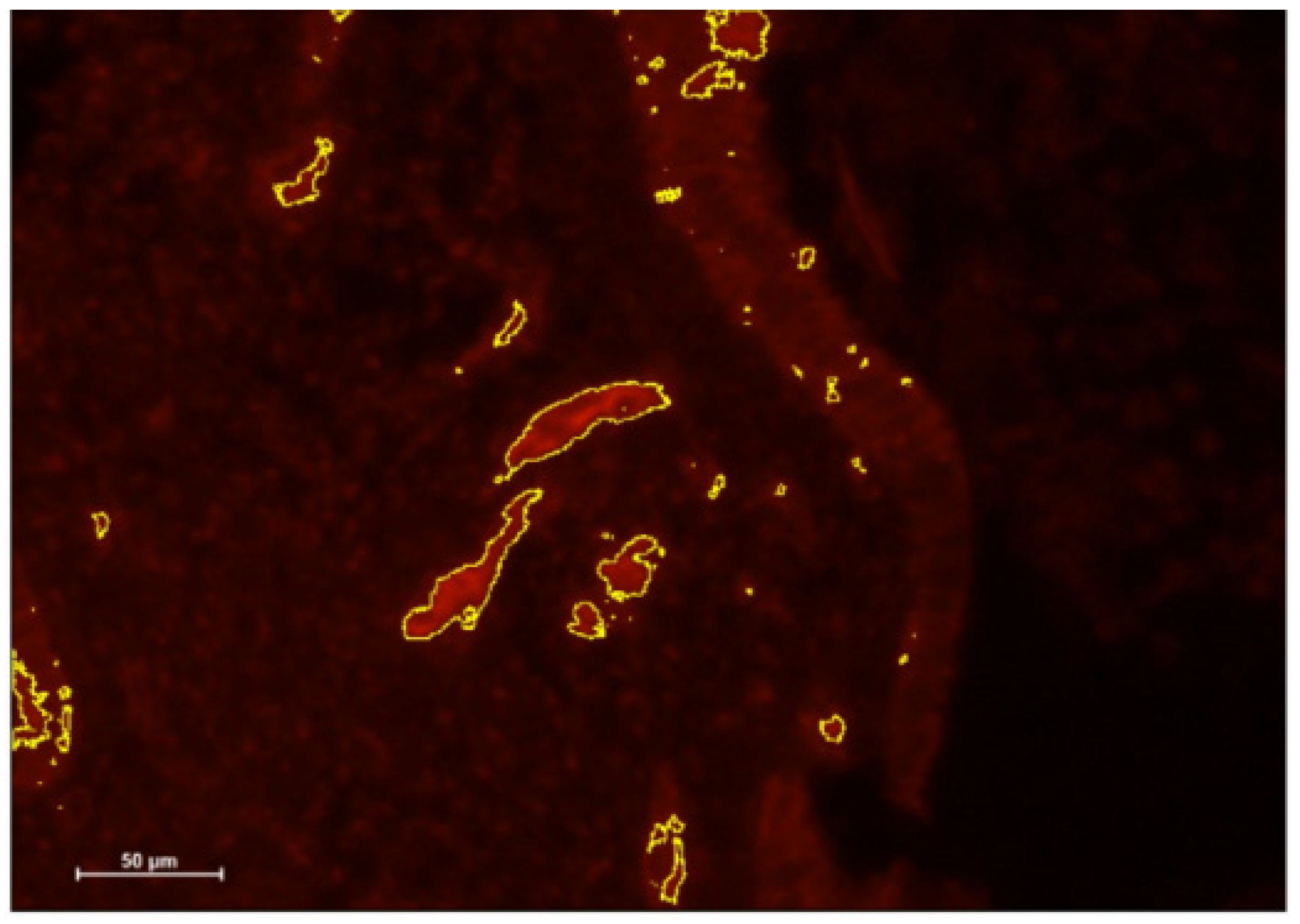
- DCs were identified using immunohistochemical (IHC) staining of CD141 and IGF-1 before and after antibiotic treatment.
2.3. Deparaffinization
2.4. Quantitative Real-Time PCR
2.5. Immunohistochemistry
3. Results
3.1. Real-Time PCR Results
3.2. Immunohistochemistry Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kitaya, K.; Matsubayashi, H.; Yamaguchi, K.; Nishiyama, R.; Takaya, Y.; Ishikawa, T.; Yasuo, T.; Yamada, H. Chronic Endometritis: Potential Cause of Infertility and Obstetric and Neonatal Complications. Am. J. Reprod. Immunol. 2016, 75, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Sethi, A. Endometritis-Diagnosis, Treatment and its impact on fertility—A Scoping Review. JBRA Assist. Reprod. 2022, 26, 538. [Google Scholar] [CrossRef] [PubMed]
- Vitagliano, A.; Saccardi, C.; Noventa, M.; Di Spiezio Sardo, A.; Saccone, G.; Cicinelli, E.; Pizzi, S.; Andrisani, A.; Litta, P.S. Effects of chronic endometritis therapy on in vitro fertilization outcome in women with repeated implantation failure: A systematic review and meta-analysis. Fertil. Steril. 2018, 110, 103–112.e1. [Google Scholar] [CrossRef] [PubMed]
- Vaduva, C.C.; Sandulescu, M.S.; Tenovici, M.; Siminel, M.A.; Novac, M.B. Results of in vitro fertilization after diagnosis and treatment of chronic endometritis. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 1069–1076. [Google Scholar] [CrossRef]
- Cicinelli, E.; McQueen, D.B.; Huepfel, B.; Vitagliano, A.; Moreno, I.; Simon, C.; Pirtea, P.; Scott, R.T.; Bellavia, M.; De Ziegler, D. Should patients be screened for chronic endometritis before assisted reproductive technology? Fertil. Steril. 2022, 118, 639–652. [Google Scholar] [CrossRef]
- Di Spiezio Sardo, A.; Palma, F.; Calagna, G.; Zizolfi, B.; Bifulco, G. Chronic Endometritis. In Genital Infections and Infertility; Darwish, A.M., Ed.; InTech: Takasago, Japan, 2016; ISBN 978-953-51-2488-7. [Google Scholar]
- Cicinelli, E.; Matteo, M.; Trojano, G.; Mitola, P.C.; Tinelli, R.; Vitagliano, A.; Crupano, F.M.; Lepera, A.; Miragliotta, G.; Resta, L. Chronic endometritis in patients with unexplained infertility: Prevalence and effects of antibiotic treatment on spontaneous conception. Am. J. Reprod. Immunol. 2018, 79, e12782. [Google Scholar] [CrossRef]
- Espinós, J.J.; Fabregues, F.; Fontes, J.; García-Velasco, J.A.; Llácer, J.; Requena, A.; Checa, M.Á.; Bellver, J. Impact of chronic endometritis in infertility: A SWOT analysis. Reprod. Biomed. Online 2021, 42, 939–951. [Google Scholar] [CrossRef]
- Cicinelli, E.; Matteo, M.; Tinelli, R.; Lepera, A.; Alfonso, R.; Indraccolo, U.; Marrocchella, S.; Greco, P.; Resta, L. Prevalence of chronic endometritis in repeated unexplained implantation failure and the IVF success rate after antibiotic therapy. Hum. Reprod. 2015, 30, 323–330. [Google Scholar] [CrossRef]
- Kasius, J.C. The impact of chronic endometritis on reproductive outcome. Fertil. Steril. 2011, 96, 1451–1456. [Google Scholar] [CrossRef]
- Cicinelli, E.; Matteo, M.; Tinelli, R.; Pinto, V.; Marinaccio, M.; Indraccolo, U.; De Ziegler, D.; Resta, L. Chronic Endometritis Due to Common Bacteria Is Prevalent in Women with Recurrent Miscarriage as Confirmed by Improved Pregnancy Outcome After Antibiotic Treatment. Reprod. Sci. 2014, 21, 640–647. [Google Scholar] [CrossRef]
- Kushnir, V.A.; Solouki, S.; Sarig-Meth, T.; Vega, M.G.; Albertini, D.F.; Darmon, S.K.; Deligdisch, L.; Barad, D.H.; Gleicher, N. Systemic inflammation and autoimmunity in women with chronic endometritis. Am. J. Reprod. Immunol. 2016, 75, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Pietro, C.D.; Cicinelli, E.; Guglielmino, M.R.; Ragusa, M.; Farina, M.; Palumbo, M.A.; Cianci, A. Altered Transcriptional Regulation of Cytokines, Growth Factors, and Apoptotic Proteins in the Endometrium of Infertile Women with Chronic Endometritis. Am. J. Reprod. Immunol. 2013, 69, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, S. Evaluation of peripheral and uterine immune status of chronic endometritis in patients with recurrent reproductive failure. Fertil. Steril. 2020, 113, 11. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, C.; Caruso, S.; Battaglia, R.; Iraci Sareri, M.; La Ferlita, A.; Strino, F.; Bonaventura, G.; Di Mauro, M.; Barcellona, M.L.; Perciavalle, V.; et al. MiR-27a-3p and miR-124-3p, upregulated in endometrium and serum from women affected by Chronic Endometritis, are new potential molecular markers of endometrial receptivity. Am. J. Reprod. Immunol. 2018, 80, e12858. [Google Scholar] [CrossRef]
- Liu, S.; Wei, H.; Li, Y.; Huang, C.; Lian, R.; Xu, J.; Chen, L.; Zeng, Y. Downregulation of ILT4+ dendritic cells in recurrent miscarriage and recurrent implantation failure. Am. J. Reprod. Immunol. 2018, 80, e12998. [Google Scholar] [CrossRef]
- Rutanen, E.-M. Insulin-like growth factors in endometrial function. Gynecol. Endocrinol. 1998, 12, 399–406. [Google Scholar] [CrossRef]
- Tang, X.M.; Rossi, M.J.; Masterson, B.J.; Chegini, N. Insulin-like growth factor I (IGF-I), IGF-I receptors, and IGF binding proteins 1-4 in human uterine tissue: Tissue localization and IGF-I action in endometrial stromal and myometrial smooth muscle cells in vitro. Biol. Reprod. 1994, 50, 1113–1125. [Google Scholar] [CrossRef]
- Daftary, G.S.; Taylor, H.S. Molecular markers of implantation: Clinical implications. Curr. Opin. Obstet. Gynecol. 2001, 13, 269–274. [Google Scholar] [CrossRef]
- Nazarenko, T.A.; Kalinina, E.A.; Knyazeva, E.A.; Kiselev, V.I.; Smolnikova, V.Y.; Sukhikh, G.T. The role of abnormal hypermethylation of the HOXA10 and HOXA11 promoters in implantation failures in IVF programs. Gynecol. Endocrinol. 2019, 35, 31–34. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, Y.S.; Yoon, T.K.; Lee, W.S. Chronic endometritis and infertility. Clin. Exp. Reprod. Med. 2016, 43, 185. [Google Scholar] [CrossRef]
- Kitaya, K.; Matsubayashi, H.; Takaya, Y.; Nishiyama, R.; Yamaguchi, K.; Takeuchi, T.; Ishikawa, T. Live birth rate following oral antibiotic treatment for chronic endometritis in infertile women with repeated implantation failure. Am. J. Reprod. Immunol. 2017, 78, e12719. [Google Scholar] [CrossRef] [PubMed]
- Cicinelli, E.; Vitagliano, A.; Kumar, A.; Lasmar, R.B.; Bettocchi, S.; Haimovich, S.; Kitaya, K.; de Ziegler, D.; Simon, C.; Moreno, I.; et al. Unified diagnostic criteria for chronic endometritis at fluid hysteroscopy: Proposal and reliability evaluation through an international randomized-controlled observer study. Fertil. Steril. 2019, 112, 162–173.e2. [Google Scholar] [CrossRef] [PubMed]
- Hirata, K.; Kimura, F.; Nakamura, A.; Kitazawa, J.; Morimune, A.; Hanada, T.; Takebayashi, A.; Takashima, A.; Amano, T.; Tsuji, S.; et al. Histological diagnostic criterion for chronic endometritis based on the clinical outcome. BMC Womens Health 2021, 21, 94. [Google Scholar] [CrossRef] [PubMed]
| Oligo Name | Sequence (5′-3′) |
|---|---|
| HOXA 10-F | CAACTGGCTCACGGCAAAGA |
| HOXA 10-R | TTCAGTTTCATCCTGCGGTTC |
| IGF1-F | GTGGAGACAGGGGCTTTTATT |
| IGF1-R | CTCCAGCCTCCTTAGATCACA |
| Program | Step 1 | Step 2 | Step 3 | Step 4 |
|---|---|---|---|---|
| Temperature (°C) | 25 | 37 | 85 | 4 |
| Time | 10 min | 120 min | 5 min | ∞ (Forever/10 min) |
| Variable | Study Group (N = 10) |
|---|---|
| Age (years) | 35.1 ± 7.6 |
| Infertility duration (years) | 3.4 ± 2.01 |
| Nulliparous, n (%) | 6 (60%) |
| Baseline FSH (Follicular stimulation hormone) (IU/L) | 6.65 ± 1.91 |
| Baseline LH(luteinizng hormone) (IU/L) | 6.36 ± 4.03 |
| Baseline E2 (Estradiol) (pmole/mL) | 211.4 ± 98.5 |
| Hysteroscopy indication, n | |
| Endometrial polyp | 3 |
| Repeated implantation failure | 3 |
| Repeated pregnancy loss | 2 |
| Other (previous cesarean section, prolonged infertility) | 2 |
| Last endometrial thickness (mm) before transfer | 6.94 ± 3.51 |
| Antibiotic treatment, n (%) | |
| First line (doxycycline) | 9 (90%) |
| Second line (ciprofloxacin + metronidazole) | 8 (80%) |
| IVF (In vitro fertilization) cycles before treatment | 4 ± 3.09 |
| IVF cycles after treatment | 3.3 ± 2.7 |
| Pregnancy (positive hCG(Human chorionic gonadotropin), n (%) | 8 (80%) |
| Live birth, n (%) | 6 (60%) |
| Pathologic signs of CE (chronic enndometritis) in hysteroscopy, n (%) | |
| Hyperemia | 3 (30%) |
| Micropolyps | 4 (40%) |
| Focal edema | 3 (30%) |
| Normal pathology | 2 (20%) |
| Not described | 2 (20%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darawshe, B.; Hantisteanu, S.; Meisel Sharon, S.; Groisman, G.; Haimovich, S.; Shalom-Paz, E. Alterations in Implantation Genes and Dendritic Cells in Endometrial Samples After Antibiotic Treatment. J. Clin. Med. 2025, 14, 834. https://doi.org/10.3390/jcm14030834
Darawshe B, Hantisteanu S, Meisel Sharon S, Groisman G, Haimovich S, Shalom-Paz E. Alterations in Implantation Genes and Dendritic Cells in Endometrial Samples After Antibiotic Treatment. Journal of Clinical Medicine. 2025; 14(3):834. https://doi.org/10.3390/jcm14030834
Chicago/Turabian StyleDarawshe, Baraa, Shay Hantisteanu, Shilhav Meisel Sharon, Gabriel Groisman, Sergio Haimovich, and Einat Shalom-Paz. 2025. "Alterations in Implantation Genes and Dendritic Cells in Endometrial Samples After Antibiotic Treatment" Journal of Clinical Medicine 14, no. 3: 834. https://doi.org/10.3390/jcm14030834
APA StyleDarawshe, B., Hantisteanu, S., Meisel Sharon, S., Groisman, G., Haimovich, S., & Shalom-Paz, E. (2025). Alterations in Implantation Genes and Dendritic Cells in Endometrial Samples After Antibiotic Treatment. Journal of Clinical Medicine, 14(3), 834. https://doi.org/10.3390/jcm14030834





