Efficacy of Tocopherol vs. Chlorhexidine in the Management of Oral Biopsy Site: A Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Participants
- Attached gingiva: attached gingiva, retromolar trigone, edentulous ridge;
- Loose mucosa: buccal mucosa, tongue, floor of mouth, lips, retrocommissure.
2.3. Outcomes
2.4. Sample Size
2.5. Blinding
2.6. Statistical Methods
3. Results
3.1. VAS Stratified Between Those Who Have and Those Who Have Not Taken Painkillers
3.2. Quality of Wound Healing
3.3. Quality of Wound Healing Stratified Between Smokers and Non-Smokers
3.4. Quality of Wound Healing: Comparison Between “Attached” and “Loose”
3.5. Adverse Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Treccani [Internet]. Biopsia—Enciclopedia. Available online: https://www.treccani.it/enciclopedia/biopsia_(Universo-del-Corpo)/ (accessed on 29 June 2024).
- Patel, K.J.; De Silva, H.L.; Tong, D.C.; Love, R.M. Concordance between clinical and histopathologic diagnoses of oral mucosal lesions. J. Oral. Maxillofac. Surg. Off. J. Am. Assoc. Oral. Maxillofac. Surg. 2011, 69, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Chiapasco, M.; Casentini, P.; Coggiola, A.; Fusari, P.; Garattini, G.; Gatti, F.; Maccarini, L.; Micolani, R.; Montinari, A.; Rossi, A.; et al. Manuale Illustrato di Chirurgia Orale, 4th ed.; Edra S.p.A.: Milano, Italy, 2020; 625p. [Google Scholar]
- Kearns, H.P.; McCartan, B.E.; Lamey, P.J. Patients’ pain experience following oral mucosal biopsy under local anaesthesia. Br. Dent. J. 2001, 13, 33–35. [Google Scholar] [CrossRef]
- López-Jornet, P.; Camacho-Alonso, F.; Martinez-Canovas, A. Clinical evaluation of polyvinylpyrrolidone sodium hyaluronate gel and 0.2% chlorhexidine gel for pain after oral mucosa biopsy: A preliminary study. J. Oral. Maxillofac. Surg. Off. J. Am. Assoc. Oral. Maxillofac. Surg. 2010, 68, 2159–2163. [Google Scholar] [CrossRef] [PubMed]
- Pilloni, A.; Ceccarelli, S.; Bosco, D.; Gerini, G.; Marchese, C.; Marini, L.; Rojas, M.A. Effect of Chlorhexidine Digluconate in Early Wound Healing of Human Gingival Tissues. A Histological, Immunohistochemical and Biomolecular Analysis. Antibiotics 2021, 10, 1192. [Google Scholar] [CrossRef] [PubMed]
- Ros-Llor, I.; Lopez-Jornet, P. Cytogenetic analysis of oral mucosa cells, induced by chlorhexidine, essential oils in ethanolic solution and triclosan mouthwashes. Environ. Res. 2014, 132, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Balloni, S.; Locci, P.; Lumare, A.; Marinucci, L. Cytotoxicity of three commercial mouthrinses on extracellular matrix metabolism and human gingival cell behaviour. Toxicol. Vitro Int. J. Publ. Assoc. BIBRA 2016, 34, 88–96. [Google Scholar] [CrossRef]
- Reda, B.; Hollemeyer, K.; Trautmann, S.; Hannig, M.; Volmer, D.A. Determination of chlorhexidine retention in different oral sites using matrix-assisted laser desorption/ionization-time of flight mass spectrometry. Arch. Oral. Biol. 2020, 110, 104623. [Google Scholar] [CrossRef]
- Leard, A.; Addy, M. The propensity of different brands of tea and coffee to cause staining associated with chlorhexidine. J. Clin. Periodontol. 1997, 24, 115–118. [Google Scholar] [CrossRef]
- Lang, N.P.; Catalanotto, F.A.; Knöpfli, R.U.; Antczak, A.A.A. Quality-specific taste impairment following the application of chlorhexidine digluconate mouthrinses. J. Clin. Periodontol. 1988, 15, 43–48. [Google Scholar] [CrossRef]
- Lockhart, A.S.; Harle, C.C. Anaphylactic reactions due to chlorohexidine (multiple letters) [2]. Br. J. Anaesth. 2001, 87, 940–941. [Google Scholar] [PubMed]
- Mariotti, A.J.; Rumpf, D.A. Chlorhexidine-induced changes to human gingival fibroblast collagen and non-collagen protein production. J. Periodontol. 1999, 70, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Hammad, H.M.; Hammad, M.M.; Abdelhadi, I.N.; Khalifeh, M.S. Effects of topically applied agents on intra-oral wound healing in a rat model: A clinical and histomorphometric study. Int. J. Dent. Hyg. 2011, 9, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, C.; Kallenberger, A. Influence of chlorhexidine rinsing on the healing of oral mucosa and osseous lesions. J. Clin. Periodontol. 1980, 7, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Lindhe, J.; Heyden, G.; Svanberg, G.; Löe, H.; Schiott, C.R. Effect of local applications of chlorhexidine on the oral mucosa of the hamster. J. Periodontal Res. 1970, 5, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Arduino, P.G.; Gambino, A.; Cabras, M.; Sciannameo, V.; Nimot, Y.; Karimi, D.; Ricceri, F.; Broccoletti, R. Effect of two different alcohol-free chlorhexidine formulations in mouthrinses on the immediate postoperative period for oral mucosal biopsies. J. Oral Sci. 2020, 62, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Schiott, C.R.; Löe, H.; Jensen, S.B.; Kilian, M.; Davies, R.M.; Glavind, K. The effect of chlorhexidine mouthrinses on the human oral flora. J. Periodontal Res. 1970, 5, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Palaia, G.; Tenore, G.; Tribolati, L.; Russo, C.; Gaimari, G.; Del Vecchio, A.; Romeo, U. Evaluation of wound healing and postoperative pain after oral mucosa laser biopsy with the aid of compound with chlorhexidine and sodium hyaluronate: A randomized double blind clinical trial. Clin. Oral. Investig. 2019, 23, 3141–3151. [Google Scholar] [CrossRef]
- Brookes, Z.L.; A Belfield, L.; Ashworth, A.; Casas-Agustench, P.; Raja, M.; Pollard, A.J.; Bescos, R. Effects of chlorhexidine mouthwash on the oral microbiome. J. Dent. 2021, 113, 103768. [Google Scholar] [CrossRef]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Wadleigh, R.G.; Redman, R.S.; Graham, M.L.; Krasnow, S.H.; Anderson, A.; Cohen, M.H. Vitamin E in the treatment of chemotherapy-induced mucositis. Am. J. Med. 1992, 92, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Im, G.J.; An, Y.S.; Lee, S.H.; Jung, H.H.; Park, S.Y. The effects of the antioxidant α-tocopherol succinate on cisplatin-induced ototoxicity in HEI-OC1 auditory cells. Int. J. Pediatr. Otorhinolaryngol. 2016, 86, 9–14. [Google Scholar] [CrossRef]
- Diplock, A.T.; Xu, G.L.; Yeow, C.L.; Okikiola, M. Relationship of tocopherol structure to biological activity, tissue uptake, and prostaglandin biosynthesis. Ann. N. Y. Acad. Sci. 1989, 570, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Trevithick, J.R.; Xiong, H.; Lee, S.; Shum, D.T.; Sanford, S.E.; Karlik, S.J.; Norley, C.; Dilworth, G.R. Topical tocopherol acetate reduces post-UVB, sunburn-associated erythema, edema, and skin sensitivity in hairless mice. Arch. Biochem. Biophys. 1992, 296, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Panganamala, R.V.; Cornwell, D.G. The effects of vitamin E on arachidonic acid metabolism. Ann. N. Y. Acad. Sci. 1982, 393, 376–391. [Google Scholar] [CrossRef]
- Nizam, N.; Discioglu, F.; Saygun, I.; Bal, V.; Avcu, F.; Ozkan, C.K.; Serdar, M.A. The effect of α-tocopherol and selenium on human gingival fibroblasts and periodontal ligament fibroblasts in vitro. J. Periodontol. 2014, 85, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Testa, D.; Marcuccio, G.; Panin, G.; Bianco, A.; Tafuri, D.; Thyrion, F.Z.; Nunziata, M.; Piombino, P.; Guerra, G.; Motta, G. Nasal mucosa healing after endoscopic sinus surgery in chronic rhinosinusitis of elderly patients: Role of topic alpha-tocopherol acetate. Aging Clin. Exp. Res. 2017, 29, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Alberts, D.S.; Goldman, R.; Xu, M.J.; Dorr, R.T.; Quinn, J.; Welch, K.; Guillen-Rodriguez, J.; Aickin, M.; Peng, Y.M.; Loescher, L.; et al. Disposition and metabolism of topically administered alpha-tocopherol acetate: A common ingredient of commercially available sunscreens and cosmetics. Nutr. Cancer 1996, 26, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Tovar, J.; Llavero, C. Perianal Application of Glyceryl Trinitrate Ointment Versus Tocopherol Acetate Ointment in the Treatment of Chronic Anal Fissure: A Randomized Clinical Trial. Dis. Colon Rectum 2022, 65, 406. [Google Scholar] [CrossRef]
- Panin, G.; Strumia, R.; Ursini, F. Topical alpha-tocopherol acetate in the bulk phase: Eight years of experience in skin treatment. Ann. N. Y. Acad. Sci. 2004, 1031, 443–447. [Google Scholar] [CrossRef]
- Fiori, G.; Galluccio, F.; Braschi, F.; Amanzi, L.; Miniati, I.; Conforti, M.L.; Del Rosso, A.; Generini, S.; Candelieri, A.; Magonio, A.; et al. Vitamin E gel reduces time of healing of digital ulcers in systemic sclerosis. Clin. Exp. Rheumatol. 2009, 27 (Suppl. S54), 51–54. [Google Scholar] [PubMed]
- Cohen, R.E.; Ciancio, S.G.; Mather, M.L.; Curro, F.A. Effect of vitamin E gel, placebo gel and chlorhexidine on periodontal disease. Clin. Prev. Dent. 1991, 13, 20–24. [Google Scholar]
- Bacci, C.; Vanzo, V.; Frigo, A.C.; Stellini, E.; Sbricoli, L.; Valente, M. Topical tocopherol for treatment of reticular oral lichen planus: A randomized, double-blind, crossover study. Oral Dis. 2017, 23, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.J.; Bullen, M.; Piccenna, L.; McNeil, J.J. Vitamin E supplementation and mortality in healthy people: A meta-analysis of randomised controlled trials. Cardiovasc. Drugs Ther. 2014, 28, 563–573. [Google Scholar] [CrossRef]
- Jiang, S.; Pan, Z.; Li, H.; Li, F.; Song, Y.; Qiu, Y. Meta-analysis: Low-dose intake of vitamin E combined with other vitamins or minerals may decrease all-cause mortality. J. Nutr. Sci. Vitaminol. 2014, 60, 194–205. [Google Scholar] [CrossRef]
- Abner, E.L.; Schmitt, F.A.; Mendiondo, M.S.; Marcum, J.L.; Kryscio, R.J. Vitamin E and all-cause mortality: A meta-analysis. Curr. Aging Sci. 2011, 4, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 Spiegazione ed Elaborazione: Linee guida aggiornate per il reporting di trial randomizzati a gruppi paralleli. Evidence 2012, 4, e1000024. [Google Scholar]
- Kerner, S.; Etienne, D.; Malet, J.; Mora, F.; Monnet-Corti, V.; Bouchard, P. Root coverage assessment: Validity and reproducibility of an image analysis system. J. Clin. Periodontol. 2007, 34, 969–976. [Google Scholar] [CrossRef]
- Alman, A.C.; Johnson, L.R.; Calverley, D.C.; Grunwald, G.K.; Lezotte, D.C.; Hokanson, J.E. Validation of a method for quantifying carotid artery calcification from panoramic radiographs. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2013, 116, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Grbatinić, I.; Milošević, N.T. Incipient UV-Induced Structural Changes in Neutrophil Granulocytes: Morphometric and Texture Analysis of Two-Dimensional Digital Images. Microsc. Microanal. Off. J. Microsc. Soc. Am. Microbeam Anal. Soc. Microsc. Soc. Can. 2016, 22, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Moshrefi, A. Chlorhexidine. J. West. Soc. Periodontol. Abstr. 2002, 50, 5–9. [Google Scholar]
- Margarone, J.E.; Natiella, J.R.; Vaughan, C.D. Artifacts in oral biopsy specimens. J. Oral. Maxillofac. Surg. Off. J. Am. Assoc. Oral. Maxillofac. Surg. 1985, 43, 163–172. [Google Scholar] [CrossRef]
- Avon, S.L.; Klieb, H.B.E. Oral soft-tissue biopsy: An overview. J. Can. Dent. Assoc. 2012, 78, c75. [Google Scholar]
- Jeng, P.Y.; Chang, M.C.; Chiang, C.P.; Lee, C.F.; Chen, C.F.; Jeng, J.H. Oral soft tissue biopsy surgery: Current principles and key tissue stabilization techniques. J. Dent. Sci. 2024, 19, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Balaji, S.M. Tobacco smoking and surgical healing of oral tissues: A review. Indian J. Dent. Res. Off. Publ. Indian Soc. Dent. Res. 2008, 19, 344–348. [Google Scholar] [CrossRef] [PubMed]





| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Patients scheduled for oral cavity biopsy at the University of Padua Dental Clinic Patients able to read and understand the information sheet and express informed consent Patients who, after being informed in detail about the treatment usage methods, adhered to the administration scheme | Underage patients Patients on antiangiogenic therapy Patients on immunomodulator therapy Patients with known hypersensitivity to the device |
| Test Group (n = 40) | Control Group (n = 37) | |
|---|---|---|
| sex, n (%): | 27 (68%) | 24 (65%) |
| women | 13 (32%) | 13 (35%) |
| men | 27 (68%) | 24 (65%) |
| age, years: median (IQR) | 64 (54–74) | 61 (56–70) |
| mucosal site, n (%): | ||
| attached | 18 (45%) | 18 (49%) |
| loose | 22 (55%) | 19 (51%) |
| painkillers, n (%) | 14 (35%) | 8 (22%) |
| smoker, n (%): | ||
| no | 26 (65%) | 26 (70%) |
| yes | 9 (23%) | 4 (11%) |
| ex | 5 (12%) | 7 (19%) |
| (a) | ||||
| Test Group (n = 40) | Control Group (n = 37) | Median Difference (95% Bootstrap Confidence Interval) | p Value | |
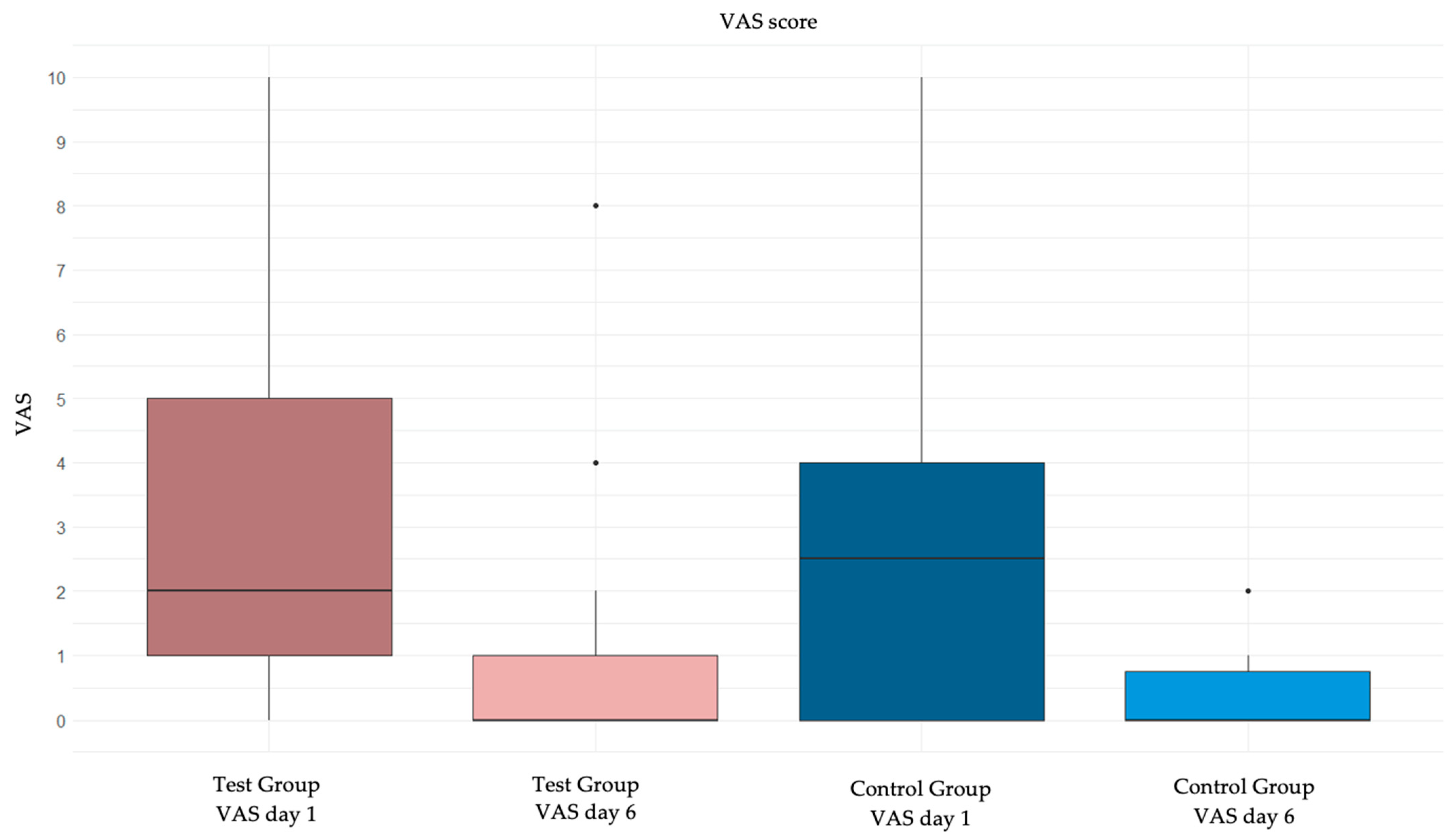
| VAS day 1: median (IQR) | 2 (1; 4) | 2 (0; 4) | ||
| VAS day 6: median (IQR) | 0 (0; 1) | 0 (0; 0) | ||
| Variation in VAS: median (IQR) | 2 (1; 3) | 2 (0; 4) | 0 (from −1 to 1) | 0.99 |
| (b) | ||||
| Test Group (n = 35) | Control Group (n = 35) | Median Difference (95% Bootstrap Confidence Interval) | p Value | |
| VAS day 1: median (IQR) | 2 (1; 4) | 2 (0; 4) | ||
| VAS day 6: median (IQR) | 0 (0; 1) | 0 (0; 1) | ||
| Variation in VAS: median (IQR) | 2 (1; 3) | 2 (0; 4) | 0 (from −1 to 1) | 0.99 |
| Test Group (n = 26) | Control Group (n = 29) | Median Difference (95% Bootstrap Confidence Interval) | p Value | |
|---|---|---|---|---|
| VAS day 1: median (IQR) | 2 (0; 4) | 2 (0; 4) | ||
| VAS day 6: median (IQR) | 0 (0; 0) | 0 (0; 0) | ||
| Variation in VAS: median (IQR) | 2 (0; 3) | 2 (0; 3) | 0 (from −1 to 2) | 0.83 |
| Test Group (n = 14) | Control Group (n = 8) | Median Difference (95% Bootstrap Confidence Interval) | p Value | |
|---|---|---|---|---|
| VAS day 1: median (IQR) | 5 (2; 8) | 4 (2; 5) | ||
| VAS day 6: median (IQR) | 0 (0; 3) | 0 (0; 1) | ||
| Variation in VAS: median (IQR) | 2 (1; 6) | 2 (2; 4) | 0 (from −4 to 3) | 0.92 |
| (a) | ||||
| Test Group (n = 37) | Control Group (n = 30) | Median Difference (95% Bootstrap Confidence Interval) | p Value | |
| Height at day 1: median (IQR) | 6.7 (5.1; 8.0) | 6.4 (5.4; 7.3) | ||
| Height at day 6: median (IQR) | 4.0 (2.3; 5.6) | 3.5 (2.4; 4.8) | ||
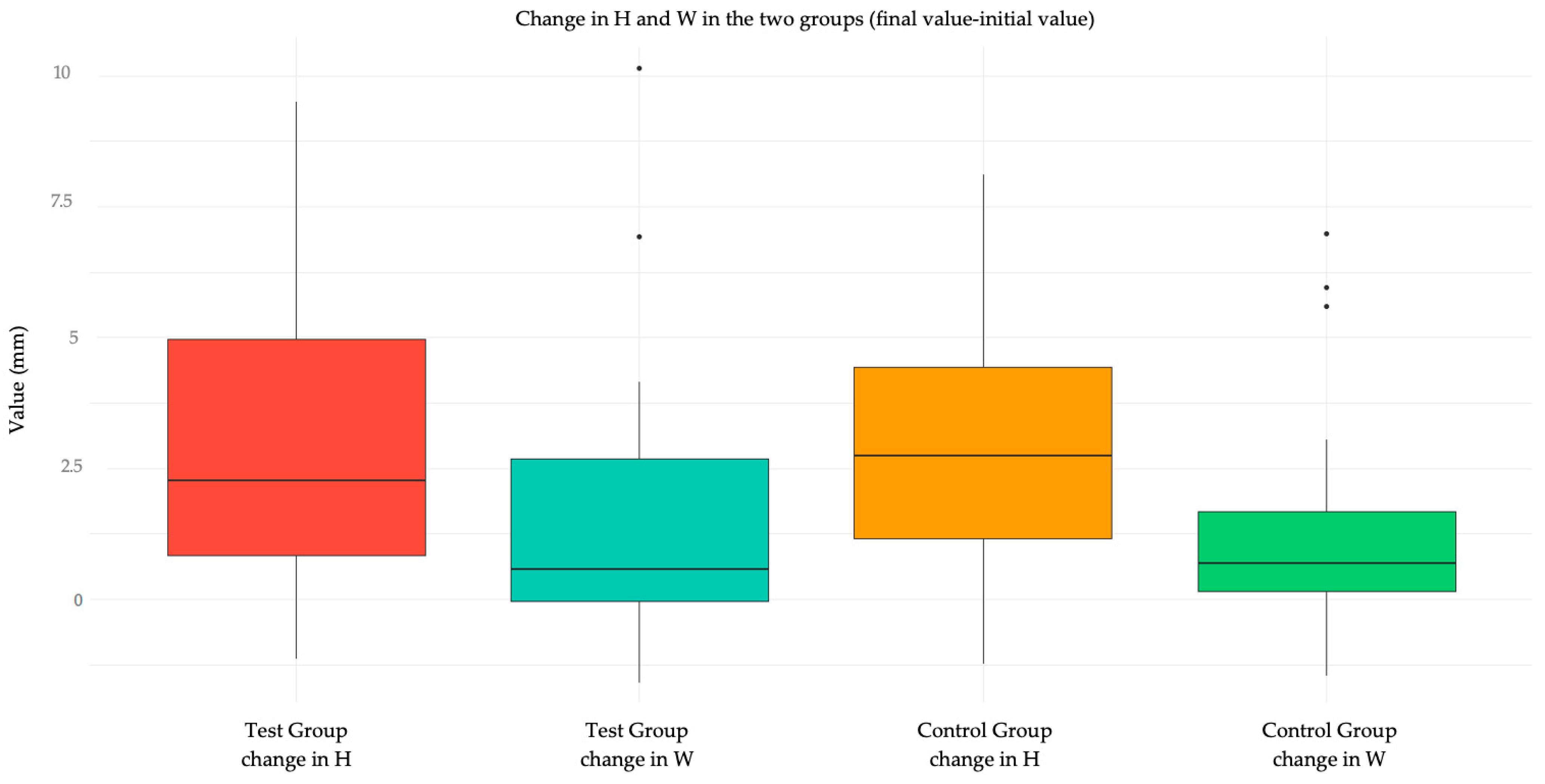
| Variation in height: median (IQR) | 2.2 (0.8; 4.9) | 2.7 (1.2; 4.1) | −0.5 (from −1.6 to 1.7) | 0.91 |
| (b) | ||||
| Test Group (n = 33) | Control Group (n = 28) | Median Difference (95% Bootstrap Confidence Interval) | p Value | |
| Height at day 1: median (IQR) | 6.7 (5.1; 8.1) | 6.4 (5.4; 7.3) | ||
| Height at day 6: median (IQR) | 4.2 (2.3; 6.0) | 3.5 (2.4; 4.8) | ||
| Variation in height: median (IQR) | 2.0 (0.8; 4.9) | 2.5 (1.0; 4.2) | −0.5 (from −2.4 to 1.0) | 0.85 |
| (a) | ||||
| Test Group (n = 37) | Control Group (n = 30) | Median Difference (95% Bootstrap Confidence Interval) | p Value | |
| Width at day 1: median (IQR) | 2.5 (1.5; 6.6) | 2.2 (1.4; 3.5) | ||
| Width at day 6: median (IQR) | 1.7 (1.1; 3.9) | 1.4 (1.1; 2.0) | ||
| Variation in width: median (IQR) | 0.4 (−0.1; 2.7) | 0.7 (0.2; 1.5) | −0.2 (from −0.9 to 0.6) | 0.71 |
| (b) | ||||
| Test Group (n = 33) | Control Group (n = 28) | Median Difference (95% Bootstrap Confidence Interval) | p Value | |
| Width at day 1: median (IQR) | 2.5 (1.5; 6.5) | 2.4 (1.4; 3.9) | ||
| Width at day 6: median (IQR) | 1.7 (1.1; 3.9) | 1.5 (1.1; 2.2) | ||
| Variation in width: median (IQR) | 0.5 (−0.2; 1.6) | 0.7 (0.2; 1.5) | −0.2 (from −1.1 to 0.5) | 0.56 |
| Test Group (n = 24) | Control Group (n = 20) | Median Difference (95% Bootstrap Confidence Interval) | p Value | |
|---|---|---|---|---|
| Height at day 1: median (IQR) | 6.6 (5.1; 7.3) | 6.4 (5.4; 7.3) | ||
| Height at day 6: median (IQR) | 3.1 (2.0; 5.4) | 2.8 (2.4; 3.9) | ||
| Variation in height: median (IQR) | 2.4 (1.1; 4.4) | 2.9 (1.3; 4.7) | −0.5 (from −2.1 to 1.6) | 0.63 |
| Test Group (n = 24) | Control Group (n = 20) | Median Difference (95% Bootstrap Confidence Interval) | p Value | |
|---|---|---|---|---|
| Width at day 1: median (IQR) | 2.9 (1.2; 5.8) | 2.2 (1.2; 3.0) | ||
| Width at day 6: median (IQR) | 1.6 (0.9; 3.3) | 1.4 (1.1; 1.7) | ||
| Variation in width: median (IQR) | 0.6 (−0.2; 2.8) | 0.6 (0.1; 1.4) | 0.0 (from −1.1 to 1.8) | 0.86 |
| Test Group (n = 14) | Control Group (n = 11) | Median Difference (95% Bootstrap Confidence Interval) | p Value | |
|---|---|---|---|---|
| Height at day 1: median (IQR) | 6.9 (5.6; 9.8) | 6.3 (5.7; 7.1) | ||
| Height at day 6: median (IQR) | 5.2 (4.0; 6.7) | 4.9 (2.5; 5.2) | ||
| Variation in height: median (IQR) | 1.7 (0.7; 5.2) | 2.6 (0.9; 3.3) | −0.9 (from −2.4 to 3.8) | 0.65 |
| Test Group (n = 14) | Control Group (n = 11) | Median Difference (95% Bootstrap Confidence Interval) | p Value | |
|---|---|---|---|---|
| Width at day 1: median (IQR) | 2.5 (1.9; 6.6) | 2.1 (1.6; 5.4) | ||
| Width at day 6: median (IQR) | 2.7 (1.3; 5.6) | 2.0 (1.1; 3.0) | ||
| Variation in width: median (IQR) | 0.4 (0.1; 1.0) | 0.9 (0.5; 1.8) | −0.5 (from −1.8 to 0.5) | 0.38 |
| Attached Gingiva (n = 32) | Loose Mucosa (n = 35) | Median Difference (95% Bootstrap Confidence Interval) | p Value | |
|---|---|---|---|---|
| Height at day 1: median (IQR) | 6.2 (5.1; 6.8) | 6.9 (5.6; 8.0) | ||
| Height at day 6: median (IQR) | 4.1 (2.4; 5.2) | 3.1 (2.3; 5.2) | ||
| Variation in height: median (IQR) | 1.4 (0.5; 3.6) | 3.2 (.9; 5.1) | −1.8 (from −3.4 to −0.2) | 0.008 |
| Attached Gingiva (n = 32) | Loose Mucosa (n = 35) | Median Difference (95% Bootstrap Confidence Interval) | p Value | |
|---|---|---|---|---|
| Width at day 1: median (IQR) | 5.5 (2.8; 7.1) | 1.5 (1.1; 2.2) | ||
| Width at day 6: median (IQR) | 2.6 (1.3; 4.8) | 1.3 (1.1; 1.8) | ||
| Variation in width: median (IQR) | 1.3 (0.6; 3.2) | 0.1 (−0.5; 0.7) | 1.1 (from 0.6 to 2.7) | <0.0001 |
| (a) | ||||
| Test Group (n = 40) | Control Group (n = 37) | Risk Ratio (95% Bootstrap Confidence Interval) | p Value | |
| Adverse effects, n (%) | 0 (0%) | 3 (8%) | 0.15 (from 0.01 to 2.94) | 0.11 |
| (b) | ||||
| Test Group (n = 35) | Control Group (n = 35) | Risk Ratio (95% Bootstrap Confidence Interval) | p Value | |
| Adverse effects, n (%) | 0 (0%) | 3 (9%) | 0.16 (from 0.01 to 3.16) | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldin, A.; Nucibella, C.; Manera, C.; Bacci, C. Efficacy of Tocopherol vs. Chlorhexidine in the Management of Oral Biopsy Site: A Randomized Clinical Trial. J. Clin. Med. 2025, 14, 788. https://doi.org/10.3390/jcm14030788
Baldin A, Nucibella C, Manera C, Bacci C. Efficacy of Tocopherol vs. Chlorhexidine in the Management of Oral Biopsy Site: A Randomized Clinical Trial. Journal of Clinical Medicine. 2025; 14(3):788. https://doi.org/10.3390/jcm14030788
Chicago/Turabian StyleBaldin, Arianna, Clara Nucibella, Claudia Manera, and Christian Bacci. 2025. "Efficacy of Tocopherol vs. Chlorhexidine in the Management of Oral Biopsy Site: A Randomized Clinical Trial" Journal of Clinical Medicine 14, no. 3: 788. https://doi.org/10.3390/jcm14030788
APA StyleBaldin, A., Nucibella, C., Manera, C., & Bacci, C. (2025). Efficacy of Tocopherol vs. Chlorhexidine in the Management of Oral Biopsy Site: A Randomized Clinical Trial. Journal of Clinical Medicine, 14(3), 788. https://doi.org/10.3390/jcm14030788






