Efficacy of a Novel Dual-Layer Plastic Stents for Malignant Biliary Obstruction
Abstract
1. Introduction
2. Methods Study Design and Patients
2.1. Inside Stents (ISs)
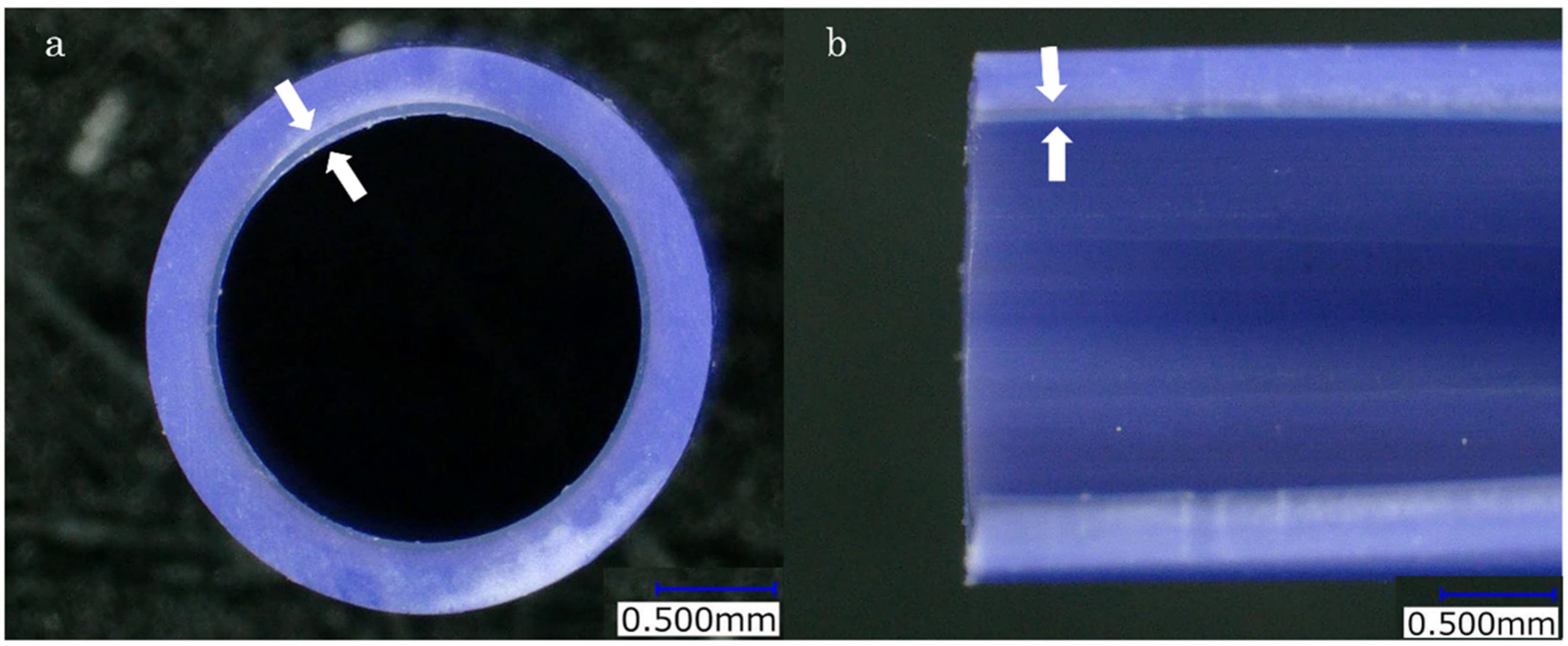
2.2. The Novel Dual-Layer Plastic Stent
2.3. Procedure
2.4. Statistical Analyses
Ethical Aspects and Consent to Participate
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wiggers, J.K.; Coelen, R.J.; Rauws, E.A.; van Delden, O.M.; van Eijck, C.H.; de Jonge, J.; Porte, R.J.; Buis, C.I.; Dejong, C.H.; Molenaar, I.Q.; et al. Preoperative endoscopic versus percutaneous transhepatic biliary drainage in potentially resectable perihilar cholangiocarcinoma (DRAINAGE trial): Design and rationale of a randomized controlled trial. BMC Gastroenterol. 2015, 15, 20. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kawakami, H.; Kuwatani, M.; Onodera, M.; Haba, S.; Eto, K.; Ehira, N.; Yamato, H.; Kudo, T.; Tanaka, E.; Hirano, S.; et al. Endoscopic nasobiliary drainage is the most suitable preoperative biliary drainage method in the management of patients with hilar cholangiocarcinoma. J. Gastroenterol. 2011, 46, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Yamamoto, R.; Matsuyama, M.; Sakai, Y.; Takayama, Y.; Ushio, J.; Ito, Y.; Kitamura, K.; Ryozawa, S.; Imamura, T.; et al. Multicenter study of endoscopic preoperative biliary drainage for malignant hilar biliary obstruction: E-POD hilar study. J. Gastroenterol. Hepatol. 2018, 33, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Nagino, M.; Hirano, S.; Yoshitomi, H.; Aoki, T.; Uesaka, K.; Unno, M.; Ebata, T.; Konishi, M.; Sano, K.; Shimada, K.; et al. Clinical practice guidelines for the management of biliary tract cancers 2019: The 3rd English edition. J. Hepato-Biliary-Pancreat. Sci. 2021, 28, 26–54. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Stevens, T.; Parsi, M.A.; Bhatt, A.; Kichler, A.; Vargo, J.J. Superiority of Self-Expandable Metallic Stents Over Plastic Stents in Treatment of Malignant Distal Biliary Strictures. Clin. Gastroenterol. Hepatol. 2022, 20, e182–e195. [Google Scholar] [CrossRef] [PubMed]
- Kwon, C.I.; Lehman, G.A. Mechanisms of Biliary Plastic Stent Occlusion and Efforts at Prevention. Clin. Endosc. 2016, 49, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Kanno, Y.; Ito, K.; Nakahara, K.; Kawaguchi, S.; Masaki, Y.; Okuzono, T.; Kato, H.; Kuwatani, M.; Ishii, S.; Murabayashi, T.; et al. Suprapapillary placement of plastic versus metal stents for malignant biliary hilar obstructions: A multicenter, randomized trial. Gastrointest. Endosc. 2023, 98, 211–221.e3. [Google Scholar] [CrossRef] [PubMed]
- Okuno, M.; Iwata, K.; Iwashita, T.; Mukai, T.; Shimojo, K.; Ohashi, Y.; Iwasa, Y.; Senju, A.; Iwata, S.; Tezuka, R.; et al. Evaluating optimal bilateral biliary stenting in endoscopic reintervention after initial plastic stent dysfunction for unresectable malignant hilar biliary obstruction: Retrospective cross-sectional study. Dig. Endosc. 2024, 36, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Deng, J.; Hu, Y.; Hong, J. Meta-analysis on clinical outcomes of suprapapillary versus transpapillary stent insertion in malignant biliary obstruction. Surg. Endosc. 2023, 37, 8178–8195. [Google Scholar] [CrossRef] [PubMed]
- Ishiwatari, H.; Hayashi, T.; Ono, M.; Sato, T.; Kato, J. Newly designed plastic stent for endoscopic placement above the sphincter of Oddi in patients with malignant hilar biliary obstruction. Dig. Endosc. 2013, 25 (Suppl. S2), 94–99. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ’EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- De Souza, G.M.V.; Ribeiro, I.B.; Funari, M.P.; de Moura, D.T.H.; Scatimburgo, M.V.C.V.; de Freitas Júnior, J.R.; Sánchez-Luna, S.A.; Baracat, R.; de Moura, E.T.H.; Bernardo, W.M.; et al. Endoscopic retrograde cholangiopancreatography drainage for palliation of malignant hilar biliary obstruction—Stent-in-stent or side-by-side? A systematic review and meta-analysis. World J. Hepatol. 2021, 13, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Ruth He, A.; Qin, S.; Chen, L.T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Ah Lee, M.; Kitano, M.; et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evid. 2022, 1, EVIDoa2200015. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.K.; Ueno, M.; Yoo, C.; Finn, R.S.; Furuse, J.; Ren, Z.; Yau, T.; Klümpen, H.J.; Chan, S.L.; Ozaka, M.; et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023, 401, 1853–1865. [Google Scholar] [CrossRef] [PubMed]
- Kurita, A.; Uza, N.; Asada, M.; Yoshimura, K.; Takemura, T.; Yazumi, S.; Kodama, Y.; Seno, H. Stent placement above the sphincter of Oddi is a useful option for patients with inoperable malignant hilar biliary obstruction. Surg. Endosc. 2022, 36, 2869–2878. [Google Scholar] [CrossRef] [PubMed]
- Kogure, H.; Kato, H.; Kawakubo, K.; Ishiwatari, H.; Katanuma, A.; Okabe, Y.; Ueki, T.; Ban, T.; Hanada, K.; Sugimori, K.; et al. A Prospective Multicenter Study of “Inside Stents” for Biliary Stricture: Multicenter Evolving Inside Stent Registry (MEISteR). J. Clin. Med. 2021, 10, 2936. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Ishii, Y.; Serikawa, M.; Tsuboi, T.; Kawamura, R.; Tsushima, K.; Hirano, T.; Mori, T.; Uemura, K.; Chayama, K. Utility of the inside stent as a preoperative biliary drainage method for patients with malignant perihilar biliary stricture. J. Hepatobiliary Pancreat. Sci. 2021, 28, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Ishiwatari, H.; Kawabata, T.; Kawashima, H.; Nakai, Y.; Miura, S.; Kato, H.; Shiomi, H.; Fujimori, N.; Ogura, T.; Inatomi, O.; et al. Clinical Outcomes of Inside Stents and Conventional Plastic Stents as Bridge-to-Surgery Options for Malignant Hilar Biliary Obstruction. Dig. Dis. Sci. 2023, 68, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Coene, P.P.; Groen, A.K.; Cheng, J.; Out, M.M.; Tytgat, G.N.; Huibregtse, K. Clogging of biliary endoprostheses: A new perspective. Gut 1990, 31, 913–917. [Google Scholar] [CrossRef] [PubMed]


| Group R (n = 48) | Group IS (n = 30) | p Value | |
|---|---|---|---|
| Gender | |||
| Male Female | 28 | 23 | 0.145 |
| 20 | 7 | ||
| Age, median (range) | 73 (44–91) | 74.5 (58–86) | 0.406 |
| Etiology | |||
| Biliary tract cancer | 43 | 22 | 0.0338 |
| Liver metastasis | 2 | 7 | |
| HCC | 1 | 1 | |
| Metastasis of lymph node | 1 | 0 | |
| Disseminated nodule | 1 | 0 |
| Group R (n = 48) | Group IS (n = 30) | p Value | |
|---|---|---|---|
| Previous endoscopic sphincterotomy | 48 | 30 | N.S. |
| Diameter and size of the placed stent | |||
| 7 Fr. 7 cm | 6 | 0 | N.S. |
| 7 Fr. 9 cm | 0 | 7 | |
| 7 Fr. 10 cm | 25 | 0 | |
| 7 Fr. 12 cm | 37 | 39 | |
| 7 Fr. 15 cm | 11 | 0 | |
| 8.5 Fr. 7 cm | 2 | 0 | |
| 8.5 Fr. 10 cm | 4 | 0 | |
| 8.5 Fr. 12 cm | 7 | 0 | |
| 8.5 Fr. 15 cm | 7 | 0 | |
| Number of the stent | |||
| 1 | 8 | 16 | 0.00208 |
| 2 | 29 | 12 | |
| 3 | 11 | 2 | |
| Acute cholangitis rate | 56.7% (17/30) | 50% (24/48) | 0.644 |
| TIME to RBO, medium (range) | 74 (62–113) | 118 (54–322) | 0.219 |
| Early adverse events | 1 | 4 | N.S. |
| High-amylase | 1 | 3 | |
| Cholangitis | 0 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekine, M.; Ijima, M.; Noguchi, S.; Kurihara, E.; Kobatake, T.; Mizutani, T.; Hashimoto, R.; Aoyama, K.; Sasaki, G.; Sato, A.; et al. Efficacy of a Novel Dual-Layer Plastic Stents for Malignant Biliary Obstruction. J. Clin. Med. 2025, 14, 764. https://doi.org/10.3390/jcm14030764
Sekine M, Ijima M, Noguchi S, Kurihara E, Kobatake T, Mizutani T, Hashimoto R, Aoyama K, Sasaki G, Sato A, et al. Efficacy of a Novel Dual-Layer Plastic Stents for Malignant Biliary Obstruction. Journal of Clinical Medicine. 2025; 14(3):764. https://doi.org/10.3390/jcm14030764
Chicago/Turabian StyleSekine, Masanari, Masashi Ijima, Satoaki Noguchi, Eishin Kurihara, Tsutomu Kobatake, Taku Mizutani, Ryo Hashimoto, Kayoko Aoyama, Goya Sasaki, Azumi Sato, and et al. 2025. "Efficacy of a Novel Dual-Layer Plastic Stents for Malignant Biliary Obstruction" Journal of Clinical Medicine 14, no. 3: 764. https://doi.org/10.3390/jcm14030764
APA StyleSekine, M., Ijima, M., Noguchi, S., Kurihara, E., Kobatake, T., Mizutani, T., Hashimoto, R., Aoyama, K., Sasaki, G., Sato, A., Kojima, S., & Mashima, H. (2025). Efficacy of a Novel Dual-Layer Plastic Stents for Malignant Biliary Obstruction. Journal of Clinical Medicine, 14(3), 764. https://doi.org/10.3390/jcm14030764






