Predicting Early Atrial Fibrillation Recurrence Post-Electrical Cardioversion: A Critical Look at Bilateral Atrial Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Transthoracic Echocardiography
2.3. Vascular Stiffness
2.4. Statistical Analysis
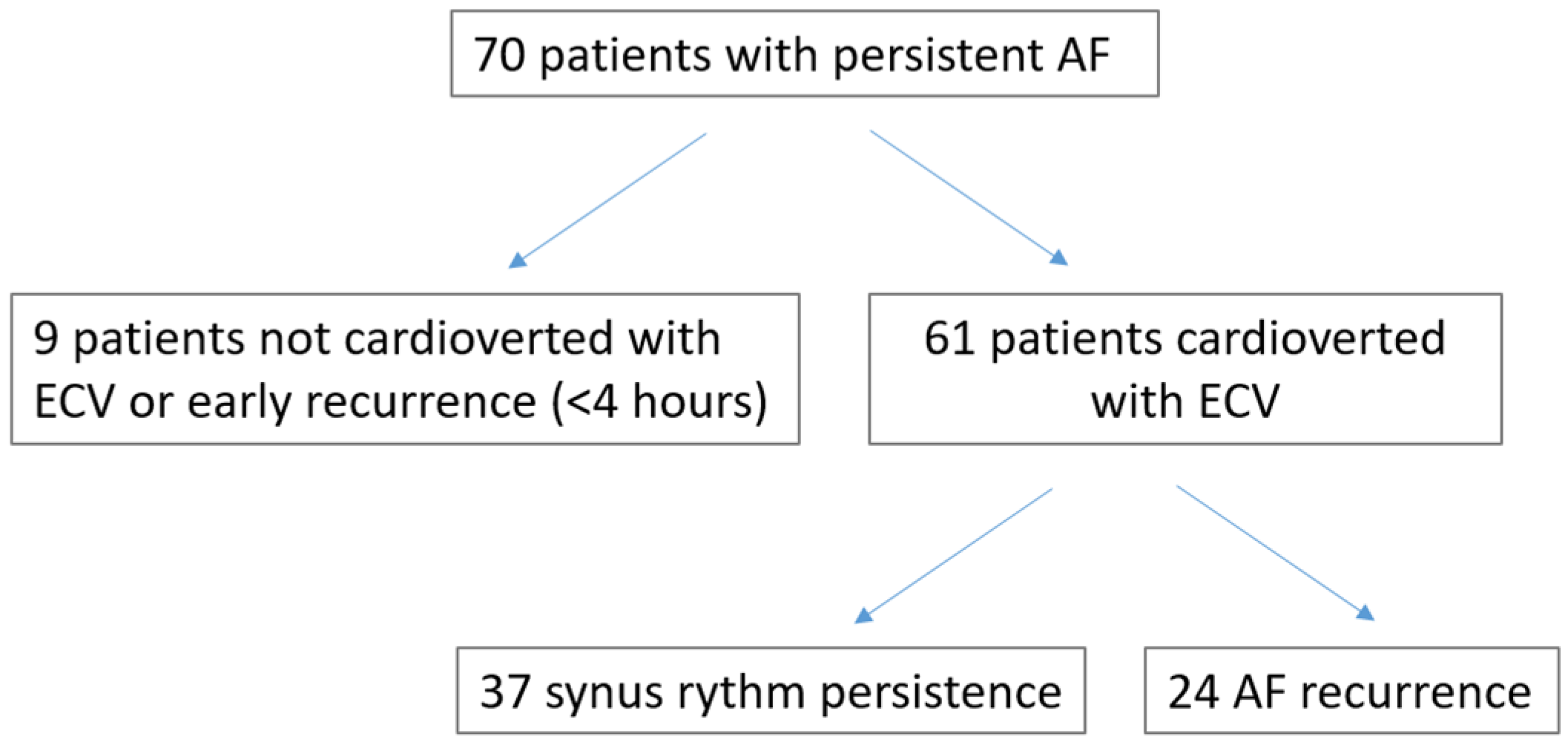
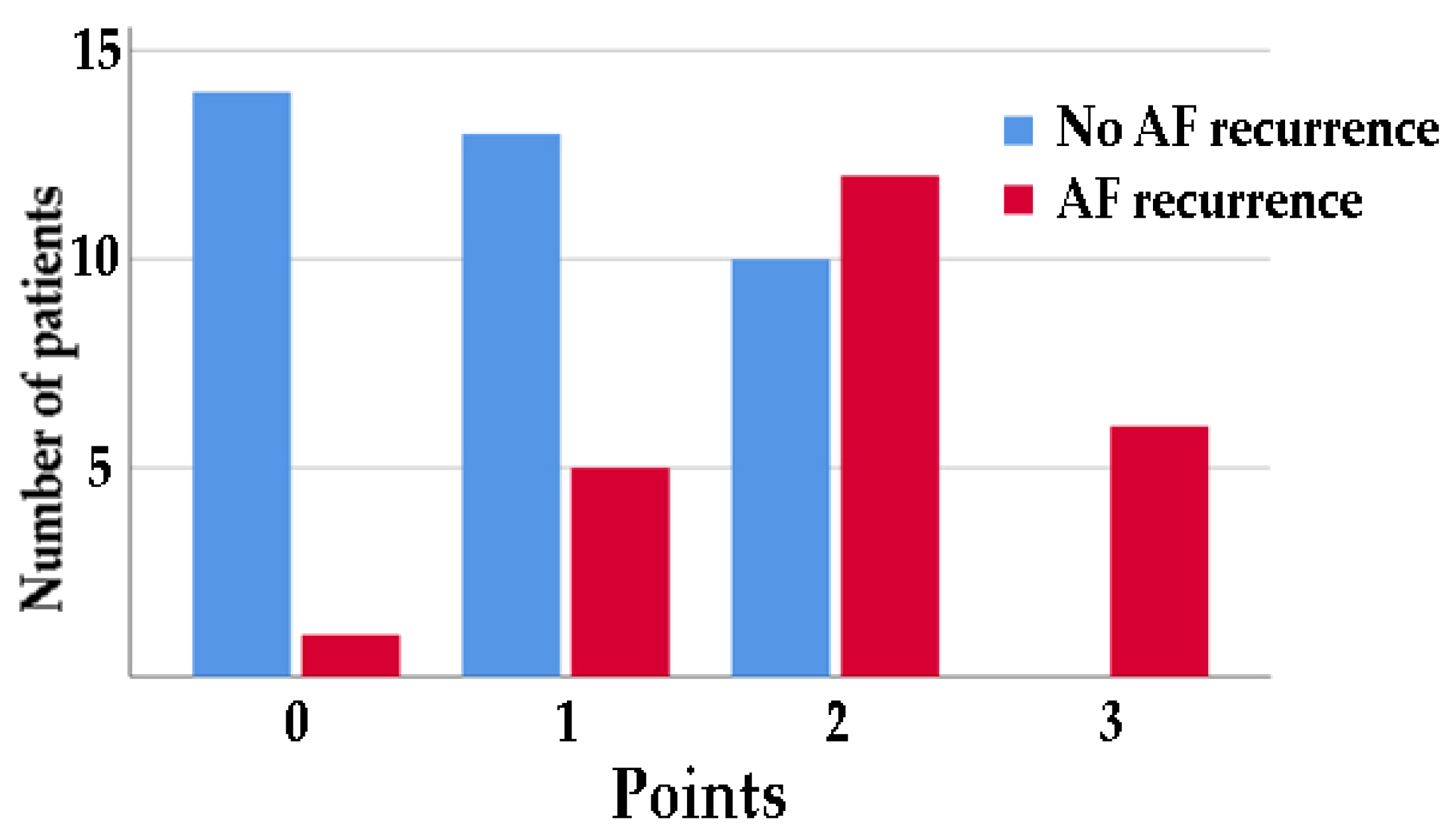
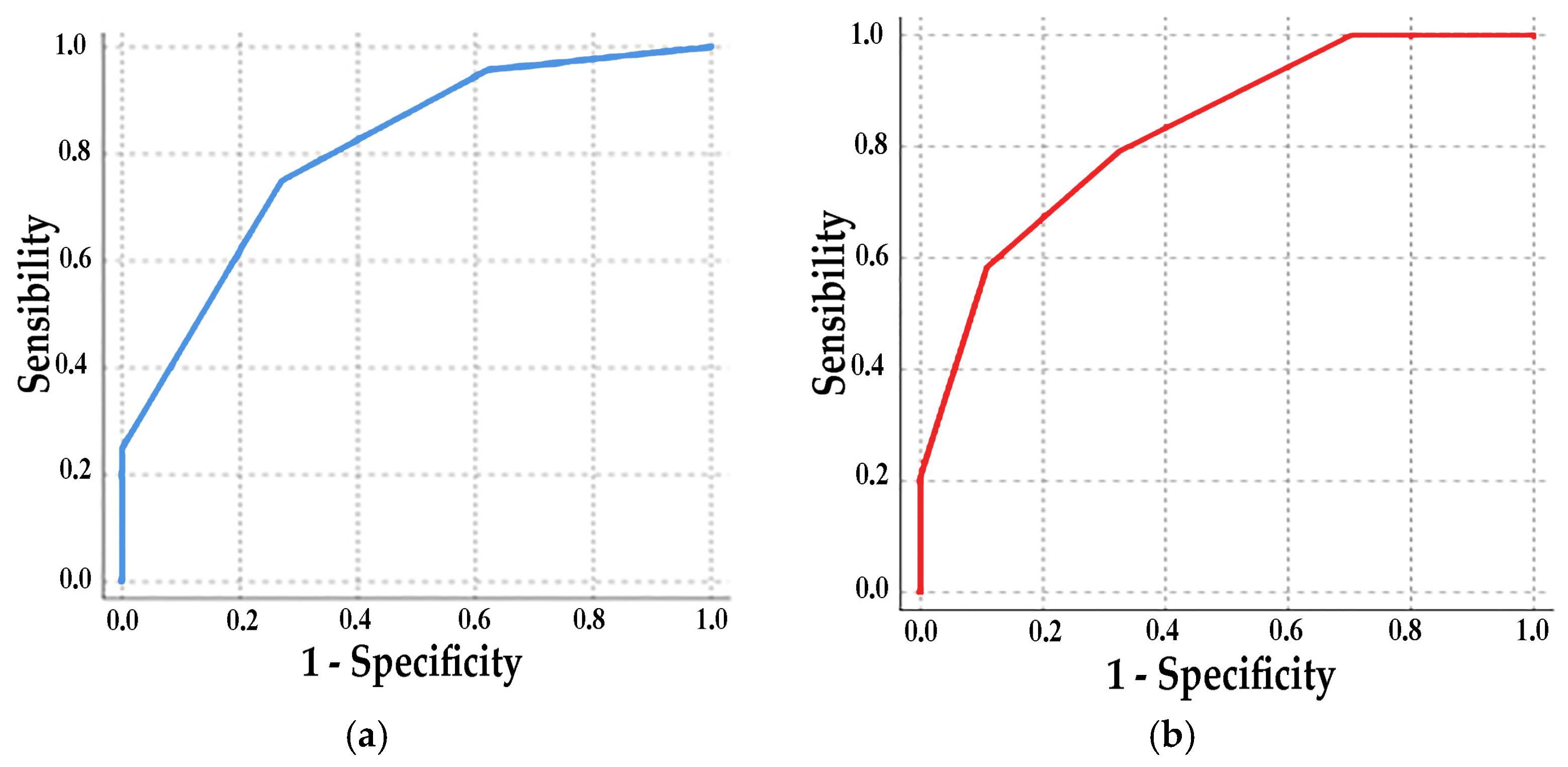
3. Results
4. Discussion
4.1. AF Progression and Atrial Remodeling
4.2. Clinical Implication
5. Conclusions
Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vizzardi, E.; Curnis, A.; Latini, M.G.; Salghetti, F.; Rocco, E.; Lupi, L.; Rovetta, R.; Quinzani, F.; Bonadei, I.; Bontempi, L.; et al. Risk factors for atrial fibrillation recurrence: A literature review. J. Cardiovasc. Med. 2014, 15, 235–253. [Google Scholar] [CrossRef] [PubMed]
- Allessie, M.; Ausma, J.; Schotten, U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc. Res. 2002, 54, 230–246. [Google Scholar] [CrossRef] [PubMed]
- Roberts-Thomson, K.C.; Stevenson, I.; Kistler, P.M.; Haqqani, H.M.; Spence, S.J.; Goldblatt, J.C.; Sanders, P.; Kalman, J.M. The role of chronic atrial stretch and atrial fibrillation on posterior left atrial wall conduction. Hear. Rhythm 2009, 6, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, F. Mechano-electric feedback and atrial fibrillation. Prog. Biophys. Mol. Biol. 2003, 82, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Teh, A.W.; Medi, C.; Kistler, P.M.; Morton, J.B.; Kalman, J.M. Atrial remodeling in varying clinical substrates within beating human hearts: Relevance to atrial fibrillation. Prog. Biophys. Mol. Biol. 2012, 110, 278–294. [Google Scholar] [CrossRef]
- Everett IV, T.H.; Olgin, J.E. Atrial fibrosis and the mechanisms of atrial fibrillation. Hear. Rhythm 2007, 4, S24–S27. [Google Scholar] [CrossRef]
- Quah, J.X.; Dharmaprani, D.; Tiver, K.; Lahiri, A.; Hecker, T.; Perry, R.; Selvanayagam, J.B.; Joseph, M.X.; McGavigan, A.; Ganesan, A. Atrial fibrosis and substrate based characterization in atrial fibrillation: Time to move forwards. J. Cardiovasc. Electrophysiol. 2021, 32, 1147–1160. [Google Scholar] [CrossRef]
- Hori, Y.; Nakahara, S.; Fukuda, R.; Sato, H.; Ukaji, T.; Koshikawa, Y.; Nishiyama, N.; Ishikawa, T.; Kobayashi, S.; Taguchi, I. Atrial reverse remodeling represented by the atrial conduction time in persistent atrial fibrillation patients after catheter ablation: Its impact on predicting late atrial fibrillation recurrence. J. Cardiol. 2020, 75, 521–528. [Google Scholar] [CrossRef]
- Wałek, P.; Ciesla, E.; Gorczyca, I.; Wożakowska-Kapłon, B. Left atrial wall dyskinesia assessed during contractile phase as a predictor of atrial fibrillation recurrence after electrical cardioversion performed due to persistent atrial fibrillation. Medicine 2020, 99, e23333. [Google Scholar] [CrossRef]
- Moreno-Ruiz, L.A.; Madrid-Miller, A.; Martínez-Flores, J.E.; González-Hermosillo, J.A.; Arenas-Fonseca, J.; Zamorano-Velázquez, N.; Mendoza-Pérez, B. Left atrial longitudinal strain by speckle tracking as independent predictor of recurrence after electrical cardioversion in persistent and long standing persistent non-valvular atrial fibrillation. Int. J. Cardiovasc. Imaging 2019, 35, 1587–1596. [Google Scholar] [CrossRef]
- Den Uijl, D.W.; Gawrysiak, M.; Tops, L.F.; Trines, S.A.; Zeppenfeld, K.; Schalij, M.J.; Bax, J.J.; Delgado, V. Prognostic value of total atrial conduction time estimated with tissue Doppler imaging to predict the recurrence of atrial fibrillation after radiofrequency catheter ablation. Europace 2011, 13, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Maenosono, R.; Mizukami, N.; Ichiki, H.; Oketani, N.; Namino, F.; Masamoto, I.; Yuasa, T.; Yamakuchi, M.; Ohishi, M.; Hashiguchi, T. Total atrial conduction time as a possible predictor of atrial fibrillation recurrence after catheter ablation for paroxysmal atrial fibrillation: Relationship between electrical atrial remodeling and structural atrial remodeling time courses. J. Med. Ultrason. 2021, 48, 295–306. [Google Scholar] [CrossRef]
- Fuenmayor, A.A.J.; Moreno, G.; Landaeta, A.; Fuenmayor, P.A.M. Inter-atrial conduction time shortens after blood pressure control in hypertensive patients with left ventricular hypertrophy. Int. J. Cardiol. 2005, 102, 443–446. [Google Scholar] [CrossRef]
- Demirkan, B.; Güray, Y.; İpek, E.G. The atrial conduction time in patients with normal atrial size. Anatol. J. Cardiol. 2016, 16, 221–222. [Google Scholar] [CrossRef]
- de Groot, J.R.; Linz, D. Arterial stiffness and atrial fibrillation recurrence: Another risk marker or a call for better management of concomitant disease? Neth. Hear. J. 2022, 30, 187–189. [Google Scholar] [CrossRef]
- Charitakis, E.; Dragioti, E.; Stratinaki, M.; Korela, D.; Tzeis, S.; Almroth, H.; Liuba, I.; Jönsson, A.H.; Charalambous, G.; Karlsson, L.O.; et al. Predictors of recurrence after catheter ablation and electrical cardioversion of atrial fibrillation: An umbrella review of meta-analyses. EP Eur. 2023, 25, 40–48. [Google Scholar] [CrossRef]
- Dereli, S.; Bayramoğlu, A.; Yontar, O.C.; Cerşit, S.; Gürsoy, M.O. Epicardial fat thickness: A new predictor of successful electrical cardioversion and atrial fibrillation recurrence. Echocardiography 2018, 35, 1926–1931. [Google Scholar] [CrossRef]
- Xu, X.; Tang, Y. Relationship between brain natriuretic peptide and recurrence of atrial fibrillation after successful electrical cardioversion: An updated meta-analysis. Braz. J. Cardiovasc. Surg. 2017, 32, 530–535. [Google Scholar] [CrossRef]
- Dentali, F.; Gianni, M.; Squizzato, A.; Ageno, W.; Castiglioni, L.; Maroni, L.; Hylek, E.M.; Grandi, A.M.; Cazzani, E.; Venco, A.; et al. Use of statins and recurrence of atrial fibrillation after catheter ablation or electrical cardioversion. A systematic review and meta-analysis. Thromb. Haemost. 2011, 106, 363–370. [Google Scholar] [CrossRef]
- Galderisi, M.; Cosyns, B.; Edvardsen, T.; Cardim, N.; Delgado, V.; Di Salvo, G.; Donal, E.; Sade, L.E.; Ernande, L.; Garbi, M.; et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: An expert consensus document of the European Association of Cardiovascular Ima. Eur. Hear. J. Cardiovasc. Imaging 2017, 18, 1301–1310. [Google Scholar] [CrossRef]
- Otterstad, J.E. Measuring left ventricular volume and ejection fraction with the biplane Simpson’s method. Heart 2002, 88, 559–560. [Google Scholar] [CrossRef] [PubMed]
- Potter, E.; Marwick, T.H. Assessment of Left Ventricular Function by Echocardiography: The case for routinely adding global longitudinal strain to ejection fraction. JACC. Cardiovasc. Imaging 2018, 11, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Deniz, A.; Sahiner, L.; Aytemir, K.; Kaya, B.; Kabakci, G.; Tokgozoglu, L.; Oto, A. Tissue Doppler echocardiography can be a useful technique to evaluate atrial conduction time. Cardiol. J. 2012, 19, 487–493. [Google Scholar] [CrossRef]
- Yamaura, G.; Watanabe, T.; Tamura, H.; Tsuchiya, H.; Hashimoto, N.; Wanezaki, M.; Nishiyama, S.; Arimoto, T.; Takahashi, H.; Yamauchi, S.; et al. Prolonged total atrial conduction time evaluated with tissue Doppler imaging predicts poor cardiac prognosis in patients with heart failure. Heart Vessels 2019, 34, 1769–1776. [Google Scholar] [CrossRef]
- Van Bortel, L.M.; Laurent, S.; Boutouyrie, P.; Chowienczyk, P.; Cruickshank, J.K.; De Backer, T.; Filipovsky, J.; Huybrechts, S.; Mattace-Raso, F.U.S.; Protogerou, A.D.; et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J. Hypertens. 2012, 30, 445–448. [Google Scholar] [CrossRef]
- Grillo, A.; Salvi, P.; Furlanis, G.; Baldi, C.; Rovina, M.; Salvi, L.; Faini, A.; Bilo, G.; Fabris, B.; Carretta, R.; et al. Mean arterial pressure estimated by brachial pulse wave analysis and comparison with currently used algorithms. J. Hypertens. 2020, 38, 2161–2168. [Google Scholar] [CrossRef]
- Fornengo, C.; Antolini, M.; Frea, S.; Gallo, C.; Grosso Marra, W.; Morello, M.; Gaita, F. Prediction of atrial fibrillation recurrence after cardioversion in patients with left-atrial dilation. Eur. Hear. J. Cardiovasc. Imaging 2015, 16, 335–341. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Hu, C.; Liu, Y.; Cheng, Y.; Chen, H.; Shu, X. Left atrial strain superior to structural remodeling in identifying occult atrial fibrillation. J. Clin. Ultrasound 2023, 51, 1301–1307. [Google Scholar] [CrossRef]
- Luong, C.; Thompson, D.J.S.; Bennett, M.; Gin, K.; Jue, J.; Barnes, M.E.; Colley, P.; Tsang, T.S.M. Right atrial volume is superior to left atrial volume for prediction of atrial fibrillation recurrence after direct current cardioversion. Can. J. Cardiol. 2015, 31, 29–35. [Google Scholar] [CrossRef]
- Anastasio, F.; Testa, M.; Ferreri, C.; Rossi, A.; Ruocco, G.; Feola, M. The Analysis of Arterial Stiffness in Heart Failure Patients: The Prognostic Role of Pulse Wave Velocity, Augmentation Index and Stiffness Index. J. Clin. Med. 2022, 11, 3507. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Fragakis, N.; Patoulias, D.; Theofilis, P.; Kassimis, G.; Karamitsos, T.; El-Tanani, M.; Rizzo, M. Effects of glucagon-like peptide 1 receptor agonists on atrial fibrillation recurrence after catheter ablation: A systematic review and meta-analysis. Adv. Ther. 2024, 41, 3749–3756. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 61) | No AF Recurrence (n = 37) | AF Recurrence (n = 24) | p | |
|---|---|---|---|---|
| Sex (male) | 51 (83.6%) | 31 (83.8%) | 20 (83.3%) | 0.67 |
| Age (years) | 66.4 ± 9.1 | 66.2 ± 10.2 | 66.7 ± 7.5 | 0.97 |
| BMI (kg/m2) | 27.0 ± 5.4 | 27.1 ± 5.6 | 26.9 ± 5.1 | 0.88 |
| Charlson Comorbidity Index | 3 [2–4] | 3 [2–5] | 3 [2–3] | 0.88 |
| Hypertension | 49 (80.3%) | 30 (81.1%) | 19 (79.2%) | 0.61 |
| Coronary artery disease | 6 (9.8%) | 3 (8.1%) | 3 (12.5%) | 0.54 |
| Heart failure | 11 (18.0%) | 7 (18.9%) | 4 (16.7%) | 0.61 |
| History of cardioversion | 21 (34.4%) | 13 (35.1%) | 8 (33.3%) | 0.94 |
| Antiarrhythmic drug | 22 (36.1%) | 13 (35.1%) | 9 (37.5%) | 0.75 |
| Class III anti-arrhythmic drug | 18 (29.5%%) | 10 (27.2%) | 8 (33.3%) | 0.34 |
| Class IC anti-arrhythmic drug | 4 (6.5%) | 3 (8.1%) | 1 (4.2%) | 0.46 |
| TIC | 14 (22.9%) | 10 (27.0%) | 4 (16.7%) | 0.48 |
| Hemoglobin (g/dL) | 14.3 ± 1.5 | 14.4 ± 1.4 | 14.1 ± 1.7 | 0.42 |
| Creatinine (mg/dL) | 1.0 ± 0.3 | 1.0 ± 0.2 | 1.0 ± 0.3 | 0.63 |
| Potassium (mEq/L) | 4.0 ± 0.4 | 4.1 ± 0.4 | 3.9 ± 0.3 | 0.13 |
| Pro-BNP (pg/mL) | 1537 [719–3063] | 1118 [587–1976] | 2521 [911–3550] | 0.15 |
| TSH (mU/L) | 1.3 ± 0.6 | 1.6 ± 0.8 | 1.3 ± 0.5 | 0.24 |
| Physical activity | 13 (21.3%) | 13 (35.1%) | 0 (0%) | 0.02 |
| Alcohol consumption | 44 (72.1%) | 26 (70.2%) | 18 (75.0%) | 0.54 |
| School | 1 [1–2] | 1 [1–2] | 1 [1–2] | 0.43 |
| Income | 4 [3–6] | 4 [3–6] | 4 [3–6] | 0.62 |
| AF recurrence at 1 month | 16 (26.2%) | |||
| AF recurrence at 6 months | 24 (39.4%) |
| Total (n = 61) | No AF Recurrence (n = 37) | AF Recurrence (n = 24) | p | |
|---|---|---|---|---|
| LVEDV (mL) | 116 ± 29 | 119 ± 31 | 112 ± 27 | 0.54 |
| SV (mL) | 63 ± 13 | 65 ± 13 | 60 ± 12 | 0.25 |
| LV mass index | 100 ± 29 | 98 ± 26 | 105 ± 32 | 0.63 |
| RWT (mm) | 0.44 ± 0.08 | 0.44 ± 0.07 | 0.44 ± 0.09 | 0.86 |
| GLS (%) | −15.6 ± 3.4 | −16.3 ± 3.7 | −14.6 ± 2.9 | 0.15 |
| PSD (ms) | 56.2 ± 22.2 | 54.7 ± 26.5 | 58.3 ± 15.5 | 0.48 |
| LA volume (mL) | 97 ± 34 | 101 ± 34 | 92 ± 34 | 0.46 |
| LAVi (mL/m2) | 50 ± 19 | 51 ±19 | 48 ± 19 | 0.64 |
| LA strain S-R (%) | 13.2 ± 7.2 | 15.8 ± 7.7 | 9.0 ± 4.2 | 0.01 |
| LA strain S-CT (%) | −7.1 ± 5.1 | −8.6 ± 5.3 | −4.7 ± 3.4 | 0.06 |
| LA strain S-CD (%) | −7.3 ± 5.8 | −9.1 ± 6.5 | −4.5 ± 3.4 | 0.07 |
| RA volume (mL) | 69 ± 19 | 64 ± 18 | 78 ± 18 | 0.06 |
| RAVi (mL/m2) | 35 ± 10 | 32 ± 8 | 40 ± 10 | 0.02 |
| RV diameter (mm) | 41 ± 4 | 41 ± 4 | 42 ± 4 | 0.84 |
| TAPSE (mm) | 20 ± 4 | 21 ± 3 | 20 ± 4 | 0.65 |
| TV gradient (mmHg) | 22 ± 6 | 21 ± 6 | 23 ± 5 | 0.24 |
| Septal e/e′ | 9 ± 5 | 9 ± 6 | 9 ± 3 | 0.64 |
| Lateral e/e′ | 10 ± 5 | 11 ± 6 | 10 ± 4 | 0.16 |
| Tricuspid e/e′ | 8 ± 3 | 8 ± 3 | 9 ± 3 | 0.16 |
| Septal a′ (cm/s) | 4 ± 2 | 4 ± 2 | 3 ± 1 | 0.15 |
| Lateral a′ (cm/s) | 4 ± 3 | 5 ± 3 | 3 ± 1 | 0.008 |
| Tricuspid a′ (cm/s) | 6 ± 3 | 7 ± 3 | 4 ± 2 | 0.009 |
| a’ average (cm/s) | 5 ± 2 | 6 ± 2 | 3 ± 1 | 0.004 |
| P wave (ms) | 97 ± 34 | 97 ± 40 | 96 ± 28 | 0.68 |
| Time p-a (ms) | 156 ± 42 | 161 ± 60 | 149 ± 20 | 0.79 |
| Time p-a′ septal (ms) | 146 ± 56 | 147 ±71 | 144 ± 31 | 0.98 |
| Time p-a′ lateral (ms) | 155 ± 51 | 164 ± 65 | 142 ± 24 | 0.54 |
| Time p-a′ tricuspid (ms) | 131 ± 56 | 138 ± 67 | 120 ± 24 | 0.41 |
| Epicardial Fat Thickness (mm) | 7 ± 3 | 7 ± 3 | 7 ± 3 | 0.88 |
| Presence of SVES | 22 (36.1%) | 12 (32.4%) | 10 (41.7%) | 0.32 |
| P wave dispersion | 42 (68.9%) | 23 (62.2%) | 19 (79.2%) | 0.13 |
| Total (n = 61) | No AF Recurrence (n = 37) | AF Recurrence (n = 24) | p | |
|---|---|---|---|---|
| SBP (mmHg) | 127 ± 17 | 124 ± 15 | 132 ± 20 | 0.24 |
| DBP (mmHg) | 77 ± 12 | 76 ± 10 | 78 ± 14 | 0.75 |
| HR (beat/min) | 63 ± 12 | 62 ± 12 | 65 ± 12 | 0.47 |
| Central SBP (mmHg) | 120 ± 19 | 118 ± 21 | 123 ± 18 | 0.55 |
| Central DBP (mmHg) | 77 ± 12 | 75 ± 11 | 78 ± 14 | 0.59 |
| AIx75 | 30 ± 13 | 26 ± 13 | 37 ± 12 | 0.02 |
| PWV (m/s) | 11 ± 2 | 10 ± 2 | 12 ±2 | 0.13 |
| OR (95% CI) | p | VIF | |
|---|---|---|---|
| LA strain S-R | 0.70 (0.56–0.87) | 0.003 | 1.32 |
| RAVi | 1.07 (1.01–1.15) | 0.03 | 1.08 |
| Lateral a′ | 0.65 (0.45–0.92) | 0.01 | 1.08 |
| Tricuspid a′ | 0.72 (0.55–0.94) | 0.02 | 1.07 |
| A′ average | 0.62 (0.44–0.88) | 0.005 | 1.02 |
| Aix75 | 1.09 (1.02–1.16) | 0.01 | 1.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anastasio, F.; Pastorini, G.; Pucci, G.; Gonella, A.; Tardivo, V.; Feola, M. Predicting Early Atrial Fibrillation Recurrence Post-Electrical Cardioversion: A Critical Look at Bilateral Atrial Function. J. Clin. Med. 2025, 14, 749. https://doi.org/10.3390/jcm14030749
Anastasio F, Pastorini G, Pucci G, Gonella A, Tardivo V, Feola M. Predicting Early Atrial Fibrillation Recurrence Post-Electrical Cardioversion: A Critical Look at Bilateral Atrial Function. Journal of Clinical Medicine. 2025; 14(3):749. https://doi.org/10.3390/jcm14030749
Chicago/Turabian StyleAnastasio, Fabio, Guido Pastorini, Giacomo Pucci, Alessandro Gonella, Valentina Tardivo, and Mauro Feola. 2025. "Predicting Early Atrial Fibrillation Recurrence Post-Electrical Cardioversion: A Critical Look at Bilateral Atrial Function" Journal of Clinical Medicine 14, no. 3: 749. https://doi.org/10.3390/jcm14030749
APA StyleAnastasio, F., Pastorini, G., Pucci, G., Gonella, A., Tardivo, V., & Feola, M. (2025). Predicting Early Atrial Fibrillation Recurrence Post-Electrical Cardioversion: A Critical Look at Bilateral Atrial Function. Journal of Clinical Medicine, 14(3), 749. https://doi.org/10.3390/jcm14030749







