One-Year Compliance After Calcitonin Gene-Related Peptide Monoclonal Antibody Therapy for Migraine Patients in a Real-World Setting: A Multicenter Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection
2.3. Treatment Pattern Analysis
2.4. Statistical Analysis
3. Results
3.1. Patient Demographics
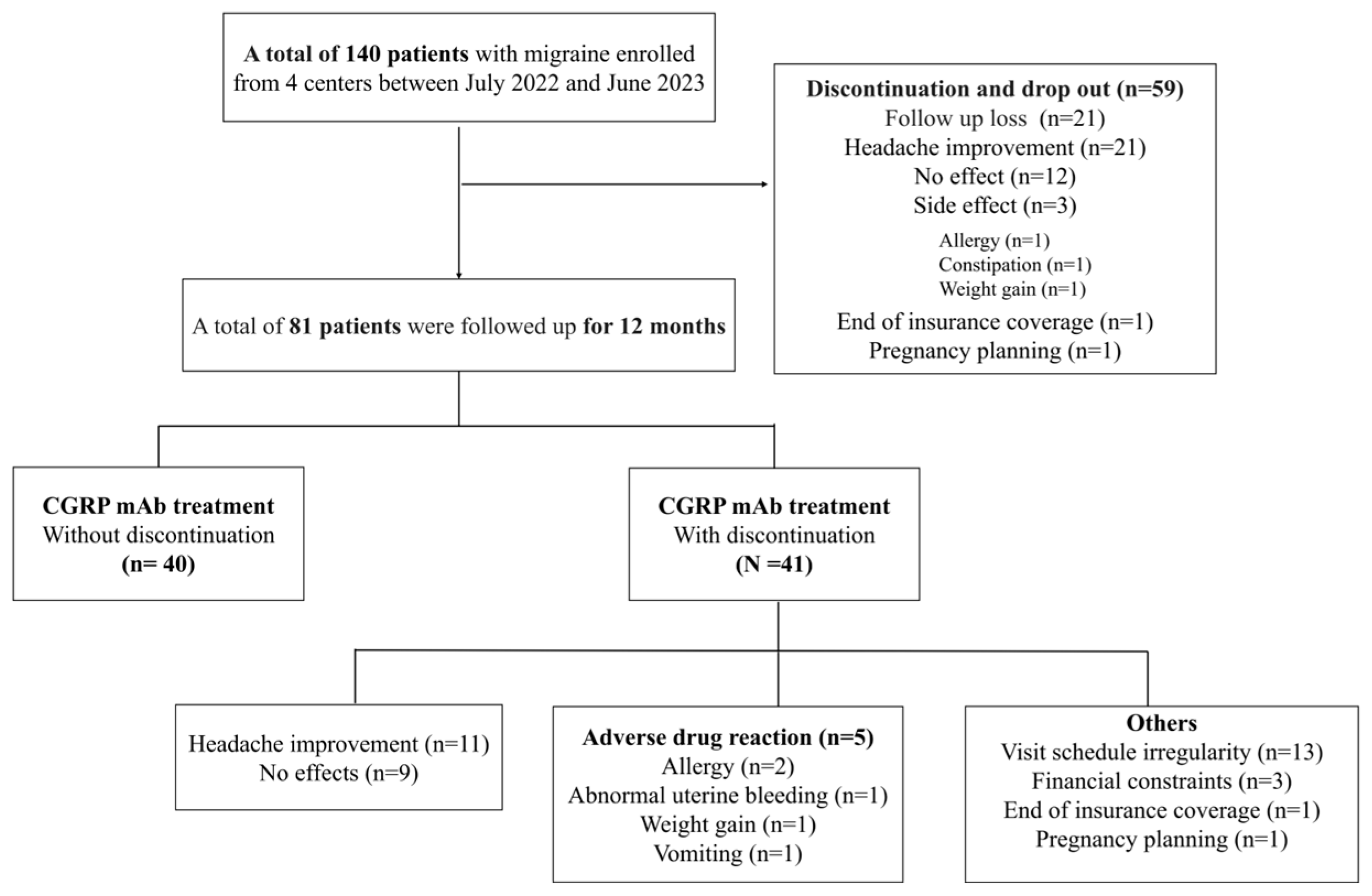
3.2. Discontinuation
3.3. Adherence
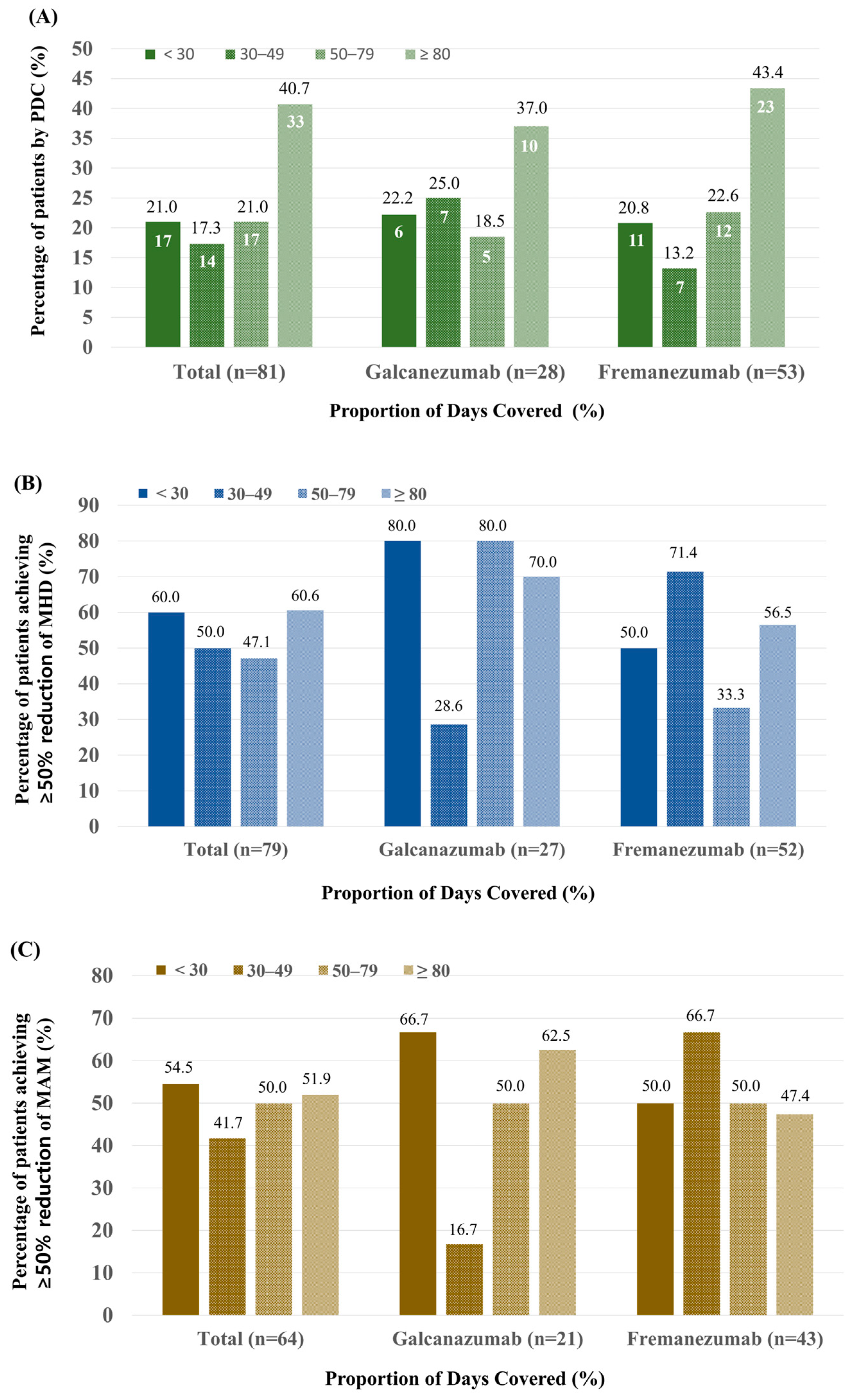
3.4. Comparison of Efficacy According to PDC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steiner, T.J.; Stovner, L.J. Global Epidemiology of Migraine and Its Implications for Public Health and Health Policy. Nat. Rev. Neurol. 2023, 19, 109–117. [Google Scholar] [CrossRef]
- Edvinsson, L.; Haanes, K.A.; Warfvinge, K.; Krause, D.N. CGRP as the Target of New Migraine Therapies—Successful Translation from Bench to Clinic. Nat. Rev. Neurol. 2018, 14, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Ray, J.C.; Kapoor, M.; Stark, R.J.; Wang, S.J.; Bendtsen, L.; Matharu, M.; Hutton, E.J. Calcitonin Gene Related Peptide in Migraine: Current Therapeutics, Future Implications and Potential Off-Target Effects. J. Neurol. Neurosurg. Psychiatry 2021, 92, 1325–1334. [Google Scholar] [CrossRef]
- Park, H.-K.; Kim, B.-K. Calcitonin Gene-Related Peptide Targeting Therapies for Migraine: A New Era for Migraine Treatment. J. Korean Neurol. Assoc. 2020, 38, 88–99. [Google Scholar] [CrossRef]
- Youn, M.S.; Kim, N.; Lee, M.J.; Kim, M. Treatment Outcome after Switching from Galcanezumab to Fremanezumab in Patients with Migraine. J. Clin. Neurol. 2024, 20, 300–305. [Google Scholar] [CrossRef]
- Charles, A.C.; Digre, K.B.; Goadsby, P.J.; Robbins, M.S.; Hershey, A.; American Headache Society; Charles, A.C.; Goadsby, P.J.; Schwedt, T.J.; Robbins, M.S.; et al. Calcitonin Gene-Related Peptide-Targeting Therapies Are a First-Line Option for the Prevention of Migraine: An American Headache Society Position Statement Update. Headache J. Head Face Pain 2024, 64, 333–341. [Google Scholar] [CrossRef]
- Sacco, S.; Amin, F.M.; Ashina, M.; Bendtsen, L.; Deligianni, C.I.; Gil-Gouveia, R.; Katsarava, Z.; MaassenVanDenBrink, A.; Martelletti, P.; Mitsikostas, D.-D. European Headache Federation Guideline on the Use of Monoclonal Antibodies Targeting the Calcitonin Gene Related Peptide Pathway for Migraine Prevention–2022 Update. J. Headache Pain 2022, 23, 67. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Chung, P.W.; Chung, J.M.; Park, K.Y.; Moon, H.S.; Park, H.K.; Bae, D.W.; Seo, J.G.; Sohn, J.H.; Song, T.J.; et al. Evidence-Based Recommendations on Pharmacologic Treatment for Migraine Prevention: A Clinical Practice Guideline from the Korean Headache Society. Headache Pain Res. 2025. [Google Scholar] [CrossRef]
- Iannone, L.F.; Fattori, D.; Benemei, S.; Chiarugi, A.; Geppetti, P.; De Cesaris, F. Long-Term Effectiveness of Three Anti-Cgrp Monoclonal Antibodies in Resistant Chronic Migraine Patients Based on the Midas Score. CNS Drugs 2022, 36, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Olesen, A.; Ashina, M. Cgrp, a Target for Preventive Therapy in Migraine and Cluster Headache: Systematic Review of Clinical Data. Cephalalgia 2019, 39, 374–389. [Google Scholar] [CrossRef]
- Raffaelli, B.; De Icco, R.; Corrado, M.; Terhart, M.; Ailani, J. Open-Label Trials for Cgrp-Targeted Drugs in Migraine Prevention: A Narrative Review. Cephalalgia 2023, 43, 3331024221137091. [Google Scholar] [CrossRef]
- Olesen, J. International Classification of Headache Disorders. Lancet Neurol. 2018, 17, 396–397. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Finnigan, K.; Clarke, S.; Barry, M.; Gorry, C. Utilization, Expenditure, and Treatment Patterns Associated with Calcitonin Gene-Related Peptide Monoclonal Antibodies Reimbursed Subject to a Managed Access Protocol in Ireland. Value Health 2024, 27, 1039–1045. [Google Scholar] [CrossRef]
- Lee, W.K.; Lee, J. Evaluation and Improvement of Adherence to Medication. J. Korean Med. Assoc. Taehan Uisa Hyophoe Chi 2021, 64, 130–136. [Google Scholar] [CrossRef]
- Prieto-Merino, D.; Mulick, A.; Armstrong, C.; Hoult, H.; Fawcett, S.; Eliasson, L.; Clifford, S. Estimating Proportion of Days Covered (Pdc) Using Real-World Online Medicine Suppliers’ Datasets. J. Pharm. Policy Pract. 2021, 14, 113. [Google Scholar] [CrossRef]
- Varnado, O.J.; Manjelievskaia, J.; Ye, W.; Perry, A.; Schuh, K.; Wenzel, R. Treatment Patterns for Calcitonin Gene-Related Peptide Monoclonal Antibodies Including Galcanezumab Versus Conventional Preventive Treatments for Migraine: A Retrospective Us Claims Study. Patient Prefer. Adherence 2022, 16, 821–839. [Google Scholar] [CrossRef]
- Schwedt, T.J.; Lee, J.; Knievel, K.; McVige, J.; Wang, W.; Wu, Z.; Gillard, P.; Shah, D.; Blumenfeld, A.M. Real-World Persistence and Costs among Patients with Chronic Migraine Treated with Onabotulinumtoxina or Calcitonin Gene-Related Peptide Monoclonal Antibodies. J. Manag. Care Spec. Pharm. 2023, 29, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Gladstone, J.; Chhibber, S.; Minhas, J.; Neish, C.S.; Power, G.S.; Lan, Z.; Rochdi, D.; Lanthier-Martel, J.; Bastien, N. Real-World Persistence of Erenumab for Preventive Treatment of Chronic and Episodic Migraine: Retrospective Real-World Study. Headache 2022, 62, 78–88. [Google Scholar] [CrossRef] [PubMed]
- de Dios, A.; Pages-Puigdemont, N.; Ojeda, S.; Riera, P.; Pelegrin, R.; Morollon, N.; Belvis, R.; Real, J.; Masip, M. Persistence, Effectiveness, and Tolerability of Anti-Calcitonin Gene-Related Peptide Monoclonal Antibodies in Patients with Chronic Migraine. Headache 2024, 65, 24–34. [Google Scholar] [CrossRef]
- Varnado, O.; Brady, B.; Zagar, A.J.; Robles, Y.; Hoyt, M.; Hall, J. Adherence and Persistence of Calcitonin Gene-Related Peptide Monoclonal Antibodies in Patients with Migraine: A Real-World Study (P12-12.007). Neurology 2023, 100, 3707. [Google Scholar] [CrossRef]
- Vandervorst, F.; Van Deun, L.; Van Dycke, A.; Paemeleire, K.; Reuter, U.; Schoenen, J.; Versijpt, J. Cgrp Monoclonal Antibodies in Migraine: An Efficacy and Tolerability Comparison with Standard Prophylactic Drugs. J. Headache Pain 2021, 22, 128. [Google Scholar] [CrossRef]
- Mascarella, D.; Matteo, E.; Favoni, V.; Cevoli, S. The Ultimate Guide to the Anti-Cgrp Monoclonal Antibodies Galaxy. Neurol. Sci. 2022, 43, 5673–5685. [Google Scholar] [CrossRef]
- An, Y.C.; Hung, K.S.; Liang, C.S.; Tsai, C.K.; Tsai, C.L.; Chen, S.J.; Lin, Y.K.; Lin, G.Y.; Yeh, P.K.; Yang, F.C. Genetic Variants Associated with Response to Anti-Cgrp Monoclonal Antibody Therapy in a Chronic Migraine Han Chinese Population. J. Headache Pain 2024, 25, 149. [Google Scholar] [CrossRef]
- Kawata, A.K.; Shah, N.; Poon, J.L.; Shaffer, S.; Sapra, S.; Wilcox, T.K.; Shah, S.; Tepper, S.J.; Dodick, D.W.; Lipton, R.B. Understanding the Migraine Treatment Landscape Prior to the Introduction of Calcitonin Gene-Related Peptide Inhibitors: Results from the Assessment of Tolerability and Effectiveness in Migraine Patients Using Preventive Treatment (Attain) Study. Headache 2021, 61, 438–454. [Google Scholar] [CrossRef]
- Pavelic, A.R.; Wober, C.; Riederer, F.; Zebenholzer, K. Monoclonal Antibodies against Calcitonin Gene-Related Peptide for Migraine Prophylaxis: A Systematic Review of Real-World Data. Cells 2022, 12, 143. [Google Scholar] [CrossRef]
- Mavridis, T.; Deligianni, C.I.; Karagiorgis, G.; Daponte, A.; Breza, M.; Mitsikostas, D.D. Monoclonal Antibodies Targeting Cgrp: From Clinical Studies to Real-World Evidence-What Do We Know So Far? Pharmaceuticals 2021, 14, 700. [Google Scholar] [CrossRef]
- Tso, A.R.; Goadsby, P.J. Anti-Cgrp Monoclonal Antibodies: The Next Era of Migraine Prevention? Curr. Treat. Options Neurol. 2017, 19, 27. [Google Scholar] [CrossRef] [PubMed]
| Age, mean (SD) | 44.6 (±12.1) |
| Sex, n (%) | |
| female | 116 (82.9) |
| male | 24 (17.1) |
| BMI, mean (SD) | 22.2 (±3.4) |
| Hypertension, n (%) | 17 (12.1) |
| DM, n (%) | 3 (2.1) |
| Migraine subtype, n (%) | |
| Migraine without aura | 131 (93.6) |
| Migraine with aura | 9 (6.4) |
| Chronic migraine | 91 (65.0) |
| PMM | 7 (5.0) |
| MRM | 12 (8.6) |
| Age of onset, years old, mean (SD) | 26.6 (±12.6) |
| Disease duration before CGRP, year, mean (SD) | 16.2 (±12.0) |
| Anti-CGRP mAbs type, n (%) | |
| Galcanezumab | 58 (41.4) |
| Change to fremanezumab | 8 (5.7) |
| Fremanezumab | 82 (58.6) |
| Change to galcanezumab | 8 (5.7) |
| Anti-CGRP continue for 12 months, n (%) * | 40 (28.6) |
| The number of days of anti-CGRP treatment, median (IQR) | 179.5 (93.0–360.0) |
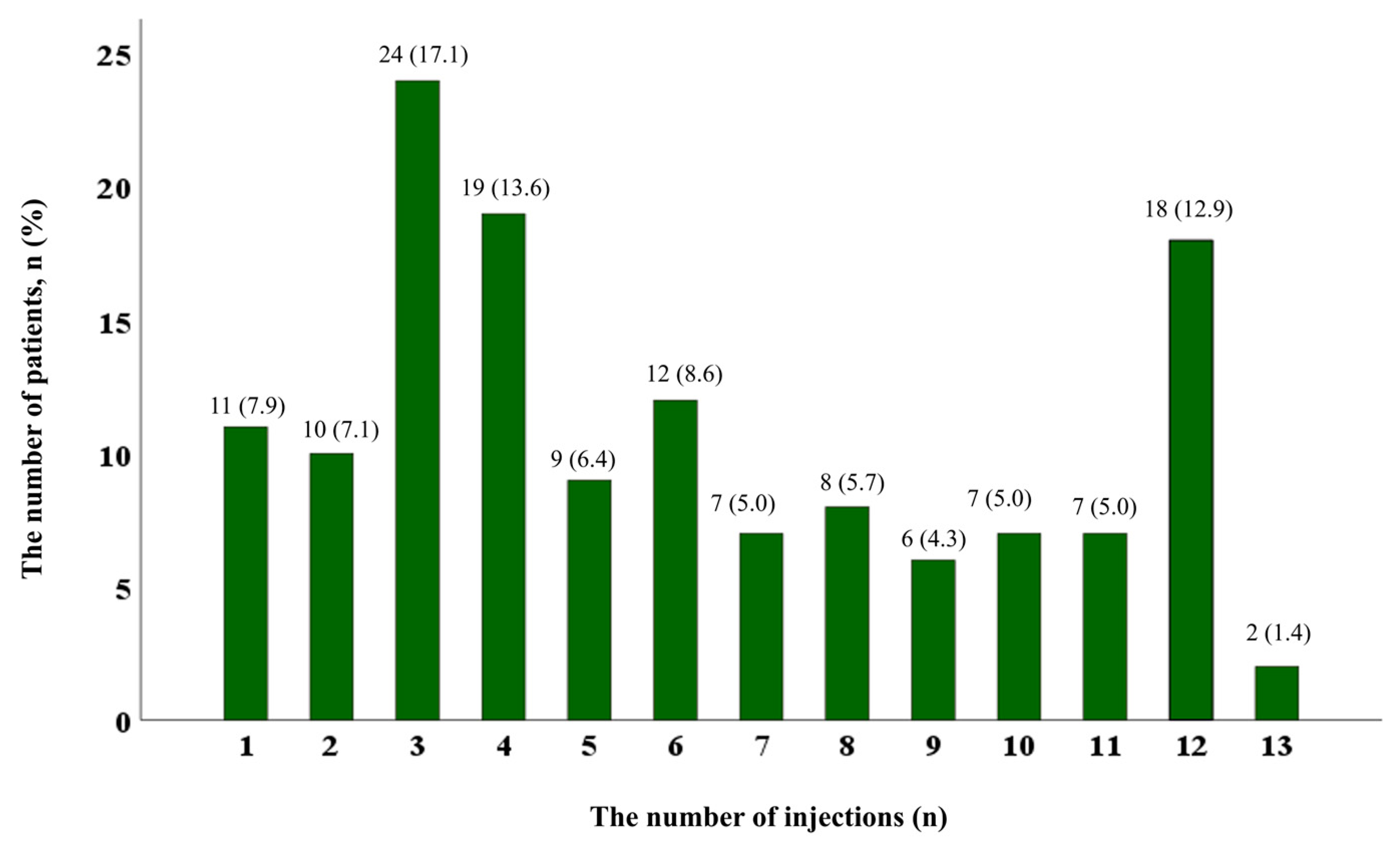
| Number of injections, median (IQR) | 5.0 (3.0–9.0) |
| Adherence, n (%) | |
| <30% | 20 (14.3) |
| 30–49% | 22 (15.7) |
| 50–79% | 26 (18.6) |
| ≥80% | 72 (51.4) |
| Intervals of injections, days, median (IQR) | 32.0 (30.0–36.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, M.-k.; Sohn, J.-H.; Cha, M.-J.; Kim, Y.H.; Hong, Y.; Im, H.-J.; Cho, S.-J. One-Year Compliance After Calcitonin Gene-Related Peptide Monoclonal Antibody Therapy for Migraine Patients in a Real-World Setting: A Multicenter Cross-Sectional Study. J. Clin. Med. 2025, 14, 734. https://doi.org/10.3390/jcm14030734
Kang M-k, Sohn J-H, Cha M-J, Kim YH, Hong Y, Im H-J, Cho S-J. One-Year Compliance After Calcitonin Gene-Related Peptide Monoclonal Antibody Therapy for Migraine Patients in a Real-World Setting: A Multicenter Cross-Sectional Study. Journal of Clinical Medicine. 2025; 14(3):734. https://doi.org/10.3390/jcm14030734
Chicago/Turabian StyleKang, Mi-kyoung, Jong-Hee Sohn, Myoung-Jin Cha, Yoo Hwan Kim, Yooha Hong, Hee-Jin Im, and Soo-Jin Cho. 2025. "One-Year Compliance After Calcitonin Gene-Related Peptide Monoclonal Antibody Therapy for Migraine Patients in a Real-World Setting: A Multicenter Cross-Sectional Study" Journal of Clinical Medicine 14, no. 3: 734. https://doi.org/10.3390/jcm14030734
APA StyleKang, M.-k., Sohn, J.-H., Cha, M.-J., Kim, Y. H., Hong, Y., Im, H.-J., & Cho, S.-J. (2025). One-Year Compliance After Calcitonin Gene-Related Peptide Monoclonal Antibody Therapy for Migraine Patients in a Real-World Setting: A Multicenter Cross-Sectional Study. Journal of Clinical Medicine, 14(3), 734. https://doi.org/10.3390/jcm14030734






