Impact of Myopia on the Utility of the Photopic Negative Response Ratio for Glaucoma Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Inclusion Criteria
2.2. Clinical Evaluation and Diagnosis
2.2.1. Optical Coherence Tomography
2.2.2. Visual Field Testing
2.2.3. Full-Field Photopic ERG
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | Area Under the Curve |
| AL | Axial Length |
| ERGs | Electroretinograms |
| GCIPL | Ganglion Cell Inner Plexiform Layer |
| MD | Mean Deviation |
| OCT | Optical Coherence Tomography |
| PhNR | Photopic Negative Response |
| RGCs | Retinal Ganglion Cells |
| RNFL | Retinal Nerve Fiber Layer |
| SD-OCT | Spectral-Domain Optical Coherence Tomography |
| VF | Visual Field |
References
- Resnikoff, S.; Pascolini, D.; Etya’ale, D.; Kocur, I.; Pararajasegaram, R.; Pokharel, G.P.; Mariotti, S.P. Global data on visual impairment in the year 2002. Bull. World Health Organ. 2004, 82, 844–851. [Google Scholar] [PubMed]
- Tan, N.Y.Q.; Sng, C.C.A.; Jonas, J.B.; Wong, T.Y.; Jansonius, N.M.; Ang, M. Glaucoma in myopia: Diagnostic dilemmas. Br. J. Ophthalmol. 2019, 103, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.K.; Yu, M.; Weinreb, R.N.; Mak, H.K.; Lai, G.; Ye, C.; Lam, D.S. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: Interpreting the RNFL maps in healthy myopic eyes. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7194–7200. [Google Scholar] [CrossRef] [PubMed]
- Kerrigan-Baumrind, L.A.; Quigley, H.A.; Pease, M.E.; Kerrigan, D.F.; Mitchell, R.S. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Investig. Ophthalmol. Vis. Sci. 2000, 41, 741–748. [Google Scholar]
- Tafreshi, A.; Racette, L.; Weinreb, R.N.; Sample, P.A.; Zangwill, L.M.; Medeiros, F.A.; Bowd, C. Pattern electroretinogram and psychophysical tests of visual function for discriminating between healthy and glaucoma eyes. Am. J. Ophthalmol. 2010, 149, 488–495. [Google Scholar] [CrossRef][Green Version]
- Wilsey, L.J.; Fortune, B. Electroretinography in glaucoma diagnosis. Curr. Opin. Ophthalmol. 2016, 27, 118–124. [Google Scholar] [CrossRef]
- Camp, A.S.; Weinreb, R.N. Will Perimetry Be Performed to Monitor Glaucoma in 2025? Ophthalmology 2017, 124, S71–S75. [Google Scholar] [CrossRef]
- Jung, K.I.; Jeon, S.; Shin, D.Y.; Lee, J.; Park, C.K. Pattern electroretinograms in preperimetric and perimetric glaucoma. Am. J. Ophthalmol. 2020, 215, 118–126. [Google Scholar] [CrossRef]
- Viswanathan, S.; Frishman, L.J.; Robson, J.G.; Harwerth, R.S.; Smith, E.L., 3rd. The photopic negative response of the macaque electroretinogram: Reduction by experimental glaucoma. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1124–1136. [Google Scholar]
- Senger, C.; Moreto, R.; Watanabe, S.E.S.; Matos, A.G.; Paula, J.S. Electrophysiology in Glaucoma. J. Glaucoma 2020, 29, 147–153. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.A.; Lee, J.; Park, C.K.; Jung, K.I. Intereye structure-function relationship using photopic negative response in patients with glaucoma or glaucoma suspect. Sci. Rep. 2022, 12, 13866. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.R.; Ly, E.; Viswanathan, S. Intensity response function of the photopic negative response (PhNR): Effect of age and test-retest reliability. Doc. Ophthalmol. 2017, 135, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Sugawara, A.; Nagashima, R.; Ikesugi, K.; Sugimoto, M.; Kondo, M. Factors Affecting Photopic Negative Response Recorded with RETeval System: Study of Young Healthy Subjects. Transl. Vis. Sci. Technol. 2020, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Chakraborty, R.; Verkicharla, P.K. Electroretinogram responses in myopia: A review. Doc. Ophthalmol. 2022, 145, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Oner, A.; Gumus, K.; Arda, H.; Karakucuk, S.; Mirza, E. Pattern electroretinographic recordings in eyes with myopia. Eye Contact Lens 2009, 35, 238–241. [Google Scholar] [CrossRef]
- Kirkiewicz, M.; Lubinski, W.; Penkala, K. Photopic negative response of full-field electroretinography in patients with different stages of glaucomatous optic neuropathy. Doc. Ophthalmol. 2016, 132, 57–65. [Google Scholar] [CrossRef][Green Version]
- Machida, S.; Gotoh, Y.; Toba, Y.; Ohtaki, A.; Kaneko, M.; Kurosaka, D. Correlation between photopic negative response and retinal nerve fiber layer thickness and optic disc topography in glaucomatous eyes. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2201–2207. [Google Scholar] [CrossRef]
- Preiser, D.; Lagreze, W.A.; Bach, M.; Poloschek, C.M. Photopic negative response versus pattern electroretinogram in early glaucoma. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1182–1191. [Google Scholar] [CrossRef]
- Sachidanandam, R.; Ravi, P.; Sen, P. Effect of axial length on full-field and multifocal electroretinograms. Clin. Exp. Optom. 2017, 100, 668–675. [Google Scholar] [CrossRef]
- Jung, K.I.; Park, H.Y.; Park, C.K. Characteristics of optic disc morphology in glaucoma patients with parafoveal scotoma compared to peripheral scotoma. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4813–4820. [Google Scholar] [CrossRef]
- Mwanza, J.C.; Durbin, M.K.; Budenz, D.L.; Sayyad, F.E.; Chang, R.T.; Neelakantan, A.; Godfrey, D.G.; Carter, R.; Crandall, A.S. Glaucoma diagnostic accuracy of ganglion cell-inner plexiform layer thickness: Comparison with nerve fiber layer and optic nerve head. Ophthalmology 2012, 119, 1151–1158. [Google Scholar] [CrossRef]
- Frishman, L.; Sustar, M.; Kremers, J.; McAnany, J.J.; Sarossy, M.; Tzekov, R.; Viswanathan, S. ISCEV extended protocol for the photopic negative response (PhNR) of the full-field electroretinogram. Doc. Ophthalmol. 2018, 136, 207–211. [Google Scholar] [CrossRef]
- Ortiz, G.; Drucker, D.; Hyde, C.; Staffetti, J.; Kremers, J.; Tzekov, R. The photopic negative response of the Light-adapted 3.0 ERG in clinical settings. Doc. Ophthalmol. 2020, 140, 115–128. [Google Scholar] [CrossRef]
- Bach, M.; Poloschek, C.M. Electrophysiology and glaucoma: Current status and future challenges. Cell Tissue Res. 2013, 353, 287–296. [Google Scholar] [CrossRef]
- Prencipe, M.; Perossini, T.; Brancoli, G.; Perossini, M. The photopic negative response (PhNR): Measurement approaches and utility in glaucoma. Int. Ophthalmol. 2020, 40, 3565–3576. [Google Scholar] [CrossRef]
- Cvenkel, B.; Sustar, M.; Perovsek, D. Ganglion cell loss in early glaucoma, as assessed by photopic negative response, pattern electroretinogram, and spectral-domain optical coherence tomography. Doc. Ophthalmol. 2017, 135, 17–28. [Google Scholar] [CrossRef]
- Horn, F.K.; Gottschalk, K.; Mardin, C.Y.; Pangeni, G.; Junemann, A.G.; Kremers, J. On and off responses of the photopic fullfield ERG in normal subjects and glaucoma patients. Doc. Ophthalmol. 2011, 122, 53–62. [Google Scholar] [CrossRef]
- Sustar, M.; Cvenkel, B.; Brecelj, J. The effect of broadband and monochromatic stimuli on the photopic negative response of the electroretinogram in normal subjects and in open-angle glaucoma patients. Doc. Ophthalmol. 2009, 118, 167–177. [Google Scholar] [CrossRef]
- Koh, V.; Tan, C.; Nah, G.; Zhao, P.; Yang, A.; Lin, S.T.; Wong, T.Y.; Saw, S.M.; Chia, A. Correlation of structural and electrophysiological changes in the retina of young high myopes. Ophthalmic Physiol. Opt. 2014, 34, 658–666. [Google Scholar] [CrossRef]

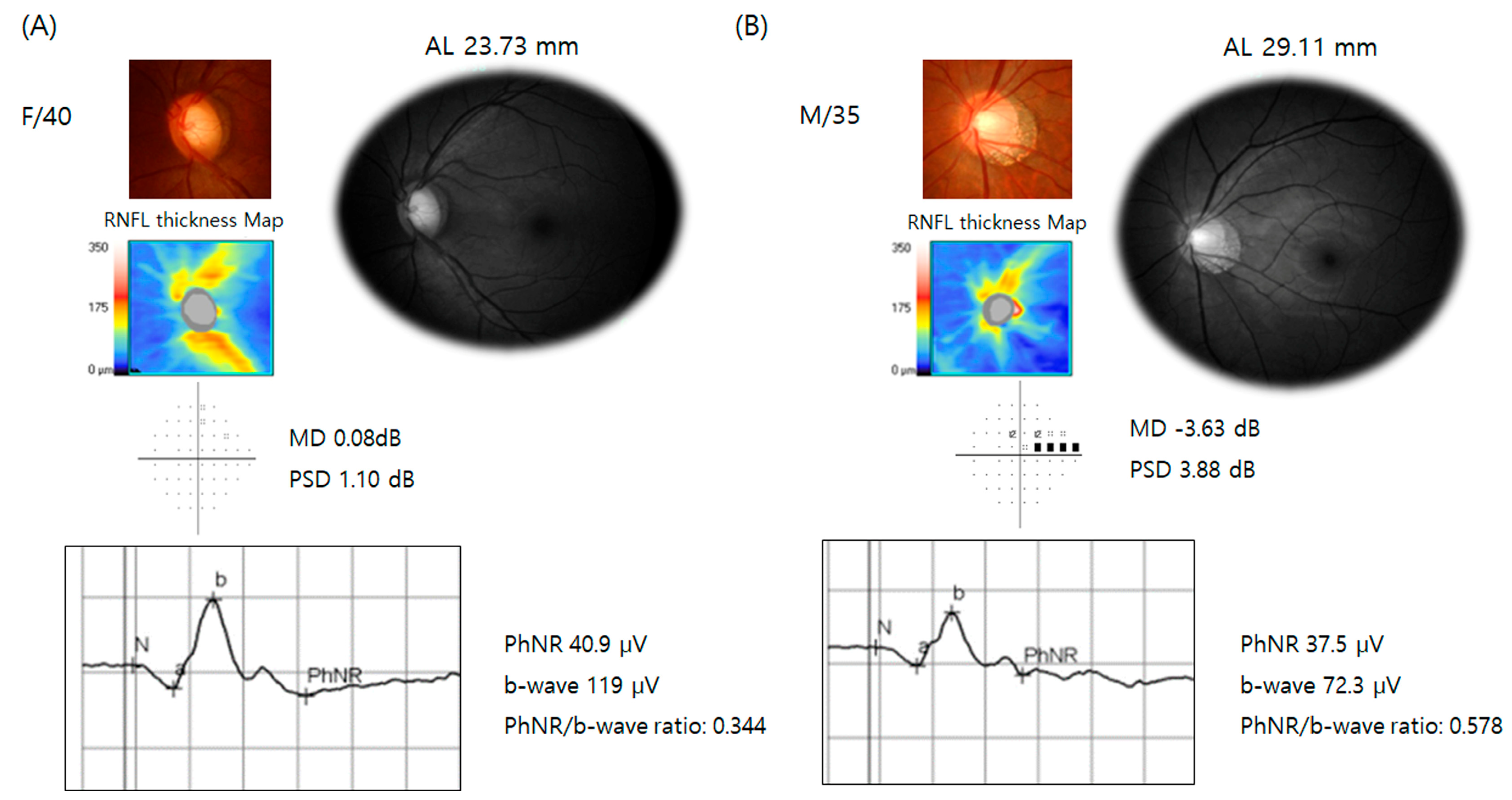
| Glaucoma Suspect (n = 19) | Glaucoma (n = 91) | p-Value | ||
|---|---|---|---|---|
| Age (years) | 50.9 ± 12.1 | 51.1 ± 12.5 | 0.950 | |
| Male/Female | 5/14 | 35/56 | 0.434 | |
| Central corneal thickness (µm) | 548.7 ± 38.2 | 534.4 ± 50.9 | 0.219 | |
| Spherical equivalent (diopter) | −2.1 ± 3.4 | −2.2 ± 2.8 | 0.846 | |
| Axial length (mm) | 24.2 ± 1.6 | 24.7 ± 1.5 | 0.138 | |
| Spectral-domain OCT | Average RNFL thickness (µm) | 90.9 ± 8.8 | 73.9 ± 10.0 | <0.001 |
| Average GCIPL thickness (µm) | 81.6 ± 4.2 | 70.3 ± 8.5 | <0.001 | |
| Minimum GCIPL thickness (µm) | 77.9 ± 5.0 | 58.8 ± 13.3 | <0.001 | |
| ERG | a-wave amplitude (µV) | 32.6 ± 10.1 | 30.4 ± 8.0 | 0.313 |
| b-wave amplitude (µV) | 112.4 ± 19.5 | 104.6 ± 25.6 | 0.213 | |
| PhNR amplitude (µV) | 35.9 ± 10.8 | 30.4 ± 10.3 | 0.035 | |
| PhNR/b-wave ratio | 0.32 ± 0.08 | 0.30 ± 0.10 | 0.370 | |
| Visual field 24-2 | MD (dB) | −1.0 ± 1.4 | −3.9 ± 3.6 | <0.001 |
| PSD (dB) | 1.6 ± 0.5 | 5.2 ± 3.4 | <0.001 | |
| Non-Myopia (n = 42, AL < 24 mm) | Myopia (n = 68, AL ≥ 24 mm) | p-Value | Non-Myopia (n = 69, AL < 25 mm) | Myopia (n = 41, AL ≥ 25 mm) | p-Value | |
|---|---|---|---|---|---|---|
| Age (years) | 57.9 ± 9.1 | 46.9 ± 12.3 | <0.001 | 54.9 ± 11.9 | 44.8 ± 10.3 | <0.001 |
| Male/Female | 6/36 | 34/34 | <0.001 | 25/62 | 15/8 | 0.001 |
| Central corneal thickness (µm) | 537.3 ± 34.6 | 535.4 ± 56.7 | 0.831 | 529.3 ± 40.1 | 547.2 ± 59.9 | 0.066 |
| Spherical equivalent (diopter) | −0.2 ± 1.4 | −3.4 ± 2.69 | <0.001 | −0.6 ± 1.4 | −4.9 ± 2.8 | <0.001 |
| AL (mm) | 23.2 ± 0.6 | 25.6 ± 1.1 | <0.001 | 23.2 ± 086 | 26.2 ± 0.9 | <0.001 |
| Parameters | Non-Myopia (n = 42, AL < 24 mm) | Myopia (n= 68, AL ≥ 24 mm) | p-Value | Non-Myopia (n = 69, AL < 25 mm) | Myopia (n = 41, AL ≥ 25 mm) | p-Value | |
|---|---|---|---|---|---|---|---|
| Spectral-domain OCT | Average RNFL thickness (µm) | 80.7 ± 10.6 | 74.5 ± 11.8 | 0.006 | 78.4 ± 11.5 | 74.3 ± 11.7 | 0.077 |
| Average GCIPL thickness (µm) | 75.4 ± 8.2 | 70.4 ± 9.0 | 0.004 | 73.8 ± 8.8 | 69.8 ± 8.7 | 0.023 | |
| Minimum GCIPL thickness (µm) | 63.1 ± 15.7 | 61.5 ± 13.4 | 0.573 | 62.2 ± 15.5 | 62.0 ± 12.0 | 0.939 | |
| ERG | a-wave amplitude (µV) | 31.59 ± 8.02 | 30.30 ± 7.85 | 0.438 | 30.85 ± 8.73 | 30.70 ± 7.85 | 0.929 |
| b-wave amplitude (µV) | 110.34 ± 20.24 | 103.21 ± 26.91 | 0.142 | 108.72 ± 25.20 | 101.25 ± 23.47 | 0.127 | |
| PhNR amplitude(µV) | 32.28 ± 9.58 | 30.73 ± 11.17 | 0.457 | 30.64 ± 10.58 | 32.47 ± 10.60 | 0.383 | |
| PhNR/b-wave ratio | 0.30 ± 0.08 | 0.31 ± 0.11 | 0.590 | 0.29 ± 0.09 | 0.33 ± 0.12 | 0.023 | |
| Visual field 24-2 | MD (dB) | −3.5 ± 3.9 | −3.3 ± 3.2 | 0.784 | −3.4 ± 3.9 | −3.3 ± 2.7 | 0.917 |
| PSD (dB) | 4.8 ± 3.9 | 4.4 ± 3.0 | 0.585 | 4.7 ± 3.6 | 4.4 ± 3.0 | 0.669 |
| ERG Parameters | r | p-Value |
|---|---|---|
| a-wave amplitude (µV) | −0.108 | 0.261 |
| b-wave amplitude (µV) | −0.230 | 0.016 |
| PhNR amplitude (µV) | 0.032 | 0.742 |
| PhNR/b-wave ratio | 0.239 | 0.012 |
| Total | Non-Myopia (AL < 24 mm) | Myopia (AL ≥ 24 mm) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | PhNR | PhNR/b-Wave | PhNR | PhNR/b-Wave | PhNR | PhNR/b-Wave | ||||||
|
β Coefficient (95% CI) | p-Value |
β Coefficient (95% CI) | p-Value |
β Coefficient (95% CI) | p-Value |
β Coefficient (95% CI) | p-Value |
β Coefficient (95% CI) | p-Value |
β Coefficient (95% CI) | p-Value | |
| Age | −0.296 (−0.486~−0.107) | 0.003 | −0.001 (−0.03~0.001) | 0.296 | −0.271 (−0.591~0.049) | 0.095 | 0.000 (−0.02~0.003) | 0.802 | −0.342 (−0.590~−0.094) | 0.008 | −0.002 (−0.004~0.000) | 0.075 |
| Gender | −3.212 (−7.778~1.353) | 0.166 | −0.045 (−0.091~0.000) | 0.049 | −9.182 (−17.445~−0.919) | 0.030 | −0.047 (−0.119~0.024) | 0.189 | −2.125 (−7.947~3.698) | 0.469 | −0.061 (−0.120~0.002) | 0.042 |
| AL | −0.718 (−2.446~1.011) | 0.412 | 0.010 (−0.007~0.028) | 0.229 | −0.592 (−5.365~4.181) | 0.803 | 0.012 (−0.029~0.054) | 0.560 | 1.191 (−1.464~3.846) | 0.373 | 0.028 (0.001~0.055) | 0.040 |
| Average GCIPL thickness | 0.373 (0.156~0.589) | 0.001 | 0.002 (0.000~0.004) | 0.036 | 0.482 (0.145~0.823) | 0.006 | 0.004 (0.001~0.007) | 0.004 | 0.290 (0.007~0.573) | 0.045 | 0.001 (−0.002~0.003) | 0.681 |
| Total | Non-Myopia (AL < 24 mm) | Myopia (AL ≥ 24 mm) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean AUC | p-Value | Sensitivity at Specificity > 80% | Cutoff |
Mean AUC (95% CI) | p-Value | Sensitivity at Specificity > 80% | Cutoff |
Mean AUC (95% CI) | p-Value | Sensitivity at Specificity > 80% | Cutoff | ||
| OCT | Average RNFL thickness (µm) | 0.901 (0.827, 0.974) | <0.001 | 82.4 | 83.5 | 0.862 (0.757, 0.967) | <0.001 | 74.2 | 83.5 | 0.921 (0.853, 0.989) | <0.001 | 86.7 | 83.5 |
| Average GCIPL thickness (µm) | 0.903 (0.842, 0.964) | <0.001 | 79.1 | 76.5 | 0.859 (0.757, 0.962) | <0.001 | 71.0 | 76.5 | 0.925 (0.866, 0.985) | <0.001 | 83.3 | 76.5 | |
| Minimum GCIPL thickness (µm) | 0.927 (0.878, 0.976) | <0.001 | 85.7 | 72.5 | 0.939 (0.875, 1.000) | <0.001 | 87.1 | 72.5 | 0.921 (0.861, 0.980) | <0.001 | 85.0 | 72.5 | |
| ERG | PhNR amplitude (µV) | 0.661 (0.536, 0.786) | 0.028 | 49.5 | 29.3 | 0.715 (0.566, 0.864) | 0.011 | 58.1 | 29.3 | 0.633 (0.498, 0.767) | 0.082 | 45.0 | 29.2 |
| PhNR/b-wave ratio (µV) | 0.616 (0.490, 0.742) | 0.114 | 38.5 | 0.26 | 0.696 (0.544, 0.849) | 0.021 | 45.2 | 0.26 | 0.574 (0.440, 0.708) | 0.332 | 35.0 | 0.26 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.G.; Park, C.K.; Jung, K.I. Impact of Myopia on the Utility of the Photopic Negative Response Ratio for Glaucoma Assessment. J. Clin. Med. 2025, 14, 682. https://doi.org/10.3390/jcm14030682
Park YG, Park CK, Jung KI. Impact of Myopia on the Utility of the Photopic Negative Response Ratio for Glaucoma Assessment. Journal of Clinical Medicine. 2025; 14(3):682. https://doi.org/10.3390/jcm14030682
Chicago/Turabian StylePark, Young Gun, Chan Kee Park, and Kyoung In Jung. 2025. "Impact of Myopia on the Utility of the Photopic Negative Response Ratio for Glaucoma Assessment" Journal of Clinical Medicine 14, no. 3: 682. https://doi.org/10.3390/jcm14030682
APA StylePark, Y. G., Park, C. K., & Jung, K. I. (2025). Impact of Myopia on the Utility of the Photopic Negative Response Ratio for Glaucoma Assessment. Journal of Clinical Medicine, 14(3), 682. https://doi.org/10.3390/jcm14030682





