Short-Term Efficacy and Safety of Scleral Lenses in the Management of Severe Dry Eye in a Chinese Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Ocular Examinations
2.2.1. Order of Ocular Examinations
2.2.2. OSDI Questionnaire
2.2.3. CHI-VFQ-25 Questionnaire
2.2.4. BCVA
2.2.5. Slit-Lamp Biomicroscopy
2.2.6. AS-OCT
2.2.7. Corneal Tomography
2.2.8. Corvis ST
2.2.9. Tear-Film Function
2.2.10. Corneal Sensitivity
2.2.11. SIT
2.3. SL Fitting
2.4. Statistical Analysis
3. Results
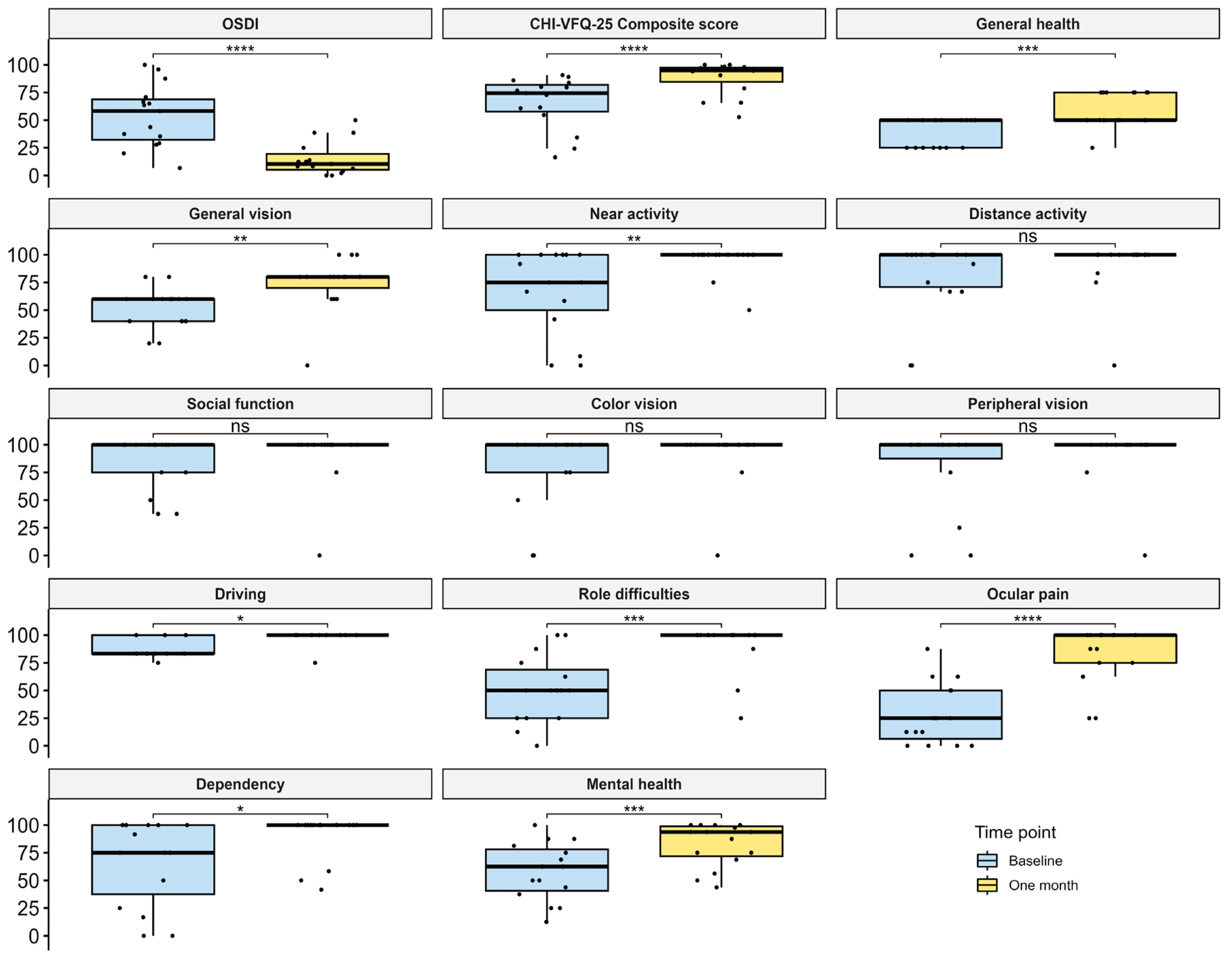
3.1. Improvement of DE Symptoms and VR-QoL
3.2. Improvement of DE Signs
3.3. Dependency on Medication
3.4. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.-K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Tsubota, K.; Yokoi, N.; Shimazaki, J.; Watanabe, H.; Dogru, M.; Yamada, M.; Kinoshita, S.; Kim, H.-M.; Tchah, H.-W.; Hyon, J.Y.; et al. New Perspectives on Dry Eye Definition and Diagnosis: A Consensus Report by the Asia Dry Eye Society. Ocul. Surf. 2017, 15, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Asian Dry Eye Society China Branch; Cross-Strait Medical and Health Exchange Association Ophthalmology Professional Committee Ocular Surface and Lacrimal Disease Study Group; Chinese Medical Doctor Association Ophthalmology Division Ocular Surface and Dry Eye Study Group. Chinese Dry Eye Expert Consensus: Definition and Classification (2020). Chin. J. Ophthalmol. 2020, 56, 418–422. [Google Scholar]
- Song, P.; Xia, W.; Wang, M.; Chang, X.; Wang, J.; Jin, S.; Wang, J.; Wei, W.; Rudan, I. Variations of Dry Eye Disease Prevalence by Age, Sex and Geographic Characteristics in China: A Systematic Review and Meta-Analysis. J. Glob. Health 2018, 8, 020503. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.; Downie, L.E.; Korb, D.; Benitez-Del-Castillo, J.M.; Dana, R.; Deng, S.X.; Dong, P.N.; Geerling, G.; Hida, R.Y.; Liu, Y.; et al. TFOS DEWS II Management and Therapy Report. Ocul. Surf. 2017, 15, 575–628. [Google Scholar] [PubMed]
- Le, Q.; Zhou, X.; Ge, L.; Wu, L.; Hong, J.; Xu, J. Impact of Dry Eye Syndrome on Vision-Related Quality of Life in a Non-Clinic-Based General Population. BMC Ophthalmol. 2012, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.; Ge, L.; Li, M.; Wu, L.; Xu, J.; Hong, J.; Gong, L. Comparison on the Vision-related Quality of Life between Outpatients and General Population with Dry Eye Syndrome. Acta Ophthalmol. 2014, 92, e124–e132. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, P.; Croteau, A. Fluid-Ventilated, Gas-Permeable Scleral Contact Lens Is an Effective Option for Managing Severe Ocular Surface Disease and Many Corneal Disorders That Would Otherwise Require Penetrating Keratoplasty. Eye Contact Lens Sci. Clin. Pract. 2005, 31, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Pecego, M.; Barnett, M.; Mannis, M.J.; Durbin-Johnson, B. Jupiter Scleral Lenses: The UC Davis Eye Center Experience. Eye Contact Lens Sci. Clin. Pract. 2012, 38, 179–182. [Google Scholar] [CrossRef]
- La Porta Weber, S.; Becco De Souza, R.; Gomes, J.Á.P.; Hofling-Lima, A.L. The Use of the Esclera Scleral Contact Lens in the Treatment of Moderate to Severe Dry Eye Disease. Am. J. Ophthalmol. 2016, 163, 167–173.e1. [Google Scholar] [CrossRef] [PubMed]
- Alipour, F.; Kheirkhah, A.; Jabarvand Behrouz, M. Use of Mini Scleral Contact Lenses in Moderate to Severe Dry Eye. Contact Lens Anterior Eye 2012, 35, 272–276. [Google Scholar] [CrossRef]
- Bavinger, J.C.; DeLoss, K.; Mian, S.I. Scleral Lens Use in Dry Eye Syndrome. Curr. Opin. Ophthalmol. 2015, 26, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Mickles, C.V.; Harthan, J.S.; Barnett, M. Assessment of a Novel Lens Surface Treatment for Scleral Lens Wearers with Dry Eye. Eye Contact Lens Sci. Clin. Pract. 2021, 47, 308–313. [Google Scholar] [CrossRef]
- Tougeron-Brousseau, B.; Delcampe, A.; Gueudry, J.; Vera, L.; Doan, S.; Hoang-Xuan, T.; Muraine, M. Vision-Related Function After Scleral Lens Fitting in Ocular Complications of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. Am. J. Ophthalmol. 2009, 148, 852–859.e2. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.-S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef]
- Chan, C.W.S.; Wong, D.; Lam, C.L.K.; McGhee, S.; Lai, W.W. Development of a Chinese Version of the National Eye Institute Visual Function Questionnaire (CHI-VFQ-25) as a Tool to Study Patients with Eye Diseases in Hong Kong. Br. J. Ophthalmol. 2009, 93, 1431–1436. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Mo, X.-H.; Li, X.-L.; Zeng, J.; Luo, W.; Huang, M.-L. Vision-Related Quality of Life and Depression in Rhegmatogenous Retinal Detachment Patients. Medicine 2019, 98, e14225. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, R.M. Reliability and Validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 2000, 118, 615. [Google Scholar] [CrossRef] [PubMed]
- CM, M. National Eye Institute Visual Function Questionnaire Field Test Investigators. Development of the 25-Item National Eye Institute Visual Function Questionnaire. Arch. Ophthalmol. 2001, 119, 1050–1058. [Google Scholar]
- Lemp, A. Report of the National Eye Institute/Industry Workshop on Clinical Trials in Dry Eyes. CLAO J. 1995, 21, 221–232. [Google Scholar] [PubMed]
- Shen, Y.; Wang, J.; Zhou, X.; Yu, Z.; Hong, J.; Le, Q. Impact of Dry Eye Disease on the Uncorrected Distance Visual Acuity after Small Incision Lenticule Extraction. J. Clin. Med. 2023, 12, 6179. [Google Scholar] [CrossRef]
- Qiu, S.X.; Fadel, D.; Hui, A. Scleral Lenses for Managing Dry Eye Disease in the Absence of Corneal Irregularities: What Is the Current Evidence? J. Clin. Med. 2024, 13, 3838. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lozano, R.E.; Gomez-Elizondo, D.E.; Colorado-Zavala, M.F.; Loya-Garcia, D.; Rodriguez-Garcia, A. Update on Indications, Complications, and Outcomes of Scleral Contact Lenses. Med. Hypothesis Discov. Innov. Ophthalmol. 2022, 10, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.; Ridges, R.; Jacobs, D.S.; Rosenthal, P. Treatment of Persistent Corneal Epithelial Defect with Overnight Wear of a Prosthetic Device for the Ocular Surface. Am. J. Ophthalmol. 2013, 156, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Van Der Worp, E.; Bornman, D.; Ferreira, D.L.; Faria-Ribeiro, M.; Garcia-Porta, N.; González-Meijome, J.M. Modern Scleral Contact Lenses: A Review. Contact Lens Anterior Eye 2014, 37, 240–250. [Google Scholar] [CrossRef]
- Schornack, M.M.; Pyle, J.; Patel, S.V. Scleral Lenses in the Management of Ocular Surface Disease. Ophthalmology 2014, 121, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gong, L.; Sun, X.; Chapin, W.J. Anxiety and Depression in Patients with Dry Eye Syndrome. Curr. Eye Res. 2011, 36, 1–7. [Google Scholar] [CrossRef]
- Kitazawa, M.; Sakamoto, C.; Yoshimura, M.; Kawashima, M.; Inoue, S.; Mimura, M.; Tsubota, K.; Negishi, K.; Kishimoto, T. The Relationship of Dry Eye Disease with Depression and Anxiety: A Naturalistic Observational Study. Transl. Vis. Sci. Technol. 2018, 7, 35. [Google Scholar] [CrossRef]
- Sayegh, R.R.; Yu, Y.; Farrar, J.T.; Kuklinski, E.J.; Shtein, R.M.; Asbell, P.A.; Maguire, M.G.; Dry Eye Assessment and Management (DREAM) Study Research Group. Ocular Discomfort and Quality of Life Among Patients in the Dry Eye Assessment and Management Study. Cornea 2021, 40, 869–876. [Google Scholar] [CrossRef]
- Mittal, R.; Patel, S.; Galor, A. Alternative Therapies for Dry Eye Disease. Curr. Opin. Ophthalmol. 2021, 32, 348–361. [Google Scholar] [CrossRef]
- Postnikoff, C.K.; Pucker, A.D.; Laurent, J.; Huisingh, C.; McGwin, G.; Nichols, J.J. Identification of Leukocytes Associated with Midday Fogging in the Post-Lens Tear Film of Scleral Contact Lens Wearers. Investig. Opthalmol. Vis. Sci. 2019, 60, 226. [Google Scholar] [CrossRef]
- Schornack, M.M.; Fogt, J.; Harthan, J.; Nau, C.B.; Nau, A.; Cao, D.; Shorter, E. Factors Associated with Patient-Reported Midday Fogging in Established Scleral Lens Wearers. Contact Lens Anterior Eye 2020, 43, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Fuller, D.G.; Wang, Y. Safety and Efficacy of Scleral Lenses for Keratoconus. Optom. Vis. Sci. 2020, 97, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.M.; Tong, L.; Yong, S.S.; Li, B.; Chaurasia, S.S.; Shui, G.; Wenk, M.R. Meibum Lipid Composition in Asians with Dry Eye Disease. PLoS ONE 2011, 6, e24339. [Google Scholar] [CrossRef]
- Suzuki, T.; Kitazawa, K.; Cho, Y.; Yoshida, M.; Okumura, T.; Sato, A.; Kinoshita, S. Alteration in Meibum Lipid Composition and Subjective Symptoms Due to Aging and Meibomian Gland Dysfunction. Ocul. Surf. 2022, 26, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.K.; Bailey, L.S.; Basso, K.B.; Redfern, R.R. Nonpolar Lipids Contribute to Midday Fogging During Scleral Lens Wear. Investig. Opthalmol. Vis. Sci. 2023, 64, 7. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qin, Q.; Liu, B.; Fu, Y.; Lin, L.; Huang, X.; Jin, X. Clinical Analysis: Aqueous-Deficient and Meibomian Gland Dysfunction in Patients with Primary Sjogren’s Syndrome. Front. Med. 2019, 6, 291. [Google Scholar] [CrossRef]
- Perez, V.L.; Mousa, H.M.; Soifer, M.; Beatty, C.; Sarantopoulos, S.; Saban, D.R.; Levy, R.B. Meibomian Gland Dysfunction: A Route of Ocular Graft-Versus-Host Disease Progression That Drives a Vicious Cycle of Ocular Surface Inflammatory Damage. Am. J. Ophthalmol. 2023, 247, 42–60. [Google Scholar] [CrossRef] [PubMed]
- García-Marqués, J.V.; Macedo-De-Araújo, R.J.; Cerviño, A.; García-Lázaro, S.; González-Méijome, J.M. Assessment of Meibomian Gland Drop-out and Visibility through a New Quantitative Method in Scleral Lens Wearers: A One-Year Follow-up Study. Contact Lens Anterior Eye 2023, 46, 101571. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Tang, M.; Li, Y.; Zhang, X.; Chu, R.; Huang, D. Anterior Segment Dimensions in Asian and Caucasian Eyes Measured by Optical Coherence Tomography. Ophthalmic Surg. Lasers Imaging Retina 2012, 43, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Hickson-curran, S.; Young, G.; Brennan, N.; Hunt, C. Chinese and Caucasian Ocular Topography and Soft Contact Lens Fit. Clin. Exp. Optom. 2016, 99, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.; Cordova, D.; Xu, J.; Deng, S.X. In Vivo Evaluation of the Limbus Using Anterior Segment Optical Coherence Tomography. Transl. Vis. Sci. Technol. 2018, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Vinciguerra, R.; Herber, R.; Wang, Y.; Zhang, F.; Zhou, X.; Bai, J.; Yu, K.; Chen, S.; Fang, X.; Raiskup, F.; et al. Corneal Biomechanics Differences Between Chinese and Caucasian Healthy Subjects. Front. Med. 2022, 9, 834663. [Google Scholar] [CrossRef] [PubMed]
| Gender | Age | Concomitant Systematic and Ocular Diseases | Previous Ocular Surface Surgeries | Eyes | Physical Treatment and Medications Before SL Therapy | Medications After SL Therapy | BCVA | Lens Materials | SAG (um) | Dia (mm) | BC (mm) | CT (mm) | BVP (D) | Cyl (D) | Axis (°) | Adverse Events | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Midday Fogging | Others | |||||||||||||||||
| Patient 1 | Female | 38 | SS | No | OD | IPL; Preserved AT (3); Preservative-free AT (1); Cyclosporine; Loteprednol | Preservative-free AT (1) | 0 | hexafocon A | 3900 | 16.00 | 8.05 | 0.30 | −5.25 | −0.75 | 80 | Yes | No |
| OS | Same as OD | Same as OD | 0 | hexafocon A | 3900 | 16.00 | 8.05 | 0.30 | −3.75 | −0.50 | 100 | Yes | No | |||||
| Patient 2 | Female | 46 | SS | No | OD | Preserved AT (1); Serum extracts; Cyclosporine; Fluorometholone | Preserved AT (1); | 0 | hexafocon A | 3700 | 15.60 | 8.25 | 0.30 | +3.50 | −0.25 | 80 | No | No |
| OS | Same as OD | Preservative-free AT (1) | 0 | hexafocon A | 3700 | 15.60 | 8.25 | 0.34 | +5.75 | −0.25 | 100 | No | No | |||||
| Patient 3 | Female | 59 | TED | No | OS | Preserved AT (1); Serum extracts; Cyclosporine | Preserved AT (1); Cyclosporine | 0 | hexafocon A | 3400 | 14.80 | 8.45 | 0.30 | +3.00 | −0.25 | 0 | Yes | No |
| Patient 4 | Female | 58 | No | Excision of pinguecula | OD | Preserved AT (1); Fluorometholone; Cyclosporine | Preservative-free AT (1); Cyclosporine | 0.22 | Boston XO2 | 3300 | 14.50 | 9.10 | 0.42 | +1.00 | −0.36 | 180 | No | No |
| Patient 5 | Female | 51 | GVHD | No | OD | IPL; Preserved AT (1); Serum extracts; Fluorometholone | Preservative-free AT (1) | 0.05 | Boston XO2 | 3500 | 14.50 | 8.20 | 0.42 | +3.00 | −0.36 | 180 | Yes | No |
| Patient 6 | Female | 46 | SS | Amniotic membrane transplantation | OD | Preserved AT (2); Preservative-free AT (1); Serum extracts; Loteprednol; Fluorometholone | Preservative-free AT (1) | 0 | Boston XO2 | 3400 | 14.50 | 8.60 | 0.42 | 0.50 | −0.36 | 180 | Yes | Subconjunctival hemorrhage of undetermined etiology |
| Patient 7 | Female | 47 | SS | No | OD | IPL; Preserved AT (1); Loteprednol | Preserved AT (1) | 0 | Boston XO2 | 3600 | 14.50 | 8.42 | 0.42 | 1.25 | −0.36 | 180 | No | No |
| Patient 8 | Female | 44 | SJS | No | OS | Preserved AT (2); Serum extracts; Cyclosporine; Tacrolimus; Fluorometholone | Preservative-free AT (1); Fluorometholone; Cyclosporine | 0.70 | hexafocon A | 3700 | 15.60 | 8.25 | 0.30 | +1.25 | −1.00 | 105 | Yes | No |
| Patient 9 | Female | 54 | RA | No | OD | IPL; Punctal Occlusion; Preserved AT (1); Preservative-free AT (1); Cyclosporine | Preserved AT (1) | 0 | Boston XO2 | 3400 | 14.50 | 8.60 | 0.30 | −2.50 | −0.36 | 180 | No | No |
| OS | Same as OD | Same as OD | 0.05 | Boston XO2 | 3400 | 14.50 | 8.60 | 0.30 | −1.00 | −0.36 | 180 | No | No | |||||
| Patient 10 | Female | 57 | No | No | OD | Preserved AT (1) | Preservative-free AT (1) | 0 | hexafocon A | 3900 | 15.60 | 8.05 | 0.30 | +3.50 | −0.25 | 0 | Yes | No |
| OS | Same as OD | Same as OD | 0 | hexafocon A | 3900 | 15.60 | 8.05 | 0.30 | +4.25 | −0.50 | 90 | Yes | No | |||||
| Patient 11 | Female | 45 | VKH | No | OD | IPL; Preserved AT (1); Preservative-free AT (1); Serum extracts; Fluorometholone | Preservative-free AT (1); Fluorometholone | 0 | Boston XO2 | 3900 | 16.00 | 9.80 | 0.42 | 5.00 | −0.36 | 180 | Yes | No |
| OS | Same as OD | Preservative-free AT (1) | 0 | Boston XO2 | 3.90 | 16.00 | 9.80 | 0.42 | 6.75 | −0.36 | 180 | Yes | No | |||||
| Patient 12 | Female | 73 | SS | No | OD | Preserved AT (1) Serum extracts | Preserved AT (1); Preservative-free AT (1) | 0.10 | hexafocon A | 3400 | 15.20 | 8.45 | 0.31 | +4.75 | −0.25 | 0 | Yes | No |
| OS | Same as OD | Same as OD | 0.10 | hexafocon A | 3400 | 15.20 | 8.45 | 0.33 | +5.50 | −0.25 | 0 | Yes | No | |||||
| Patient 13 | Female | 14 | Filamentary keratitis | No | OD | Preservative-free AT (2); Serum extracts; Cyclosporine; Loteprednol; Fluorometholone | Cyclosporine | 0 | hexafocon A | 4000 | 16.30 | 8.25 | 0.30 | −1.00 | −0.25 | 0 | No | No |
| OS | Preservative-free AT (2); Serum extracts; Tacrolimus; Loteprednol; Fluorometholone | Tacrolimus | 0 | hexafocon A | 4000 | 16.30 | 8.25 | 0.30 | −2.00 | −0.50 | 100 | No | No | |||||
| Patient 14 | Male | 39 | GVHD | No | OD | Preserved AT (1); Tacrolimus; Fluorometholone | Preserved AT (1); Tacrolimus; Fluorometholone | 0 | hexafocon A | 3700 | 15.60 | 8.25 | 0.30 | +1.75 | −1.50 | 90 | No | No |
| Patient 15 | Female | 54 | No | No | OD | Serum extracts | Preservative-free AT (1) | 0.22 | reflufocon D | 4510 | 15.80 | 8.04 | 0.31 | 2.00 | −1.50 | 145 | Yes | No |
| Baseline | One Month | p | |
|---|---|---|---|
| LogMAR BCVA | 0 (0–0.1) | 0 (0–0) | 0.015 * |
| ST (s) | 2.5 (0–9) | 2 (0–5) | 0.626 |
| TBUT | 0.6 ± 0.5 | 2.2 ± 1.0 | <0.0001 * |
| CFS | 10.2 ± 3.9 | 7 (0–12) | 0.001 * |
| First NIBUT | 3.7 (2.9–5.7) | 6.1 ± 3.3 | 0.109 |
| Average NIBUT | 5.1 (3.4–7.6) | 8.1 ± 3.9 | 0.282 |
| TMH | 0.2 (0.2–0.3) | 0.2 ± 0.1 | 0.445 |
| MG dropout scores | 2 (1–3) | 2.7 ± 1.6 | 0.039 * |
| LLC | 0.761 | ||
| Normal | 10 (45.5%) | 9 (40.9%) | |
| Abnormal | 12 (54.5%) | 13 (59.1%) | |
| Corneal sensitivity | 31.6 ± 23.9 | 39.6 ± 20.0 | 0.078 |
| CCT | 517.1 ± 49.9 | 526.7 ± 52.6 | 0.055 |
| CET | 53.4 (50.3–55.6) | 53.5 (50.2–56.1) | 0.429 |
| bIOP | 16.6 ± 3.3 | 15.3 ± 1.5 | 0.039 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, C.; Han, D.; Zeng, L.; Hong, J.; Fadel, D.; Zhou, X.; Chen, Z.; Le, Q. Short-Term Efficacy and Safety of Scleral Lenses in the Management of Severe Dry Eye in a Chinese Population. J. Clin. Med. 2025, 14, 658. https://doi.org/10.3390/jcm14030658
Lu C, Han D, Zeng L, Hong J, Fadel D, Zhou X, Chen Z, Le Q. Short-Term Efficacy and Safety of Scleral Lenses in the Management of Severe Dry Eye in a Chinese Population. Journal of Clinical Medicine. 2025; 14(3):658. https://doi.org/10.3390/jcm14030658
Chicago/Turabian StyleLu, Chuwei, Danjie Han, Li Zeng, Jiaxu Hong, Daddi Fadel, Xingtao Zhou, Zhi Chen, and Qihua Le. 2025. "Short-Term Efficacy and Safety of Scleral Lenses in the Management of Severe Dry Eye in a Chinese Population" Journal of Clinical Medicine 14, no. 3: 658. https://doi.org/10.3390/jcm14030658
APA StyleLu, C., Han, D., Zeng, L., Hong, J., Fadel, D., Zhou, X., Chen, Z., & Le, Q. (2025). Short-Term Efficacy and Safety of Scleral Lenses in the Management of Severe Dry Eye in a Chinese Population. Journal of Clinical Medicine, 14(3), 658. https://doi.org/10.3390/jcm14030658







