Paraneoplastic Dermatoses: A Clue for Underlying Malignancies
Abstract
1. Introduction
2. Obligate Paraneoplastic Dermatoses
2.1. Necrolytic Migratory Erythema
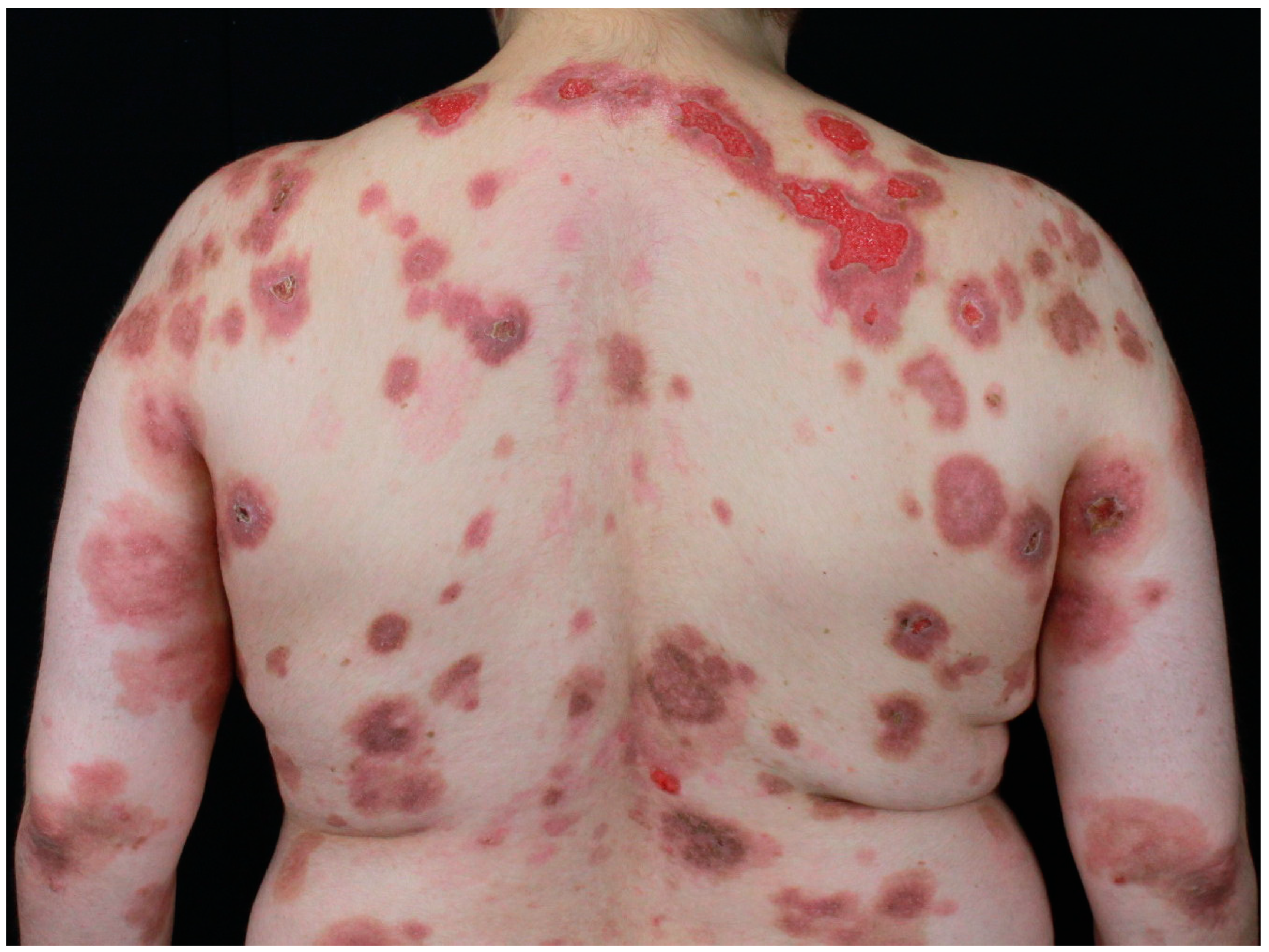
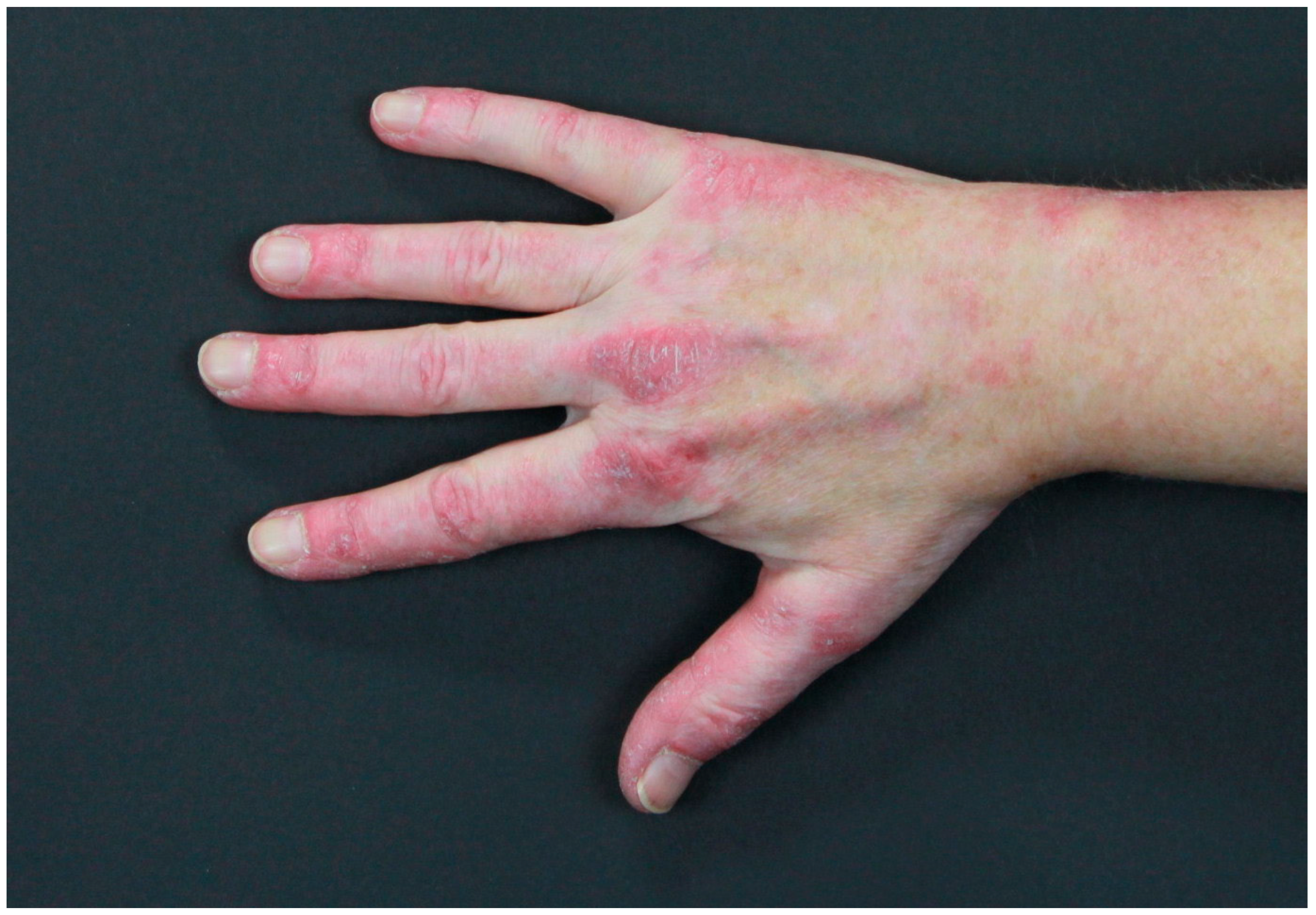
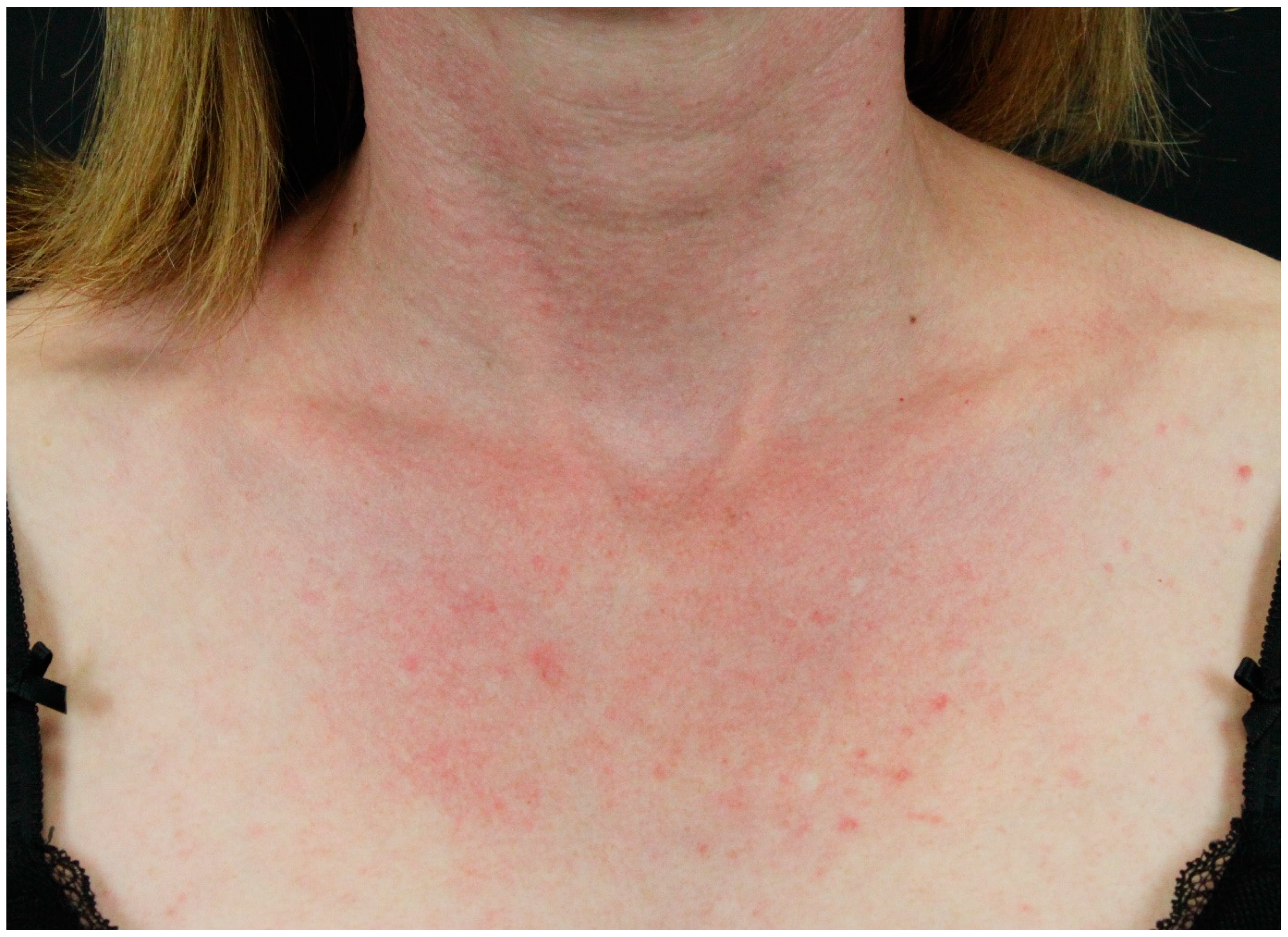
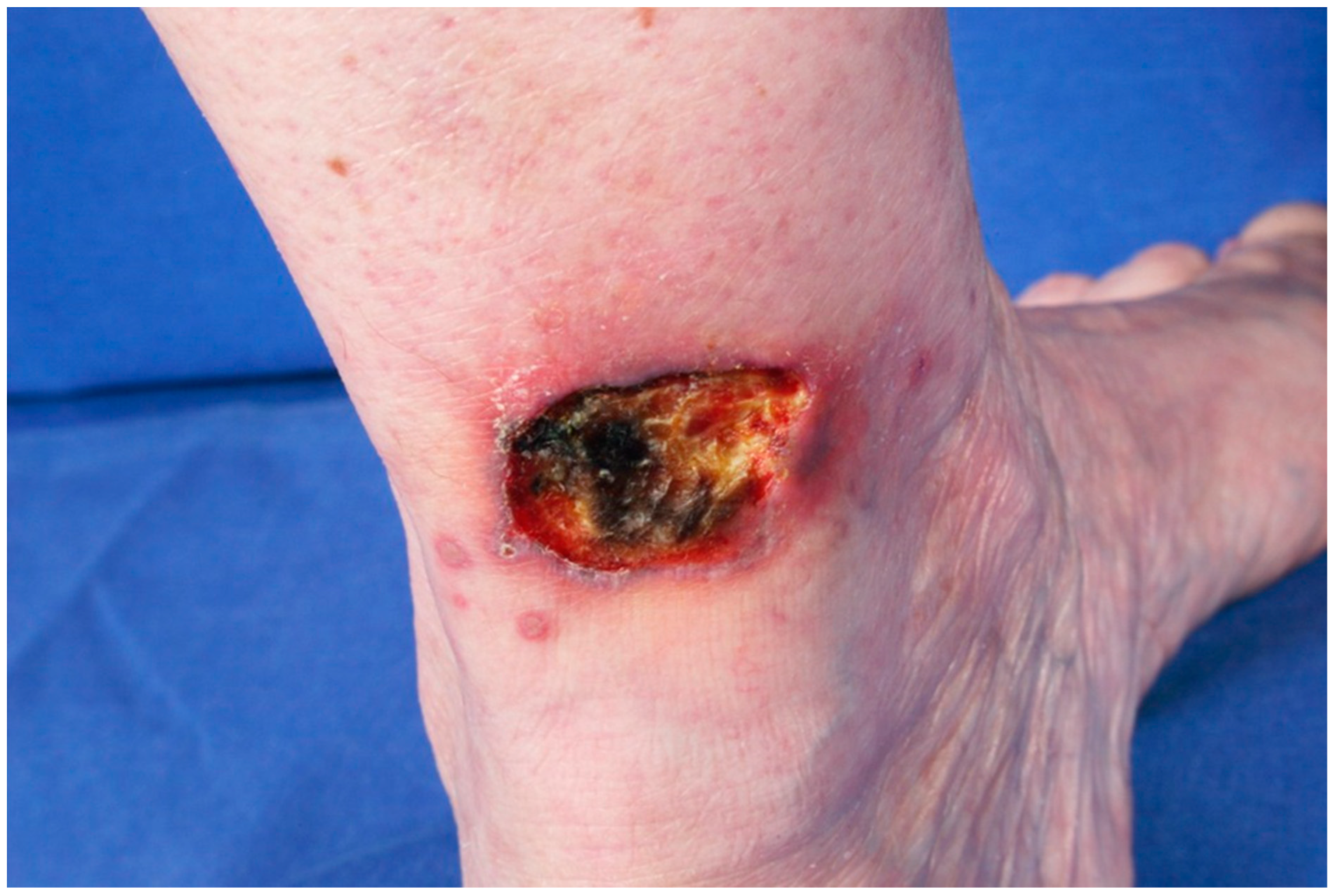
2.2. Paraneoplastic Autoimmune Multiorgan Syndrome
2.3. Tripe Palms
3. Facultative Paraneoplastic Dermatoses
3.1. Acanthosis Nigricans
3.2. Leser–Trélat’s Sign
3.3. Paraneoplastic Dermatomyositis
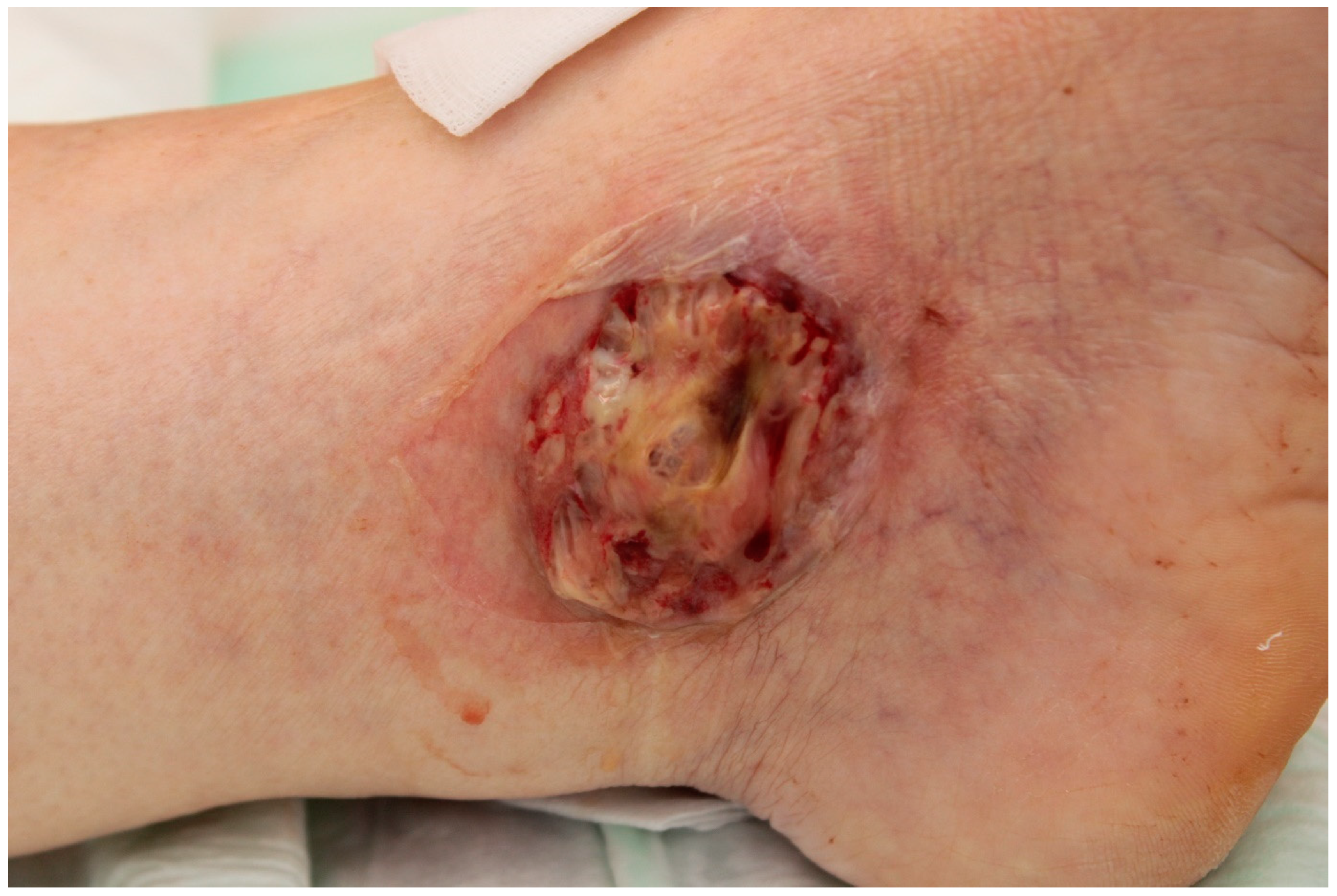
3.4. Pyoderma Gangrenosum
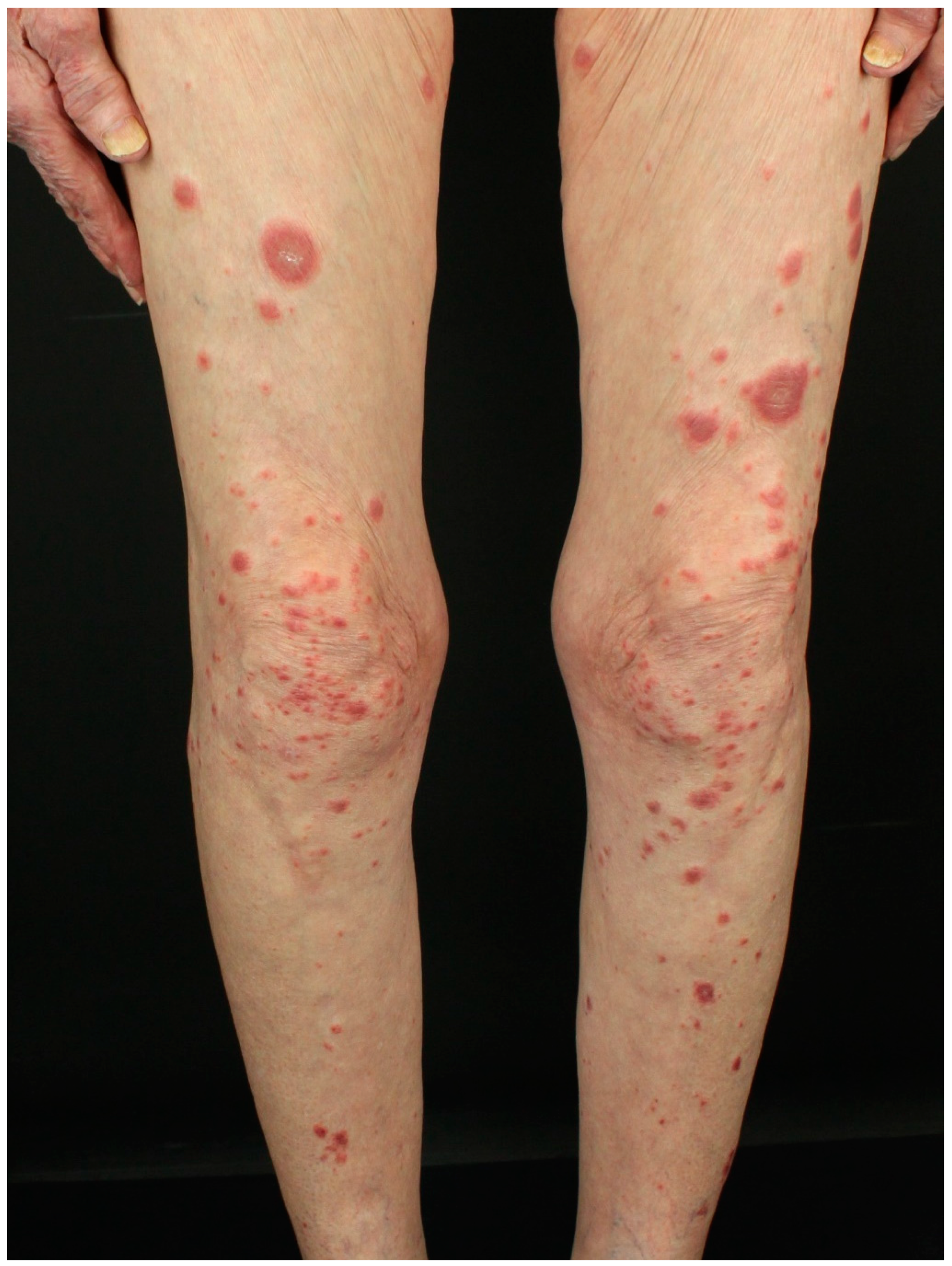
3.5. Sweet’s Syndrome
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A2ML1 | α-2 microglobulin-like 1 |
| AN | Acanthosis nigricans |
| BMZ | Basal membrane zone |
| BO | Bronchiolitis obliterans |
| BP | Bullous pemphigoid |
| DIF | Direct immunofluorescence |
| Dsg | Desmoglein |
| DM | Dermatomyositis |
| EGF-α | Epidermal growth factor α |
| ELISA | Enzyme-linked immunosorbent assay |
| EGFR | Epidermal growth factor receptor |
| EGF | Epidermal growth factor |
| ERK | Extracellular-signal-regulated kinase |
| FGF | Fibroblast growth factor |
| FGFR3 | Fibroblast growth factor receptor 3 |
| G-CSF | Granulocyte colony stimulating factor |
| GM-CSF | Granulocyte macrophage colony stimulating factor |
| HOA | Hypertrophic osteoarthropathy |
| HPV | Human papillomavirus |
| IB | Immunoblotting |
| IBD | Inflammatory bowel diseases |
| IIF | Indirect immunofluorescence |
| IL | Interleukin |
| IGF-1 | Insulin growth factor 1 |
| IP | Immunoprecipitation |
| LTS | Leser–Trélat’s sign |
| MAN | Malignant acanthosis nigricans |
| MAPK | Mitogen-activated protein kinase |
| MSH | Melanocyte-stimulating hormone |
| MF | Mycosis fungoides |
| MM | Malignant melanoma |
| MSA | Myositis-specific antibodies |
| NME | Necrolytic migratory erythema |
| PAMS | Paraneoplastic autoimmune multiorgan syndrome |
| PAPA | Pyogenic arthritis, pyoderma gangrenosum, acne |
| PsAPASH | Psoriatic arthritis, pyoderma gangrenosum, acne, hidradenitis suppurativa |
| PD | Paraneoplastic dermatosis |
| PF | Pemphigus foliaceus |
| PG | Pyoderma gangrenosum |
| PNP | Paraneoplastic pemphigus |
| PV | Pemphigus vulgaris |
| PSTPIP1 | Proline–serine–threonine phosphatase-interacting protein 1 gene |
| SK | Seborrheic keratoses |
| SS | Sweet’s syndrome |
| TGF-α | Tumor growth factor α |
| TIF1-γ | Transcription intermediary factor 1 γ |
| TNF-α | Tumor necrosis factor α |
| TP | Tripe palms |
References
- Didona, D.; Fania, L.; Didona, B.; Eming, R.; Hertl, M.; Di Zenzo, G. Paraneoplastic Dermatoses: A Brief General Review and an Extensive Analysis of Paraneoplastic Pemphigus and Paraneoplastic Dermatomyositis. Int. J. Mol. Sci. 2020, 21, 2178. [Google Scholar] [CrossRef]
- Gualtieri, B.; Hertl, M. Seltene Erkrankungen an der Haut erkennen. Internist 2019, 60, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.A.; Mesquita, K.d.C.; Igreja, A.C.d.S.M.; Lucas, I.C.R.N.; Freitas, A.F.; de Oliveira, S.M.; Costa, I.M.C.; Campbell, I.T. Paraneoplastic cutaneous manifestations: Concepts and updates. An. Bras. Dermatol. 2013, 88, 9–22. [Google Scholar] [CrossRef] [PubMed]
- John, A.M.; Schwartz, R.A. Glucagonoma syndrome: A review and update on treatment. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 2016–2022. [Google Scholar] [CrossRef]
- Toberer, F.; Hartschuh, W.; Wiedemeyer, K. Glucagonoma-Associated Necrolytic Migratory Erythema: The Broad Spectrum of the Clinical and Histopathological Findings and Clues to the Diagnosis. Am. J. Dermatopathol. 2019, 41, e29–e32. [Google Scholar] [CrossRef]
- Albers, M.B.; Sevcik, M.; Wiese, D.; Manoharan, J.; Rinke, A.; Jesinghaus, M.; Bartsch, D.K. Characteristics, therapy, and outcome of rare functioning pancreatic neuroendocrine neoplasms. Sci. Rep. 2024, 14, 18507. [Google Scholar] [CrossRef] [PubMed]
- Braverman, I.M. “Cutaneous manifestations of internal malignant tumors” by Becker, Kahn and Rothman, June 1942. Commentary: Migratory necrolytic erythema. Arch. Dermatol. 1982, 118, 784–798. [Google Scholar] [CrossRef]
- Wilkinson, D.S. Necrolytic migratory erythema with pancreatic carcinoma. Proc. R. Soc. Med. 1971, 64, 1197–1198. [Google Scholar] [CrossRef] [PubMed]
- Tierney, E.P.; Badger, J. Etiology and pathogenesis of necrolytic migratory erythema: Review of the literature. MedGenMed 2004, 6, 4. [Google Scholar] [PubMed]
- van Beek, A.P.; de Haas, E.R.M.; van Vloten, W.A.; Lips, C.J.M.; Roijers, J.F.M.; Canninga-van Dijk, M.R. The glucagonoma syndrome and necrolytic migratory erythema: A clinical review. Eur. J. Endocrinol. 2004, 151, 531–537. [Google Scholar] [CrossRef]
- Echenique-Elizondo, M.; Tuneu Valls, A.; Elorza Orúe, J.L.; Martinez de Lizarduy, I.; Ibáñez Aguirre, J. Glucagonoma and pseudoglucagonoma syndrome. JOP 2004, 5, 179–185. [Google Scholar] [PubMed]
- Manes, G.; Pellegrini, A.; Riccardi, F.; Uomo, G. Rare presentation of endocrine pancreatic tumour: A case of glucagonoma without necrolytic migratory erythema? Ital. J. Gastroenterol. 1995, 27, 248–249. [Google Scholar] [PubMed]
- Leichter, S.B. Clinical and metabolic aspects of glucagonoma. Medicine 1980, 59, 100–113. [Google Scholar] [CrossRef]
- Wahba, A.; Tan, Z.; Dillon, J.S. Management of functional neuroendocrine tumors. Curr. Probl. Cancer 2024, 52, 101130. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, V.N.; Jain, S. Acrodermatitis enteropathica. Clin. Dermatol. 2000, 18, 745–748. [Google Scholar] [CrossRef]
- Didona, D.; Schmidt, M.F.; Maglie, R.; Solimani, F. Pemphigus and pemphigoids: Clinical presentation, diagnosis and therapy. J. Dtsch. Dermatol. Ges. 2023, 21, 1188–1209. [Google Scholar] [CrossRef]
- Rebora, A.; Rongioletti, F. The red face: Seborrheic dermatitis. Clin. Dermatol. 1993, 11, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Anhalt, G.J.; Kim, S.C.; Stanley, J.R.; Korman, N.J.; Jabs, D.A.; Kory, M.; Izumi, H.; Ratrie, H.; Mutasim, D.; Ariss-Abdo, L. Paraneoplastic pemphigus. An autoimmune mucocutaneous disease associated with neoplasia. N. Engl. J. Med. 1990, 323, 1729–1735. [Google Scholar] [CrossRef]
- Krain, L.S. The association of pemphigus with thymoma or malignancy: A critical review. Br. J. Dermatol. 1974, 90, 397–405. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Ndoye, A.; Bassler, K.D.; Shultz, L.D.; Shields, M.C.; Ruben, B.S.; Webber, R.J.; Pittelkow, M.R.; Lynch, P.J.; Grando, S.A. Classification, clinical manifestations, and immunopathological mechanisms of the epithelial variant of paraneoplastic autoimmune multiorgan syndrome: A reappraisal of paraneoplastic pemphigus. Arch. Dermatol. 2001, 137, 193–206. [Google Scholar]
- Anderson, H.J.; Huang, S.; Lee, J.B. Paraneoplastic pemphigus/paraneoplastic autoimmune multiorgan syndrome: Part I. Clinical overview and pathophysiology. J. Am. Acad. Dermatol. 2024, 91, 1–10. [Google Scholar] [CrossRef]
- Kaplan, I.; Hodak, E.; Ackerman, L.; Mimouni, D.; Anhalt, G.J.; Calderon, S. Neoplasms associated with paraneoplastic pemphigus: A review with emphasis on non-hematologic malignancy and oral mucosal manifestations. Oral Oncol. 2004, 40, 553–562. [Google Scholar] [CrossRef]
- Didona, D.; Di Zenzo, G.; Joly, P. Paraneoplastic autoimmune multiorgan syndrome. Ital. J. Dermatol. Venerol. 2021, 156, 174–183. [Google Scholar] [CrossRef]
- Didona, D.; Hertl, M. Paraneoplastische Autoimmundermatosen. Hautarzt 2021, 72, 277–287. [Google Scholar] [CrossRef]
- Bazarbachi, A.; Bachelez, H.; Dehen, L.; Delmer, A.; Zittoun, R.; Dubertret, L. Lethal paraneoplastic pemphigus following treatment of chronic lymphocytic leukaemia with fludarabine. Ann. Oncol. 1995, 6, 730–731. [Google Scholar] [CrossRef] [PubMed]
- Gooptu, C.; Littlewood, T.J.; Frith, P.; Lyon, C.C.; Carmichael, A.J.; Oliwiecki, S.; MacWhannell, A.; Amagai, M.; Hashimoto, T.; Dean, D.; et al. Paraneoplastic pemphigus: An association with fludarabine? Br. J. Dermatol. 2001, 144, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Powell, A.M.; Albert, S.; Oyama, N.; Sakuma-Oyama, Y.; Bhogal, B.; Black, M.M. Paraneoplastic pemphigus secondary to fludarabine evolving into unusual oral pemphigus vegetans. J. Eur. Acad. Dermatol. Venereol. 2004, 18, 360–364. [Google Scholar] [CrossRef]
- Yildiz, O.; Ozguroglu, M.; Yanmaz, M.T.; Turna, H.; Kursunoglu, S.G.; Antonov, M.; Serdaroglu, S.; Demirkesen, C.; Buyukunal, E. Paraneoplastic pemphigus associated with fludarabine use. Med. Oncol. 2007, 24, 115–118. [Google Scholar] [CrossRef]
- Kirsner, R.S.; Anhalt, G.J.; Kerdel, F.A. Treatment with alpha interferon associated with the development of paraneoplastic pemphigus. Br. J. Dermatol. 1995, 132, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Fried, R.; Lynfield, Y.; Vitale, P.; Anhalt, G. Paraneoplastic pemphigus appearing as bullous pemphigoid-like eruption after palliative radiation therapy. J. Am. Acad. Dermatol. 1993, 29, 815–817. [Google Scholar] [CrossRef]
- Lee, M.S.; Kossard, S.; Ho, K.K.; Barnetson, R.S.; Ravich, R.B. Paraneoplastic pemphigus triggered by radiotherapy. Australas. J. Dermatol. 1995, 36, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Martel, P.; Loiseau, P.; Joly, P.; Busson, M.; Lepage, V.; Mouquet, H.; Courville, P.; Flageul, B.; Charron, D.; Musette, P.; et al. Paraneoplastic pemphigus is associated with the DRB1*03 allele. J. Autoimmun. 2003, 20, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Bu, D.-F.; Li, D.; Zhu, X.-J. Genotyping of HLA-I and HLA-II alleles in Chinese patients with paraneoplastic pemphigus. Br. J. Dermatol. 2008, 158, 587–591. [Google Scholar] [CrossRef]
- Amber, K.T.; Valdebran, M.; Grando, S.A. Paraneoplastic autoimmune multiorgan syndrome (PAMS): Beyond the single phenotype of paraneoplastic pemphigus. Autoimmun. Rev. 2018, 17, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Inaoki, M.; Kodera, M.; Fujimoto, A.; Nousari, H.C.; Anhalt, G.J.; Takehara, K. Paraneoplastic pemphigus without antidesmoglein 3 or antidesmoglein 1 autoantibodies. Br. J. Dermatol. 2001, 144, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Amagai, M.; Nishikawa, T.; Nousari, H.C.; Anhalt, G.J.; Hashimoto, T. Antibodies against desmoglein 3 (pemphigus vulgaris antigen) are present in sera from patients with paraneoplastic pemphigus and cause acantholysis in vivo in neonatal mice. J. Clin. Investig. 1998, 102, 775–782. [Google Scholar] [CrossRef]
- Paolino, G.; Didona, D.; Magliulo, G.; Iannella, G.; Didona, B.; Mercuri, S.R.; Moliterni, E.; Donati, M.; Ciofalo, A.; Granata, G.; et al. Paraneoplastic Pemphigus: Insight into the Autoimmune Pathogenesis, Clinical Features and Therapy. Int. J. Mol. Sci. 2017, 18, 2532. [Google Scholar] [CrossRef]
- Takahashi, M.; Shimatsu, Y.; Kazama, T.; Kimura, K.; Otsuka, T.; Hashimoto, T. Paraneoplastic pemphigus associated with bronchiolitis obliterans. Chest 2000, 117, 603–607. [Google Scholar] [CrossRef]
- Tsuchisaka, A.; Numata, S.; Teye, K.; Natsuaki, Y.; Kawakami, T.; Takeda, Y.; Wang, W.; Ishikawa, K.; Goto, M.; Koga, H.; et al. Epiplakin Is a Paraneoplastic Pemphigus Autoantigen and Related to Bronchiolitis Obliterans in Japanese Patients. J. Investig. Dermatol. 2016, 136, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Ohzono, A.; Sogame, R.; Li, X.; Teye, K.; Tsuchisaka, A.; Numata, S.; Koga, H.; Kawakami, T.; Tsuruta, D.; Ishii, N.; et al. Clinical and immunological findings in 104 cases of paraneoplastic pemphigus. Br. J. Dermatol. 2015, 173, 1447–1452. [Google Scholar] [CrossRef]
- Wang, R.; Li, J.; Wang, M.; Hao, H.; Chen, X.; Li, R.; Zhu, X. Prevalence of myasthenia gravis and associated autoantibodies in paraneoplastic pemphigus and their correlations with symptoms and prognosis. Br. J. Dermatol. 2015, 172, 968–975. [Google Scholar] [CrossRef]
- Frew, J.W.; Murrell, D.F. Paraneoplastic pemphigus (paraneoplastic autoimmune multiorgan syndrome): Clinical presentations and pathogenesis. Dermatol. Clin. 2011, 29, 419–425. [Google Scholar] [CrossRef]
- Antiga, E.; Bech, R.; Maglie, R.; Genovese, G.; Borradori, L.; Bockle, B.; Caproni, M.; Caux, F.; Chandran, N.S.; Corrà, A.; et al. S2k guidelines on the management of paraneoplastic pemphigus/paraneoplastic autoimmune multiorgan syndrome initiated by the European Academy of Dermatology and Venereology (EADV). J. Eur. Acad. Dermatol. Venereol. 2023, 37, 1118–1134. [Google Scholar] [CrossRef] [PubMed]
- Joly, P.; Richard, C.; Gilbert, D.; Courville, P.; Chosidow, O.; Roujeau, J.C.; Beylot-Barry, M.; D’incan, M.; Martel, P.; Lauret, P.; et al. Sensitivity and specificity of clinical, histologic, and immunologic features in the diagnosis of paraneoplastic pemphigus. J. Am. Acad. Dermatol. 2000, 43, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Amagai, M.; Watanabe, K.; Chorzelski, T.P.; Bhogal, B.S.; Black, M.M.; Stevens, H.P.; Boorsma, D.M.; Korman, N.J.; Gamou, S. Characterization of paraneoplastic pemphigus autoantigens by immunoblot analysis. J. Investig. Dermatol. 1995, 104, 829–834. [Google Scholar] [CrossRef]
- Probst, C.; Schlumberger, W.; Stöcker, W.; Recke, A.; Schmidt, E.; Hashimoto, T.; Zhu, X.J.; Zillikens, D.; Komorowski, L. Development of ELISA for the specific determination of autoantibodies against envoplakin and periplakin in paraneoplastic pemphigus. Clin. Chim. Acta 2009, 410, 13–18. [Google Scholar] [CrossRef]
- Huang, S.; Anderson, H.J.; Lee, J.B. Paraneoplastic pemphigus/paraneoplastic autoimmune multiorgan syndrome: Part II. Diagnosis and management. J. Am. Acad. Dermatol. 2024, 91, 13–22. [Google Scholar] [CrossRef]
- Brandt, O.; Rafei, D.; Podstawa, E.; Niedermeier, A.; Jonkman, M.F.; Terra, J.B.; Hein, R.; Hertl, M.; Pas, H.H.; Müller, R. Differential IgG recognition of desmoglein 3 by paraneoplastic pemphigus and pemphigus vulgaris sera. J. Investig. Dermatol. 2012, 132, 1738–1741. [Google Scholar] [CrossRef] [PubMed]
- Mimouni, D.; Anhalt, G.J.; Lazarova, Z.; Aho, S.; Kazerounian, S.; Kouba, D.J.; Mascaro, J.M.; Nousari, H.C. Paraneoplastic pemphigus in children and adolescents. Br. J. Dermatol. 2002, 147, 725–732. [Google Scholar] [CrossRef]
- Camisa, C.; Helm, T.N. Paraneoplastic pemphigus is a distinct neoplasia-induced autoimmune disease. Arch. Dermatol. 1993, 129, 883–886. [Google Scholar] [CrossRef]
- Barnadas, M.; Roe, E.; Brunet, S.; Garcia, P.; Bergua, P.; Pimentel, L.; Puig, L.; Francia, A.; García, R.; Gelpí, C.; et al. Therapy of paraneoplastic pemphigus with Rituximab: A case report and review of literature. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Boyce, M.; Flower, C. Tripe Palms. N. Engl. J. Med. 2022, 387, 355. [Google Scholar] [CrossRef]
- Khoschbin, T.; Löser, C.; Dippel, E. Paraneoplastische Hauterkrankungen. Internist 2019, 60, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Yu, P.-J.; Liu, Z.-L.; Zhu, S.-M.; Zhang, C.-W. Malignant acanthosis nigricans with Leser-Trélat sign and tripe palms: A case report. World J. Clin. Cases 2020, 8, 5632–5638. [Google Scholar] [CrossRef]
- Chantarojanasiri, T.; Buranathawornsom, A.; Sirinawasatien, A. Diffuse Esophageal Squamous Papillomatosis: A Rare Disease Associated with Acanthosis Nigricans and Tripe Palms. Case Rep. Gastroenterol. 2020, 14, 702–706. [Google Scholar] [CrossRef]
- Moore, R.L.; Devere, T.S. Epidermal manifestations of internal malignancy. Dermatol. Clin. 2008, 26, 17–29. [Google Scholar] [CrossRef]
- Schmidt, M.F.; Schaller, M.; Schmitt, L.C. Hyperplasie-assoziierte obligate Paraneoplasien der Haut. Hautarzt 2021, 72, 295–298. [Google Scholar] [CrossRef]
- Chosidow, O.; Bécherel, P.A.; Piette, J.C.; Arock, M.; Debré, P.; Francès, C. Tripe palms associated with systemic mastocytosis: The role of transforming growth factor-alpha and efficacy of interferon-alfa. Br. J. Dermatol. 1998, 138, 698–703. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, C.; Zhang, Z. Primary hypertrophic osteoarthropathy: An update. Front. Med. 2013, 7, 60–64. [Google Scholar] [CrossRef]
- Butacu, A.-I.; Toma, C.; Negulet, I.-E.; Manole, I.; Banica, A.N.; Plesea, A.; Badircea, I.A.; Iancu, I.; Tiplica, G.-S. Updates on Psoriasis in Special Areas. J. Clin. Med. 2024, 13, 7549. [Google Scholar] [CrossRef]
- Das, A.; Datta, D.; Kassir, M.; Wollina, U.; Galadari, H.; Lotti, T.; Jafferany, M.; Grabbe, S.; Goldust, M. Acanthosis nigricans: A review. J. Cosmet. Dermatol. 2020, 19, 1857–1865. [Google Scholar] [CrossRef]
- Charli-Joseph, Y.; Chong, K.; Ai, W.Z.; Leboit, P.E.; Pincus, L.B. Paraneoplastic acanthosis nigricans paralleling disease course in a patient with mycosis fungoides. Eur. J. Dermatol. 2013, 23, 907–908. [Google Scholar] [CrossRef]
- Cheng, E.; Roy, D.B.; Magro, C.M. A case of acanthosis nigricans coexisting with mycosis fungoides. Dermatol. Online J. 2015, 21, 11. [Google Scholar] [CrossRef]
- Schweitzer, W.J.; Goldin, H.M.; Bronson, D.M.; Brody, P.E. Acanthosis nigricans associated with mycosis fungoides. J. Am. Acad. Dermatol. 1988, 19, 951–953. [Google Scholar] [CrossRef]
- Kim, D.J.; van Truong, S.; Paik, A.; Kraus, C.; Harris, R.M.; Barr, R.J.; Linden, K.G.; Smith, J. A case of acanthosis nigricans-like mycosis fungoides. JAAD Case Rep. 2021, 16, 144–148. [Google Scholar] [CrossRef]
- Spigariolo, C.B.; Berti, E.; Cerri, A.; Venegoni, L.; Croci, G.; Violetti, S.A. T follicular helper phenotype mycosis fungoides associated with acanthosis nigricans. J. Cutan. Pathol. 2023, 50, 420–424. [Google Scholar] [CrossRef]
- Mohta, A.; Sarkar, R.; Narayan, R.V.; Deoghare, S.; Arora, A. Terra Firma-Forme Dermatosis-More Than Just Dirty. Indian Dermatol. Online J. 2024, 15, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Geber, A.; Hadžavdić, A.; Ljubojević Hadžavdić, S. The Leser-Trélat Sign in a Patient with Gastric Adenocarcinoma. Acta Dermatovenerol. Croat. 2023, 31, 51–52. [Google Scholar]
- Narala, S.; Cohen, P.R. Cutaneous T-cell lymphoma-associated Leser-Trélat sign: Report and world literature review. Dermatol. Online J. 2017, 23, 4. [Google Scholar] [CrossRef]
- Pereira, R.; Carvalho, S.D.; Cruz, J.F. Leser-Trélat sign in metastatic melanoma to pleura. BMJ Case Rep. 2019, 12, e228834. [Google Scholar] [CrossRef] [PubMed]
- Sardon, C.; Dempsey, T. The Leser-Trélat sign. Cleve. Clin. J. Med. 2017, 84, 918. [Google Scholar] [CrossRef]
- Gori, N.; Esposito, I.; Del Regno, L.; D’Amore, A.; Peris, K.; Di Stefani, A. Leser-Trélat sign as a rare manifestation of cutaneous melanoma. Dermatol. Rep. 2020, 12, 8665. [Google Scholar] [CrossRef]
- Lebas, E.; Quatresooz, P.; Arrese, J.E.; Nikkels, A.F. Eruptive Seborrheic Keratoses Restricted to Plaque/Patch-Stage Mycosis Fungoides. Case Rep. Dermatol. 2017, 9, 35–39. [Google Scholar] [CrossRef]
- Vidulich, K.A.; Rady, P.L.; He, Q.; Tyring, S.K.; Duvic, M. Detection of high-risk human papillomaviruses in verrucae of patients with mycosis fungoides and Sézary syndrome: A case series. Int. J. Dermatol. 2009, 48, 598–602. [Google Scholar] [CrossRef]
- Puig, L.; Musulén, E.; Fernández-Figueras, M.T.; Miralles, J.; Sitjas, D.; de Moragas, J.M. Mycosis fungoides associated with unusual epidermal hyperplasia. Clin. Exp. Dermatol. 1996, 21, 61–64. [Google Scholar] [CrossRef]
- West, L.; Carlson, M.; Wallis, L.; Goff, H.W. The Sign of Leser-Trelát and Malignant Acanthosis Nigricans Associated with Fallopian Tube Carcinoma. Obstet. Gynecol. 2018, 132, 1116–1119. [Google Scholar] [CrossRef]
- Suening, B.S.; Neidenbach, P.J. The Pseudo-Sign of Leser-Trélat: A Rare Presentation. Cureus 2023, 15, e35155. [Google Scholar] [CrossRef]
- Patton, T.; Zirwas, M.; Nieland-Fisher, N.; Jukic, D. Inflammation of seborrheic keratoses caused by cytarabine: A pseudo sign of Leser-Trelat. J. Drugs Dermatol. 2004, 3, 565–566. [Google Scholar] [PubMed]
- Handjani, F.; Radanfar, R.; Sepaskhah, M.; Dehdari Ebrahimi, N. COVID-19 infection and Leser-Trelat sign: Is there an association? Clin. Case Rep. 2023, 11, e7638. [Google Scholar] [CrossRef]
- Sahin, M.T.; Oztürkcan, S.; Ermertcan, A.T.; Saçar, T.; Türkdogan, P. Transient eruptive seborrhoeic keratoses associated with erythrodermic pityriasis rubra pilaris. Clin. Exp. Dermatol. 2004, 29, 554–555. [Google Scholar] [CrossRef]
- Gleeson, C.M.; Chan, I.; Griffiths, W.A.D.; Bunker, C.B. Eruptive seborrhoeic keratoses associated with erythrodermic pityriasis rubra pilaris. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 217–218. [Google Scholar] [CrossRef]
- D’Souza, M.; Garg, B.R.; Reddy, B.S.; Ratnakar, C. Lepromatous leprosy with extensive truncal seborrheic keratoses and acral verruca vulgaris. Int. J. Dermatol. 1994, 33, 498–500. [Google Scholar] [CrossRef]
- Inamadar, A.C.; Palit, A. Eruptive seborrhoeic keratosis in human immunodeficiency virus infection: A coincidence or ‘the sign of Leser-Trélat’? Br. J. Dermatol. 2003, 149, 435–436. [Google Scholar] [CrossRef]
- Didona, D.; Juratli, H.A.; Scarsella, L.; Eming, R.; Hertl, M. The polymorphous spectrum of dermatomyositis: Classic features, newly described skin lesions, and rare variants. Eur. J. Dermatol. 2020, 30, 229–242. [Google Scholar] [CrossRef]
- Didona, D.; Solimani, F.; Caposiena Caro, R.D.; Sequeira Santos, A.M.; Hinterseher, J.; Kussini, J.; Cunha, T.; Hertl, M.; Didona, B. Dermatomyositis: A comprehensive review of clinical manifestations, serological features, and therapeutic approaches. Ital. J. Dermatol. Venerol. 2023, 158, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Kouranloo, K.; Dey, M.; Elwell, H.; Nune, A. A systematic review of the incidence, management and prognosis of new-onset autoimmune connective tissue diseases after COVID-19. Rheumatol. Int. 2023, 43, 1221–1243. [Google Scholar] [CrossRef]
- Callen, J.P. Dermatomyositis. Lancet 2000, 355, 53–57. [Google Scholar] [CrossRef]
- Musai, J.; Mammen, A.L.; Pinal-Fernandez, I. Recent Updates on the Pathogenesis of Inflammatory Myopathies. Curr. Rheumatol. Rep. 2024, 26, 421–430. [Google Scholar] [CrossRef]
- Bohan, A.; Peter, J.B. Polymyositis and dermatomyositis (first of two parts). N. Engl. J. Med. 1975, 292, 344–347. [Google Scholar] [CrossRef]
- Bohan, A.; Peter, J.B. Polymyositis and dermatomyositis (second of two parts). N. Engl. J. Med. 1975, 292, 403–407. [Google Scholar] [CrossRef]
- Mainetti, C.; Terziroli Beretta-Piccoli, B.; Selmi, C. Cutaneous Manifestations of Dermatomyositis: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2017, 53, 337–356. [Google Scholar] [CrossRef]
- Leatham, H.; Schadt, C.; Chisolm, S.; Fretwell, D.; Chung, L.; Callen, J.P.; Fiorentino, D. Evidence supports blind screening for internal malignancy in dermatomyositis: Data from 2 large US dermatology cohorts. Medicine 2018, 97, e9639. [Google Scholar] [CrossRef] [PubMed]
- Requena, C.; Sánchez-Romero, E.; Nagore, E.; Sanmartín, O. Dermatomiositis paraneoplásica: Una serie de 25 casos de un hospital oncológico. Actas Dermosifiliogr. 2024. [Google Scholar] [CrossRef] [PubMed]
- Ramos-E-Silva, M.; Lima Pinto, A.P.F.; Pirmez, R.; Cuzzi, T.; Carneiro, S. Dermatomyositis-Part 2: Diagnosis, Association with Malignancy, and Treatment. Skinmed 2016, 14, 354–358. [Google Scholar] [PubMed]
- Callen, J.P. When and how should the patient with dermatomyositis or amyopathic dermatomyositis be assessed for possible cancer? Arch. Dermatol. 2002, 138, 969–971. [Google Scholar] [CrossRef]
- Stockton, D.; Doherty, V.R.; Brewster, D.H. Risk of cancer in patients with dermatomyositis or polymyositis, and follow-up implications: A Scottish population-based cohort study. Br. J. Cancer 2001, 85, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jia, X.; Sun, X.; Shi, L.; Lin, F.; Gan, Y.; Zhang, X.; Gao, X.; Miao, M.; Hong, D.; et al. Risk factors for cancer-associated myositis: A large-scale multicenter cohort study. Int. J. Rheum. Dis. 2021, 24, 268–273. [Google Scholar] [CrossRef]
- Didona, D.; Juratli, H.A.; Scarsella, L.; Keber, U.; Eming, R.; Hertl, M. Amyopathic and anti-TIF1 gamma-positive dermatomyositis: Analysis of a monocentric cohort and proposal to update diagnostic criteria. Eur. J. Dermatol. 2020, 30, 279–288. [Google Scholar] [CrossRef]
- Didona, D.; Mostaccioli, S.; Paolino, G.; Cantisani, C.; Caposiena Caro, R.D.; Viti, G.; Didona, B. Shiitake dermatosis in a Caucasian woman. G. Ital. Dermatol. Venereol. 2018, 153, 586–588. [Google Scholar] [CrossRef]
- Maverakis, E.; Marzano, A.V.; Le, S.T.; Callen, J.P.; Brüggen, M.-C.; Guenova, E.; Dissemond, J.; Shinkai, K.; Langan, S.M. Pyoderma gangrenosum. Nat. Rev. Dis. Primers 2020, 6, 81. [Google Scholar] [CrossRef]
- Moreno-Artero, E.; Torrelo, A. Pediatric Neutrophilic Dermatoses. Dermatol. Clin. 2024, 42, 267–283. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Balgobind, A.; Strunk, A.; Garg, A.; Alloo, A. Prevalence estimates for pyoderma gangrenosum in the United States: An age- and sex-adjusted population analysis. J. Am. Acad. Dermatol. 2020, 83, 425–429. [Google Scholar] [CrossRef]
- Su, R.; Tan, Y.; Peng, S. Clinical characteristics of pyoderma gangrenosum: Case series and literature review. Medicine 2024, 103, e39634. [Google Scholar] [CrossRef]
- Alavi, A.; French, L.E.; Davis, M.D.; Brassard, A.; Kirsner, R.S. Pyoderma Gangrenosum: An Update on Pathophysiology, Diagnosis and Treatment. Am. J. Clin. Dermatol. 2017, 18, 355–372. [Google Scholar] [CrossRef] [PubMed]
- Maronese, C.A.; Derlino, F.; Moltrasio, C.; Cattaneo, D.; Iurlo, A.; Marzano, A.V. Neutrophilic and eosinophilic dermatoses associated with hematological malignancy. Front. Med. 2023, 10, 1324258. [Google Scholar] [CrossRef]
- Maglie, R.; Genovese, G.; Solimani, F.; Guglielmo, A.; Pileri, A.; Portelli, F.; Hertl, M.; Marzano, A.V.; Antiga, E. Immune-Mediated Dermatoses in Patients with Haematological Malignancies: A Comprehensive Review. Am. J. Clin. Dermatol. 2020, 21, 833–854. [Google Scholar] [CrossRef]
- Shah, M.; Sachdeva, M.; Gefri, A.; Jfri, A. Paraneoplastic pyoderma gangrenosum in solid organ malignancy: A literature review. Int. J. Dermatol. 2020, 59, 154–158. [Google Scholar] [CrossRef]
- Łyko, M.; Ryguła, A.; Kowalski, M.; Karska, J.; Jankowska-Konsur, A. The Pathophysiology and Treatment of Pyoderma Gangrenosum-Current Options and New Perspectives. Int. J. Mol. Sci. 2024, 25, 2440. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.J.; Allantaz, F.; Bennett, L.; Zhang, D.; Gao, X.; Wood, G.; Kastner, D.L.; Punaro, M.; Aksentijevich, I.; Pascual, V.; et al. Clinical, Molecular, and Genetic Characteristics of PAPA Syndrome: A Review. Curr. Genom. 2010, 11, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.M.; Sullivan, G.P.; Clancy, D.M.; Afonina, I.S.; Kulms, D.; Martin, S.J. Neutrophil-Derived Proteases Escalate Inflammation through Activation of IL-36 Family Cytokines. Cell Rep. 2016, 14, 708–722. [Google Scholar] [CrossRef]
- Kastner, D.L.; Aksentijevich, I.; Goldbach-Mansky, R. Autoinflammatory disease reloaded: A clinical perspective. Cell 2010, 140, 784–790. [Google Scholar] [CrossRef]
- Ahn, C.; Negus, D.; Huang, W. Pyoderma gangrenosum: A review of pathogenesis and treatment. Expert Rev. Clin. Immunol. 2018, 14, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.S.; Ormerod, A.D.; Craig, F.E.; Greenlaw, N.; Norrie, J.; Mitchell, E.; Mason, J.M.; Johnston, G.A.; Wahie, S.; Williams, H.C. Clinical outcomes and response of patients applying topical therapy for pyoderma gangrenosum: A prospective cohort study. J. Am. Acad. Dermatol. 2016, 75, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Sweet, R.D. An Acute Febrile Neutrophilic Dermatosis. Br. J. Dermatol. 1964, 76, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.R. Sweet’s syndrome—A comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J. Rare Dis. 2007, 2, 34. [Google Scholar] [CrossRef]
- Calabrese, L.; Satoh, T.K.; Aoki, R.; Rubegni, G.; Neulinger-Mũnoz, M.; Stadler, P.C.; French, L.E.; Rubegni, P. Sweet syndrome: An update on clinical aspects, pathophysiology, and treatment. Ital. J. Dermatol. Venerol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.R.; Talpaz, M.; Kurzrock, R. Malignancy-associated Sweet’s syndrome: Review of the world literature. J. Clin. Oncol. 1988, 6, 1887–1897. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.R.; Kurzrock, R. Sweet’s syndrome and malignancy. Am. J. Med. 1987, 82, 1220–1226. [Google Scholar] [CrossRef]
- Cowen, E.A.; Barrios, D.M.; Pulitzer, M.P.; Moy, A.P.; Dusza, S.W.; de Wolf, S.; Geyer, M.B.; Markova, A. Acute Febrile Neutrophilic Dermatosis (Sweet Syndrome) in Acute Myeloid Leukemia Patients: A 28-Year Institutional Experience. Acta Haematol. 2024, 147, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Marzano, A.V.; Fanoni, D.; Antiga, E.; Quaglino, P.; Caproni, M.; Crosti, C.; Meroni, P.L.; Cugno, M. Expression of cytokines, chemokines and other effector molecules in two prototypic autoinflammatory skin diseases, pyoderma gangrenosum and Sweet’s syndrome. Clin. Exp. Immunol. 2014, 178, 48–56. [Google Scholar] [CrossRef]
- Orfaly, V.E.; Shakshouk, H.; Heath, M.; Hamilton, A.; Ortega-Loayza, A.G. Sweet Syndrome: A Review of Published Cases. Dermatology 2023, 239, 664–669. [Google Scholar] [CrossRef]

| Criterion |
|---|
| The onset of dermatosis must be related to the beginning of the neoplasia |
| Skin manifestations and neoplasia must show a parallel course |
| The dermatosis is not related to a genetic syndrome |
| A specific dermatosis occurs in patients with a specific tumor |
| The dermatosis is rare in the general population |
| High frequency of association between the dermatosis and the underlying neoplasia |
| Obligate Paraneoplastic Dermatosis | Neoplasia | Involved Molecular Factors |
| Necrolytic migratory erythema | Glucagonoma and small cell lung cancer | Increased level of arachidonic acid Deficit of niacin |
| Paraneoplastic autoimmune multiorgan syndrome | Castleman’s disease, chronic lymphocytic leukemia, non-Hodgkin’s lymphoma, thymoma | |
| Facultative Paraneoplastic Dermatosis | Neoplasia | |
| Leser–Trélat’s Sign | Colorectal and gastric carcinoma | EGF-α, IGF-1, TGF-α |
| Malignant acanthosis nigricans | Gastrointestinal adenocarcinoma, hepatocellular carcinoma, pancreatic adenocarcinoma | EGF-α, FGF, IGF-1, MSH-α, TGF-α |
| Paraneoplastic dermatomyositis | Lung and ovarian adenocarcinoma | |
| Pyoderma gangrenosum | Leukemia, myelodysplastic syndrome, myeloma, | Fas, FasL, IL-1b, IL-8, IL-17, IL-23 |
| Sweet’s syndrome | Acute myelogenous leukemia, myelodysplastic syndrome | G-CSF, GM-CSF, IL-1, IL-3, IL-6, IL-8 |
| Tripe palms | Gastrointestinal and lung adenocarcinoma | EGF-α and TGF-α |
| Other Paraneoplastic Dermatoses | Neoplasia | |
| Acquired hypertrichosis lanuginosa | Breast, colorectal and lung adenocarcinoma | FGF |
| Acquired ichthyosis | Hodgkin’s lymphoma and lung adenocarcinoma | Impaired vitamin A metabolism TGF-α |
| Paraneoplastic acrokeratosis | Squamous cell carcinoma of several anatomic districts (e.g., esophagus, larynx, lung, tongue) | EGFR, IGF-1, TGF-α |
| Clinical Criteria | Major Laboratory Criteria | Minor Laboratory Criteria |
|---|---|---|
| Underlying neoplasia | Detection of autoantibodies against desmoplakin, envoplakin, periplakin and/or A2ML1 by ELISA, IB or IP | Detection of linear or granular IgG and/or C3 along the BMZ by DIF |
| Chronic erosive mucositis | Detection of intercellular IgG deposition by IIF on rat bladder | Staining of the cytoplasmatic cell membrane of keratinocytes by IIF |
| Polymorphic skin lesions, including pemphigus-like and lichenoid lesions | Detection of anti-Dsg autoantibodies and at least one of the following autoantibodies (anti-desmocollin, anti-epiplakin, anti-plectin, anti-BP180 and anti-BP230) by ELISA, IB or IP |
| Criterion | Details |
|---|---|
| 1. Symmetric proximal muscle weakness | With or without dysphagia and/or diaphragmatic weakness |
| 2. Elevation of skeletal muscle enzyme levels | Creatine kinase (CK); aspartate transaminase (AST); alanine transaminase (ALT); lactate dehydrogenase (LDH) |
| 3. Abnormal electromyography results | Polyphasic motor unit action potentials (MUAPs); fibrillation potentials; positive sharp waves; increased insertional irritability; repetitive high-frequency discharges |
| 4. Muscle biopsy abnormalities | Histopathologic findings of muscle degeneration/regeneration/necrosis; interstitial mononuclear infiltrates |
| 5. Typical skin rash of dermatomyositis | Heliotrope rash or Gottron’s papules |
| Major Criteria * |
| Abrupt onset of painful erythematous plaques or nodules |
| Histopathologic evidence of a dense neutrophilic infiltrate without evidence of leukocytoclastic vasculitis |
| Minor Criteria |
| Fever |
Association with:
|
| Excellent clinical response to systemic corticosteroids or potassium iodide |
Elevation of three of the four laboratory values:
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Didona, D.; Rallo, A.; Carugno, A.; Paolino, G. Paraneoplastic Dermatoses: A Clue for Underlying Malignancies. J. Clin. Med. 2025, 14, 1014. https://doi.org/10.3390/jcm14031014
Didona D, Rallo A, Carugno A, Paolino G. Paraneoplastic Dermatoses: A Clue for Underlying Malignancies. Journal of Clinical Medicine. 2025; 14(3):1014. https://doi.org/10.3390/jcm14031014
Chicago/Turabian StyleDidona, Dario, Alessandra Rallo, Andrea Carugno, and Giovanni Paolino. 2025. "Paraneoplastic Dermatoses: A Clue for Underlying Malignancies" Journal of Clinical Medicine 14, no. 3: 1014. https://doi.org/10.3390/jcm14031014
APA StyleDidona, D., Rallo, A., Carugno, A., & Paolino, G. (2025). Paraneoplastic Dermatoses: A Clue for Underlying Malignancies. Journal of Clinical Medicine, 14(3), 1014. https://doi.org/10.3390/jcm14031014








