Long-Term Results in Minimally Invasive Non-Resectional Mitral Valve Repair for Barlow Mitral Valve Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Patient Population
2.3. Surgical Technique
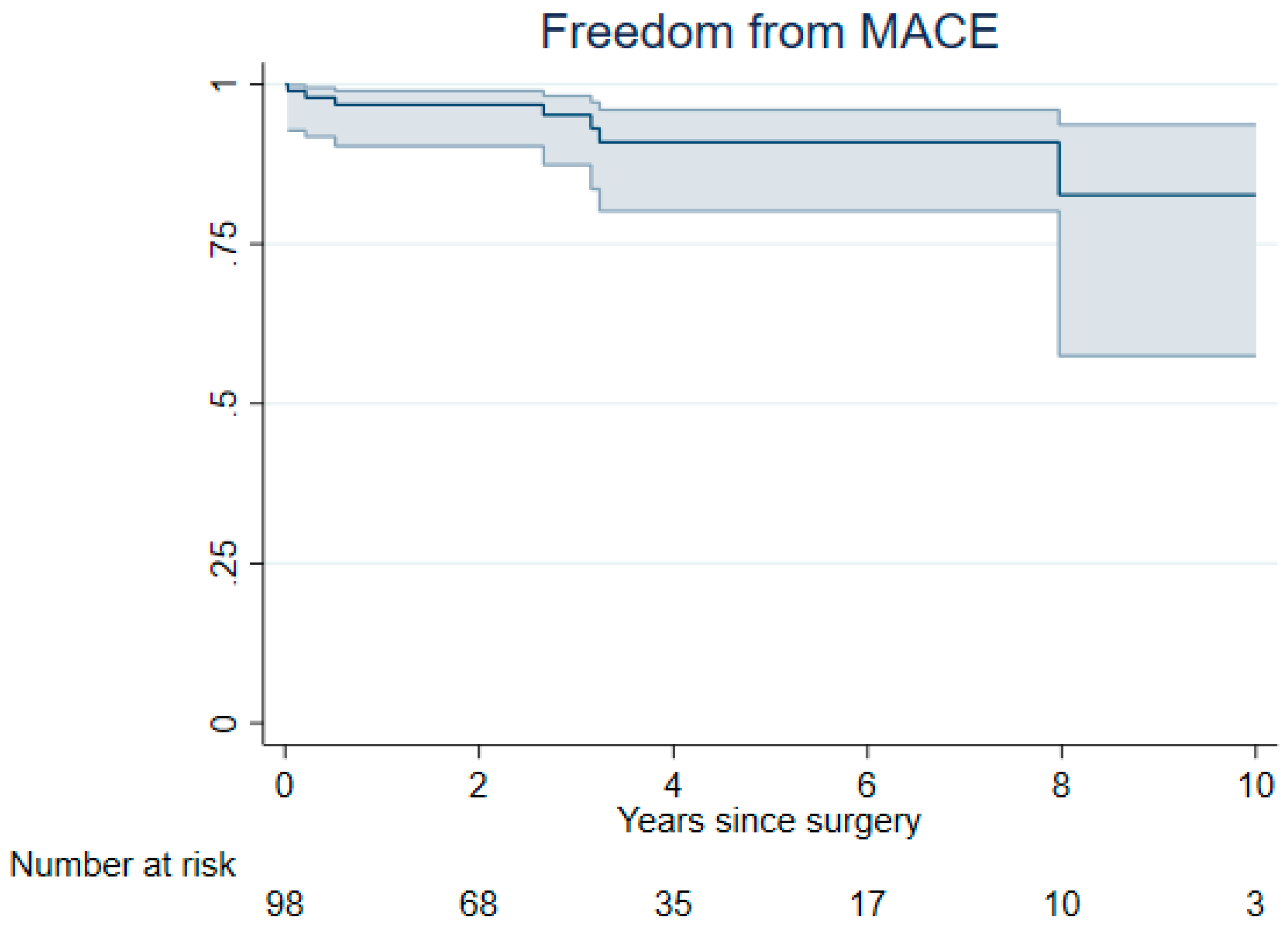
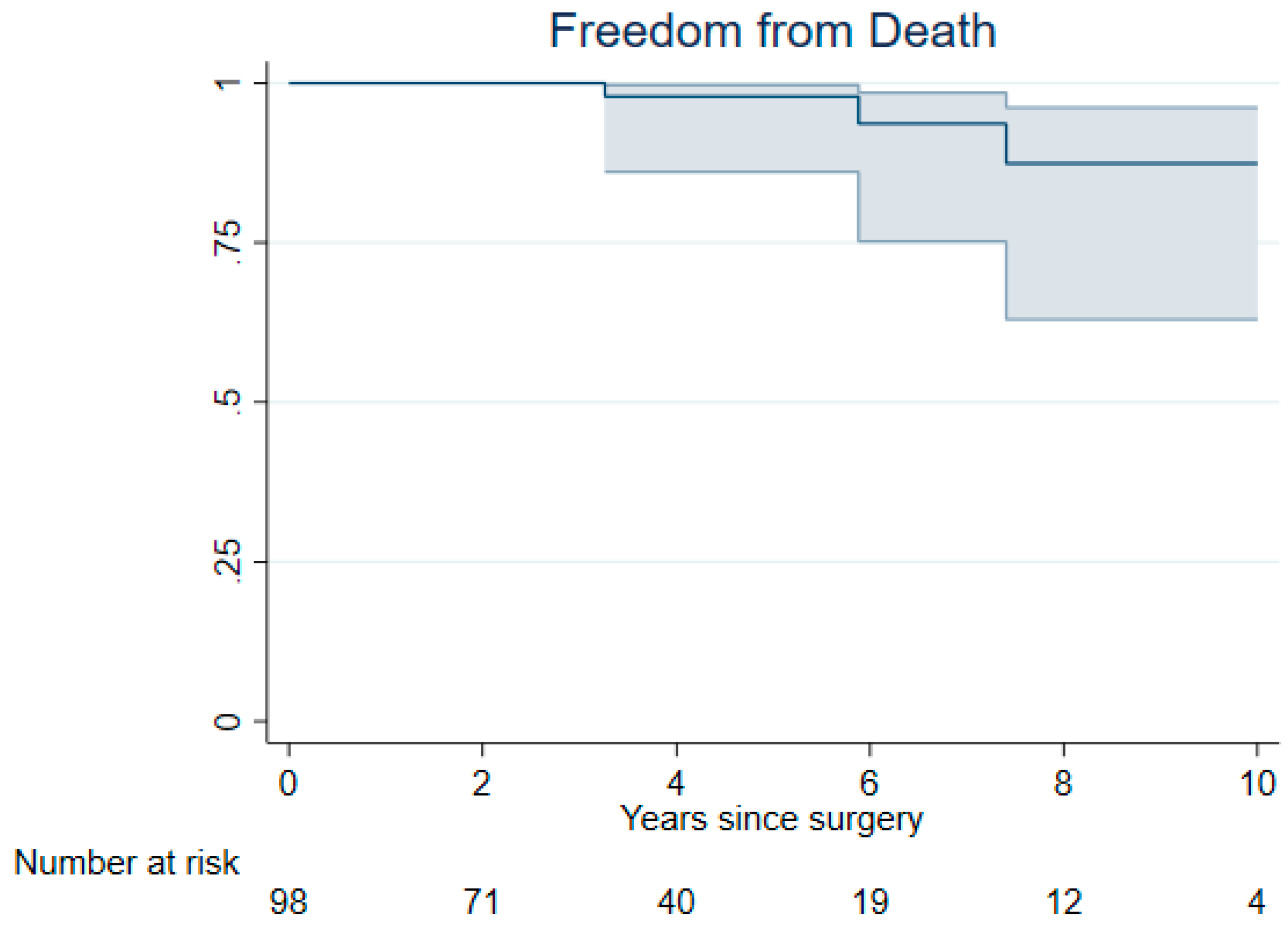
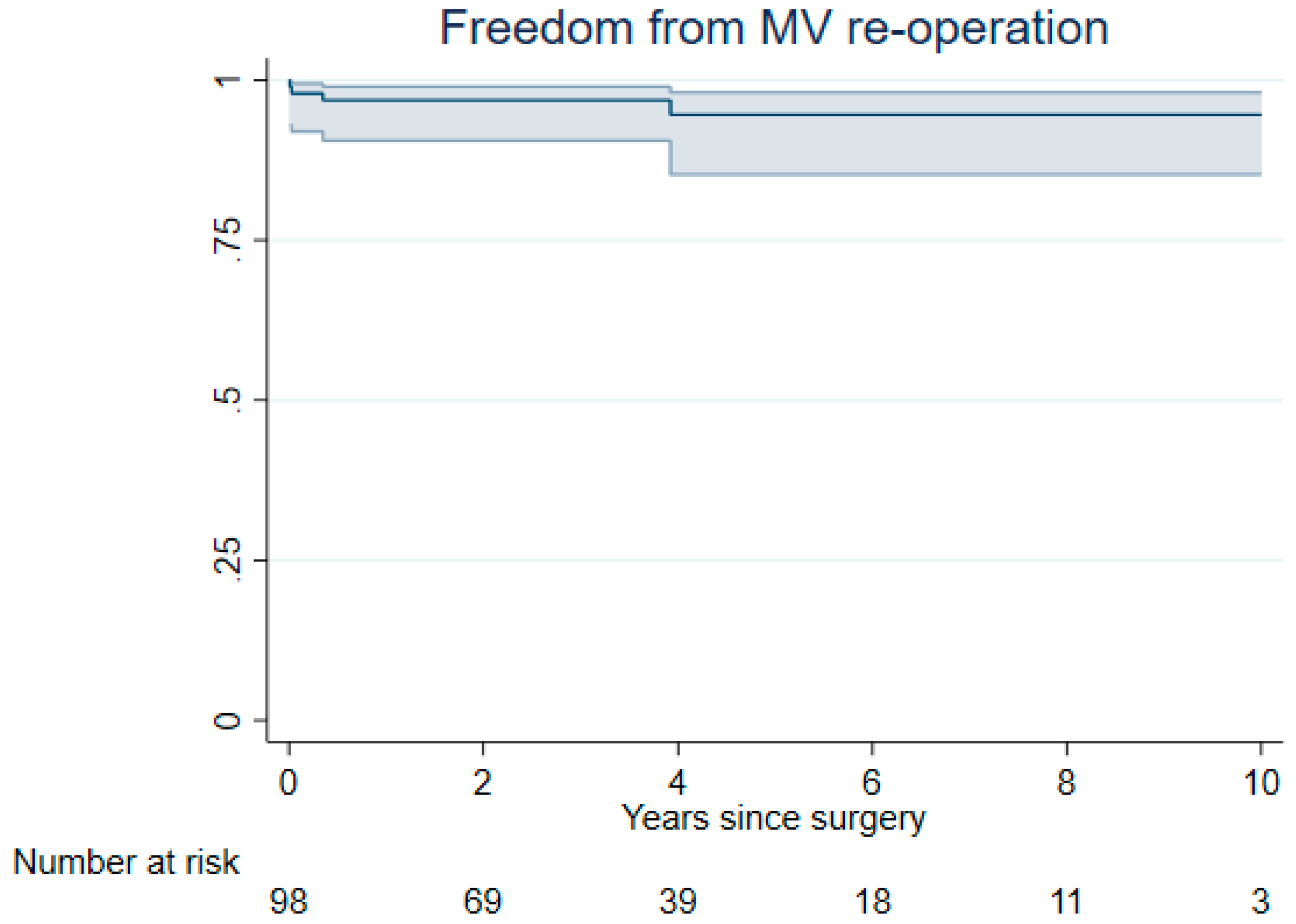
3. Results
3.1. Early Outcomes
3.2. Late Outcomes
4. Discussion
5. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LV | left ventricle |
| MV | mitral valve |
| MVR | mitral valve regurgitation |
| NYHA | New York Heart Association |
| MIMVS | minimally invasive mitral valve surgery |
| DM | Diabetes mellitus |
| IQR | interquartile range |
| MRI | magnetic resonance imaging |
| TEE | transthoracic echocardiography |
| CT | computed tomography angiogram |
| MACE | combined major adverse cardiovascular event |
| PTFE | polytetraflouroethylene |
References
- Enriquez-Sarano, M.; Akins, C.W.; Vahanian, A. Mitral regurgitation. Lancet 2009, 373, 1382–1394. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.H.; Rosenhek, R.; Falk, V. Degenerative mitral valve regurgitation: Best practice revolution. Eur. Heart J. 2010, 31, 1958–1966. [Google Scholar] [CrossRef]
- Anyanwu, A.C.; Adams, D.H. Etiologic classification of degenerative mitral valve disease: Barlow’s disease and fibroelastic deficiency. Semin. Thorac. Cardiovasc. Surg. 2007, 19, 90–96. [Google Scholar] [CrossRef]
- Anyanwu, A.C.; Adams, D.H. Bileaflet repair for barlow syndrome. Semin. Thorac. Cardiovasc. Surg. 2010, 22, 179–183. [Google Scholar] [CrossRef]
- Carpentier, A. Cardiac valve surgery–the “French correction”. J. Thorac. Cardiovasc. Surg. 1983, 86, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Fasol, R.; Mahdjoobian, K. Repair of mitral valve billowing and prolapse (Barlow): The surgical technique. Ann. Thorac. Surg. 2002, 74, 602–605. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, O.; Maisano, F. An effective technique to correct anterior mitralleaflet prolapse. J. Card. Surg. 1999, 14, 468–470. [Google Scholar] [CrossRef]
- Borger, M.A.; Mohr, F.W. Repair of bileaflet prolapse in Barlow syndrome. Semin. Thorac. Cardiovasc. Surg. 2010, 22, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Lawrie, G.M.; Earle, E.A.; Earle, N.R. Nonresectional repair of the barlow mitral valve: Importance of dynamic annular evaluation. Ann. Thorac. Surg. 2009, 88, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Suri, R.M.; Burkhart, H.M.; Schaff, H.V. A novel method of leaflet reconstruction after triangular resection for posterior mitral valve prolapse. Ann. Thorac. Surg. 2010, 89, e53–e56. [Google Scholar] [CrossRef]
- Zekry, S.B.; Spiegelstein, D.; Sternik, L.; Lev, I.; Kogan, A.; Kuperstein, R.; Raanani, E. Simple repair approach for mitral regurgitation in Barlow disease. J. Thorac. Cardiovas Surg. 2015, 150, 1071–1077.e1. [Google Scholar] [CrossRef] [PubMed]
- De Paulis, R.; Maselli, D.; Salica, A.; Leonetti, S.; Guerrieri Wolf, L.; Weltert, L.; Nardella, S.; Bellisario, A. Mitral repair with the sole use of a semi-rigid band in a sub-population of patients with Barlow’s disease: A 4-year follow-up with stress echocardiography. Interact. CardioVasc Thorac. Surg. 2015, 21, 316–321. [Google Scholar] [CrossRef]
- Immohr, M.B.; Sugimura, Y.; Kröpil, P.; Aubin, H.; Minol, J.P.; Albert, A.; Boeken, U.; Lichtenberg, A.; Akhyari, P. Impact of standardized computed tomographic angiography for minimally invasive mitral and tricuspid valve surgery. J. Cardiothorac. Surg. 2021, 16, 34. [Google Scholar] [CrossRef]
- Berdajs, D.; Miazza, J.; Koechlin, L.; Gahl, B.; Reuthebuch, O.; Eckstein, F. Minimally Invasive Nonresectional Mitral Valve Repair Long-term Results. Can. J. Cardiol. 2023, 39, 990–996. [Google Scholar] [CrossRef] [PubMed]
- VARC-3 Writing Committee; Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; et al. Valve Academic Research Consortium 3: Updated Endpoint Definitions for Aortic Valve Clinical Research. J. Am. Coll. Cardiol. 2021, 77, 2717–2746. [Google Scholar] [CrossRef]
- Perier, P.; Hohenberger, W.; Lakew, F.; Batz, G.; Urbanski, P.; Zacher, M.; Diegeler, A. Toward a new paradigm for the reconstruction of posterior leaflet prolapse: Midterm results of the “respect rather than resect” approach. Ann. Thorac. Surg. 2008, 86, 718–725. [Google Scholar] [CrossRef] [PubMed]
- von Oppell, U.O.; Mohr, F.W. Chordal replacement for both minimally invasive and conventional mitral valve surgery using premeasured Gore-Tex loops. Ann. Thorac. Surg. 2000, 70, 2166–2168. [Google Scholar] [CrossRef] [PubMed]
- Grewal, J.; Suri, R.; Mankad, S.; Tanaka, A.; Mahoney, D.W.; Schaff, H.V.; Miller, F.A.; Enriquez-Sarano, M. Mitral annular dynamics in myxomatous valve disease: New insights with real-time 3-dimensional echocardiography. Circulation 2010, 121, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, A.J.; Harrigan, P.; Popovic, A.D.; Weyman, A.E.; Levine, R.A. Papillary muscle traction in mitral valve prolapse: Quantitation by two-dimensional echocardiography. J. Am. Coll. Cardiol. 1992, 19, 564–571. [Google Scholar] [CrossRef]
- Raanani, E.; Schwammenthal, E.; Moshkovitz, Y.; Cohen, H.; Kogan, A.; Peled, Y.; Sternik, L.; Ram, E. Repair with annuloplasty only of balanced bileaflet mitral valve prolapse with severe regurgitation. Eur. J. Cardiothorac. Surg. 2021, 61, 908–916. [Google Scholar] [CrossRef]
- Adams, D.H.; Anyanwu, A.C.; Rahmanian, P.B.; Abascal, V.; Salzberg, S.P.; Filsoufi, F. Large annuloplasty rings facilitate mitral valve repair in Barlow’s disease. Ann. Thorac. Surg. 2006, 82, 2096–2100. [Google Scholar] [CrossRef]
- Pölzl, L.; Gollmann-Tepeköylü, C.; Nägele, F.; Cetin, K.; Spilka, J.; Holfeld, J.; Oezpeker, U.C.; Stastny, L.; Graber, M.; Hirsch, J.; et al. Five-year outcomes of different techniques for minimally invasive mitral valve repair in Barlow’s disease. Eur. J. Cardiothorac. Surg. 2024, 65, ezae213. [Google Scholar] [CrossRef] [PubMed]
- Borger, M.A.; Kaeding, A.F.; Seeburger, J.; Melnitchouk, S.; Hoebartner, M.; Winkfein, M.; Misfeld, M.; Mohr, F.W. Minimally invasive mitral valve repair in Barlow’s disease: Early and long-term results. J. Thorac. Cardiovasc. Surg. 2014, 148, 1379–1385. [Google Scholar] [CrossRef]
| Total (N = 98) | |
| Age | 59 (12) |
| Gender | |
| Female | 42 (43%) |
| Male | 56 (57%) |
| BMI | 24 (3.6) |
| Current smoker | 3 (3.1%) |
| Preoperative stroke | 4 (4.1%) |
| History of myocardial infarction | 2 (2%) |
| Renal impairment | 2(2%) |
| COPD | 2 (2.8%) |
| Hypertension | 44 (45%) |
| NYHA III or IV | 25 (26%) |
| Atrial fibrillation | 19 (19%) |
| EuroSCORE 2 | 0.87 (0.64 to 1.3) |
| Total (N = 98) | |
| In-hospital mortality | 0 (0%) |
| Cardiac-related death | 0 (0%) |
| Reoperation for bleeding | 8 (8.2%) |
| Atrial fibrillation at discharge | 23 (23%) |
| Pulmonary infection | 3 (3.1%) |
| Postoperative MI | 0 (0%) |
| Postoperative stroke | 0 (0%) |
| Postoperative MACE | 0 (0%) |
| Postoperative renal failure | 1 (1.0%) |
| Renal substitution therapy | 0 (0%) |
| Intubation > 72 h | 0 (0%) |
| Length of ICU stay | 2.0 (1.0 to 3.0) |
| Length of hospital stay | 8.0 (7.0 to 10) |
| Need for pacemaker implantation | 5 (5.1%) |
| MACE | Number of Events | Person Years | Rate per Patient-Year (95% CI) |
|---|---|---|---|
| Death | 3 | 399 | 0.75 (0.24 to 2.3) |
| Myocardial infarction | 4 | 382 | 1.05 (0.39 to 2.8) |
| Stroke | 4 | 384 | 1.04 (0.39 to 2.8) |
| Congestive heart failure | 0 | 399 | 0.00 (0.00 to 0.8) |
| Any MACE | 7 | 371 | 1.89 (0.90 to 4.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koch, N.A.; Chiappini, J.; Ihringer, L.M.; Caracioni, A.A.M.; Salikhanov, I.; Gahl, B.; Berdajs, D. Long-Term Results in Minimally Invasive Non-Resectional Mitral Valve Repair for Barlow Mitral Valve Disease. J. Clin. Med. 2025, 14, 1005. https://doi.org/10.3390/jcm14031005
Koch NA, Chiappini J, Ihringer LM, Caracioni AAM, Salikhanov I, Gahl B, Berdajs D. Long-Term Results in Minimally Invasive Non-Resectional Mitral Valve Repair for Barlow Mitral Valve Disease. Journal of Clinical Medicine. 2025; 14(3):1005. https://doi.org/10.3390/jcm14031005
Chicago/Turabian StyleKoch, Nicola A., Jonas Chiappini, Lisa M. Ihringer, Andrei A. M. Caracioni, Islam Salikhanov, Brigitta Gahl, and Denis Berdajs. 2025. "Long-Term Results in Minimally Invasive Non-Resectional Mitral Valve Repair for Barlow Mitral Valve Disease" Journal of Clinical Medicine 14, no. 3: 1005. https://doi.org/10.3390/jcm14031005
APA StyleKoch, N. A., Chiappini, J., Ihringer, L. M., Caracioni, A. A. M., Salikhanov, I., Gahl, B., & Berdajs, D. (2025). Long-Term Results in Minimally Invasive Non-Resectional Mitral Valve Repair for Barlow Mitral Valve Disease. Journal of Clinical Medicine, 14(3), 1005. https://doi.org/10.3390/jcm14031005






