1. Introduction
Early gastroesophageal carcinomas, including superficial esophageal squamous cell carcinomas (ESCCs), esophageal adenocarcinomas (EACs), adenocarcinomas of the gastroesophageal junction (GEJ), and gastric cancers (GCs), are intramucosal carcinomas or superficially invasive carcinomas without or with only minor risk for lymph node metastases [
1,
2]. Historically, esophagectomy or subtotal gastrectomy was the standard of care for these early cancers [
3,
4,
5]. However, although curative for most patients, esophagectomy is associated with significant morbidity and mortality and low quality of life postoperatively. The development of endoscopic resection (ER) techniques, with endoscopic mucosal resection (EMR) as first method described and applied in Japan during the 1980s, established local excision as an effective therapy for superficial gastric and esophageal cancers, later also expanding to other GI lesions [
6,
7,
8,
9,
10]. Despite its success, EMR presented limitations for larger or more complex lesions, prompting the evolution of endoscopic submucosal dissection (ESD) in Japan in the following decades [
7]. ESD enabled true En-bloc resection using electrosurgical knives, improving local control and pathological assessment for lesions not suitable for piecemeal EMR. Today, both EMR and ESD are integral tools in the endoscopic portfolio for the treatment of early upper gastrointestinal (GI) carcinomas. Continuous technical improvements and expansion of their indications worldwide has helped to significantly increase the quality of life of patients with localized gastroesophageal cancers who did not need to undergo surgery for an oncologically satisfactory treatment [
6,
7,
11,
12,
13,
14,
15].
Integration of clinical and pathological perspectives is essential for optimal patient care. Clinically, the identification of appropriate indications relies on careful endoscopic assessment and patient factors. From a pathological point of view, the meticulous examination of ESD specimens provides not only accurate histopathologic classification of the lesions but also precise evaluation of invasion depth, lymphovascular invasion, and margin status for malignant lesions [
16,
17,
18]. These histological risk factors predict the two main outcomes: risk of lymph node metastasis and risk of residual disease at the ER site.
This article aims to provide a comprehensive overview of ESD for carcinomas of the upper gastrointestinal tract by synthesizing current clinical practices and pathological evaluation and reporting standards. It will also address limitations and challenges of histopathologic work up and finally highlight the importance of a bilateral dialog between clinics and pathology.
2. Methods
This article was designed as a narrative review with the aim of synthesizing current practice of the application of ER techniques, particularly ESD on upper GI carcinomas, and pathology work up and reporting of the respective resection specimens.
A comprehensive, non-systematic search of the literature was conducted across major databases, including PubMed, covering publications from 2019 to 2025. Relevant studies, reviews, and meta-analyses were identified using combinations of the following keywords: “ESD”, “EMR”, “esophagus/esophageal”, “gastric”, “cancer”, “histopathology”, “risk”, “Barrett”, “endoscopic/endoscopy”, and “guidelines”. Articles were then selected based on their relevance to one or more of the following themes: application of ER techniques, particularly ESD; histopathology work up and reporting; and guidelines. Due to the character of a narrative review, no formal quality assessment or meta-analysis was performed. Most of the references finally included are reviews, meta-analyses, and guidelines.
3. Histopathology of Lesions Suitable for Endoscopic Resections
The histologic spectrum of lesions amenable to ESD or ER in general comprises primarily benign lesions and both precancerous conditions and early-stage malignancies. In the esophagus, the principal histologic entities are squamous and glandular lesions [
19,
20,
21].
Squamous intraepithelial neoplasia, graded as low- or high-grade dysplasia, represents the precursor stage to squamous cell carcinoma, which itself may be encountered in early, intramucosal form suitable for ER or in carcinomas with superficial invasion of the submucosa without risk factors for lymphatic metastases, as discussed more in detail later in this paper [
22].
In the glandular compartment, Barrett’s esophagus gives rise to a sequence of dysplastic changes, ranging from indefinite for dysplasia through low-grade and high-grade dysplasia, ultimately progressing to adenocarcinoma [
22]. In the stomach, the lesions relevant to ER include a variety of adenomatous dysplastic proliferations. Intestinal-type adenomas are the most frequent, besides foveolar-type and pyloric gland adenomas. Dysplasia may also present outside a polypoid adenoma, as flat or depressed lesions. Gastric carcinoma is subtyped according to the WHO classification into tubular, papillary, poorly cohesive (including signet-ring cell carcinoma), and mucinous carcinomas and several special subtypes [
21,
23,
24]. In addition to the WHO classification, the Laurén classification (intestinal vs. diffuse type) [
25] and the Japanese classification [
26] with similar detailed subtypes are widely accepted. In addition, small, well-differentiated neuroendocrine tumors (NETs) of grade 1 or 2 [
27] may be resected endoscopically, as may hyperplastic or hamartomatous polyps if they harbor focal dysplasia, or other benign, mesenchymal lesions arising in the mucosa [
28]. This review, however, primarily focusses on high-grade dysplastic lesions and squamous cell carcinomas and adenocarcinomas.
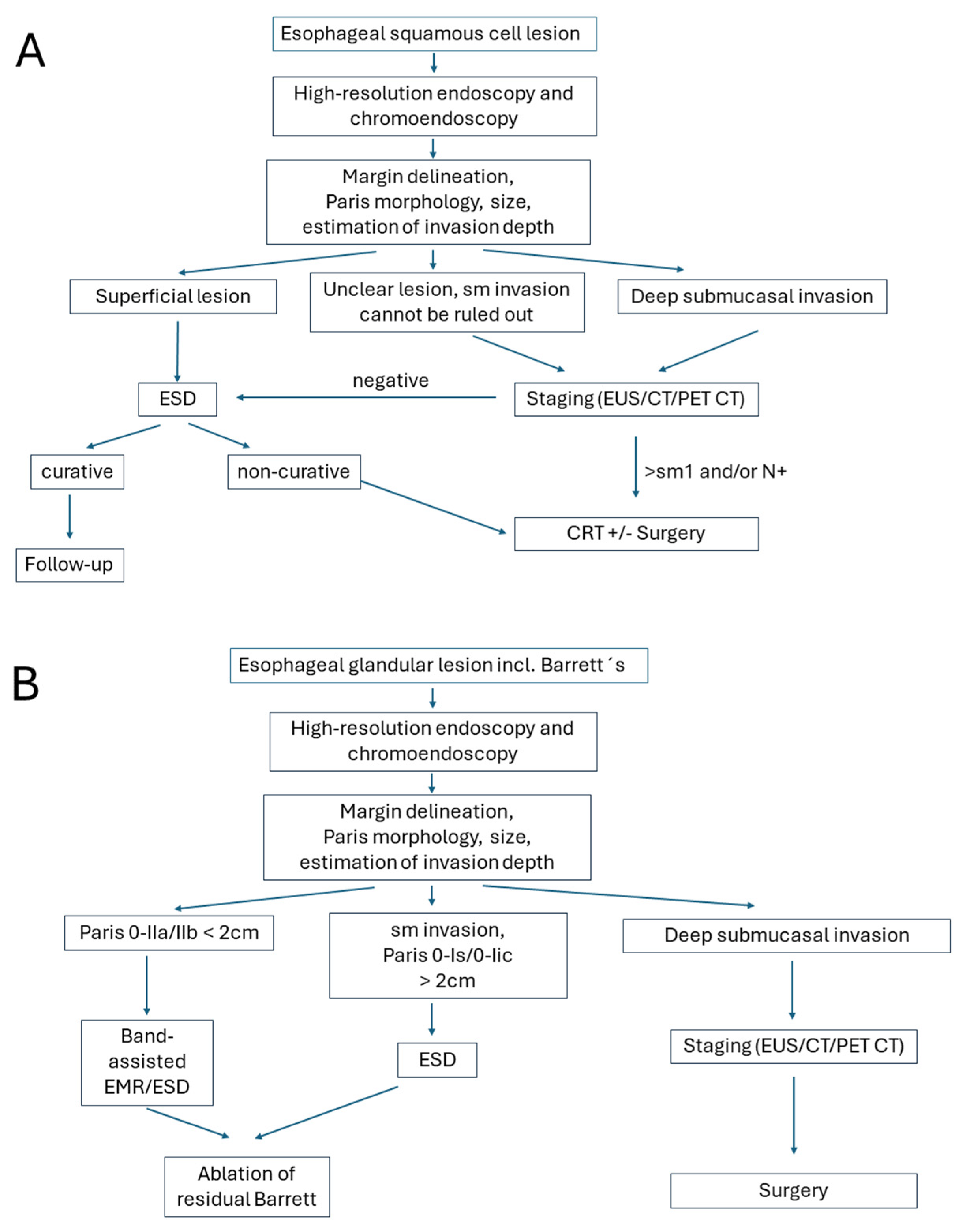
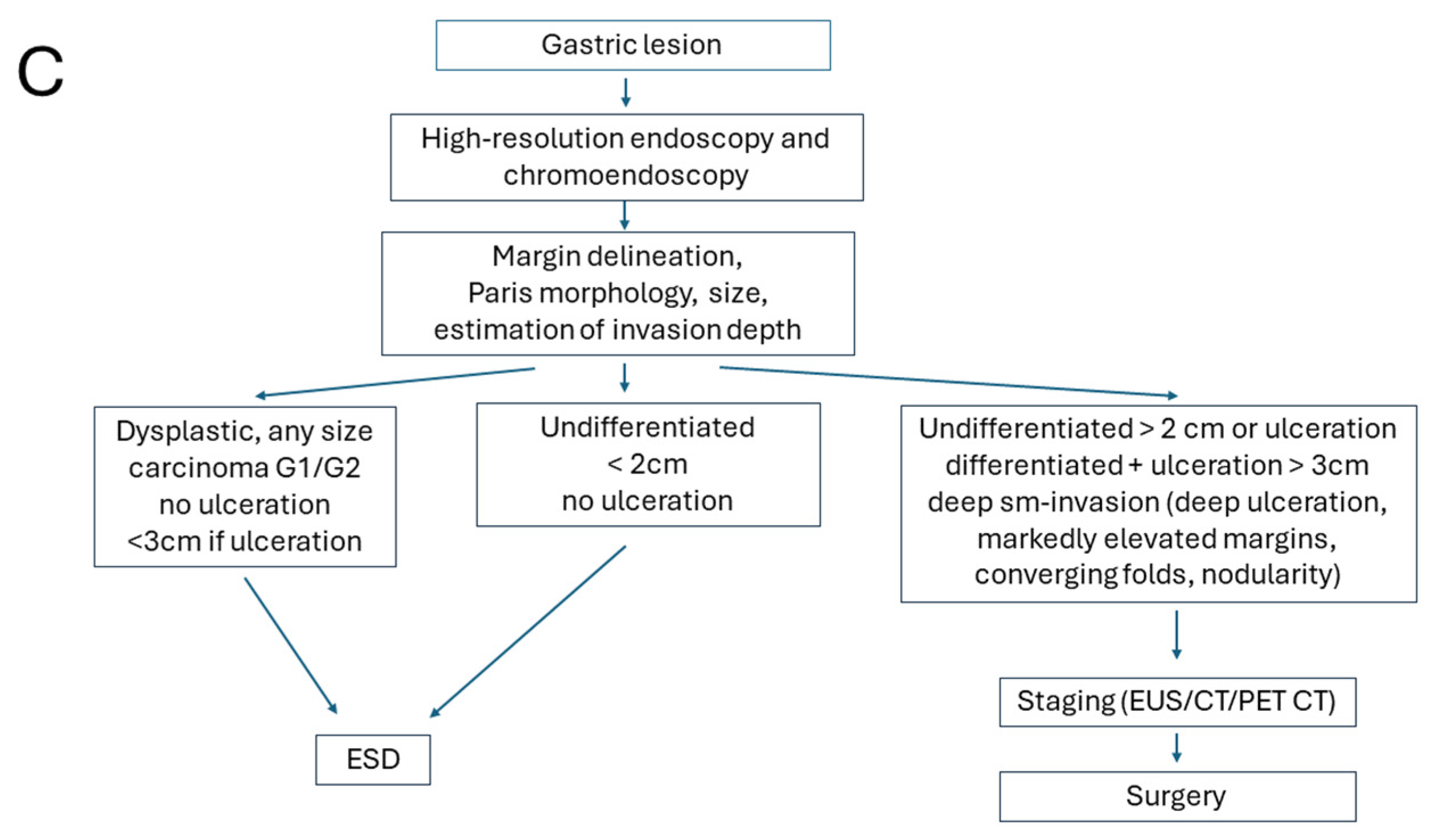
4. Clinical Indications
Endoscopic resection has emerged as the preferred first-line approach for appropriately selected patients with (high-grade) dysplastic lesions or early carcinomas across international guidelines—including those from the European Society for Medical Oncology (ESMO) [
29,
30], the National Comprehensive Cancer Network (NCCN) [
31,
32], and the Japanese Gastric Cancer and Esophageal Cancer guidelines [
33,
34,
35]. Surgical resection is reserved for carcinomas with higher risk of insufficient oncological outcomes, e.g., disseminating disease or unresectable lesions by endoscopic means. This stratification reflects a balance between cure rates, functional preservation, and procedural safety.
Apart from local indications such as multifocality, for lesions in locations or with conditions limiting complete endoscopic clearance, the risk of lymphatic spread with subsequent node metastases is the major determinator for choosing primary surgery as a curative therapeutic option. Lymphatic spread, in turn, is associated with aggressive tumor features (poor differentiation), larger tumor size, and deeper tumor infiltration, which should be determined before endoscopic intervention [
36,
37,
38]. The final assessment of risk factors with a subsequent definitive therapeutic decision (i.e., follow-up for oncologically sufficient and definite curative excision vs. completing surgery for situations with high-risk of failure of local tumor control, of lymphatic spread, or recurrence) is then performed on the resection specimen by histopathology [
16].
5. Preintervention Assessment
The most important part of the pre-intervention assessment is meticulous endoscopic evaluation. This is typically performed with high-definition white-light endoscopy to clearly delineate the lesions and their boundaries [
11,
39]. Image-enhanced modalities, including narrow-band imaging (NBI), blue laser imaging, or chromoendoscopy, further enhance visualization of subtle mucosal and vascular details. Detection of irregular microvascular (vascular) or micro-surface (epithelial) patterns can non-invasively predict which lesions are likely to harbor high-grade dysplasia, intramucosal carcinoma, or invasion into the submucosa with high accuracy [
40]. Particularly, the presence of a demarcation line separating the abnormal area from normal mucosa in combination with irregular microvascular or micro-surface patterns is highly predictive of neoplasia. Normal mucosa exhibits regular, well-organized microvascular and micro-surface features. In high-grade dysplasia or early cancer, the microvasculature becomes irregular, dilated, and tortuous (“corkscrew” vessels), or shows mesh-like, branching patterns, while the epithelial surface loses its uniformity and regular pit pattern, showing chaotic or disrupted features [
41,
42]. Specific features linked with submucosal invasion include non-dense vessel density, negative vessel regularity, and increased vessel meandering [
43]. Visible tumor lesions identified during endoscopic assessment are described according to the Paris classification, which categorizes them as polypoid (type I), flat (type II), or excavated (type III). The flat, type II lesions can be further subdivided: those with slight elevation less than 1.3 mm (IIa), entirely flat (IIb), or superficially depressed (IIc). Flat, depressed, and excavated lesion types are associated with a considerably increased probability of submucosal invasion, which impacts the suitability for endoscopic resection [
44].
Endoscopic ultrasound (EUS) is not always required routinely, but in certain cases it serves as an important adjunct, particularly for esophageal lesions [
45,
46]. Here, EUS can help to additionally assess the depth of tumor infiltration and exclude frank involvement of the muscularis propria, However, EUS accuracy drops for subtle submucosal invasion and is not recommended routinely for further assessment of these lesions. Routine cross-sectional imaging (CT, MRI, and PET-CT) is not typically indicated before endoscopic resection, except in scenarios with suspected advanced disease [
29,
30].
For histologic confirmation, targeted samples from representative lesion areas are preferable. Taking multiple or deep biopsies may risk inducing fibrosis and complicating subsequent ESD. This is in contrast to the recommendations for advanced carcinomas, where multiple biopsies are required for subsequent biomarker analysis, particularly in view of the intratumoral heterogeneity in these larger tumors [
47,
48]. In histological biopsy diagnosis, a distinction is made between squamous and glandular lesions, the presence or grade of dysplasia, and the presence of invasive carcinomas [
21]. For glandular lesions, reporting according to the Vienna Classification is recommended, which recognizes the following: Category 1: Negative for neoplasia/dysplasia; Category 2: Indefinite for neoplasia/dysplasia (i.e., uncertain diagnosis, often due to inflammation or regeneration obscuring clear assessment, with close follow-up monitoring recommended); Category 3: Non-invasive low-grade dysplasia; Category 4: Non-invasive high-grade dysplasia, carcinoma in situ, intramucosal carcinoma, or suspicion of invasive carcinoma; Category 5: Invasive neoplasia (intramucosal or submucosal infiltration, or beyond) [
49] (
Table 1).
EMR may be sufficient for non-ulcerated, polypoid, well- or moderately differentiated smaller lesions < 15–20 mm without endoscopic features of deep invasion. ESD is favored for smaller, superficial lesions—but usually less than 2 cm in the esophagus or less than 2–3 cm in the stomach—that are well- or moderately differentiated by histology and lack ulceration or a flat or depressed macroscopic morphology [
50]. These features correlate with a high likelihood of curative resection and a negligible risk of lymph node metastasis. Other high-risk histology features beyond size, subtype of the lesion, or differentiation, namely lymphovascular invasion or deep submucosal penetration—as described more in detail below—can be assessed by histology only after ER has already been performed [
16].
The location of the lesion may also contribute to therapeutic decision-making: ideal for ESD are better accessible and technically favorable areas such as the gastric antrum, mid/distal esophagus, or lesser curvature of the stomach. These sites facilitate easier scope maneuverability, En bloc resection, and lower complication rates. In contrast, the gastric cardia, fundus, greater curvature, upper/middle esophagus, or near anatomic bends and previous surgical sites are anatomically challenging regions posing significant technical difficulties for ESD. These locations increase the risk of incomplete resection, perforation, and procedural failure. In such cases, surgery may be preferred, especially if expertise or specialized equipment is limited. Algorithms for clinical decision-making are presented in
Figure 1.
6. Endoscopic Submucosal Dissection Procedure
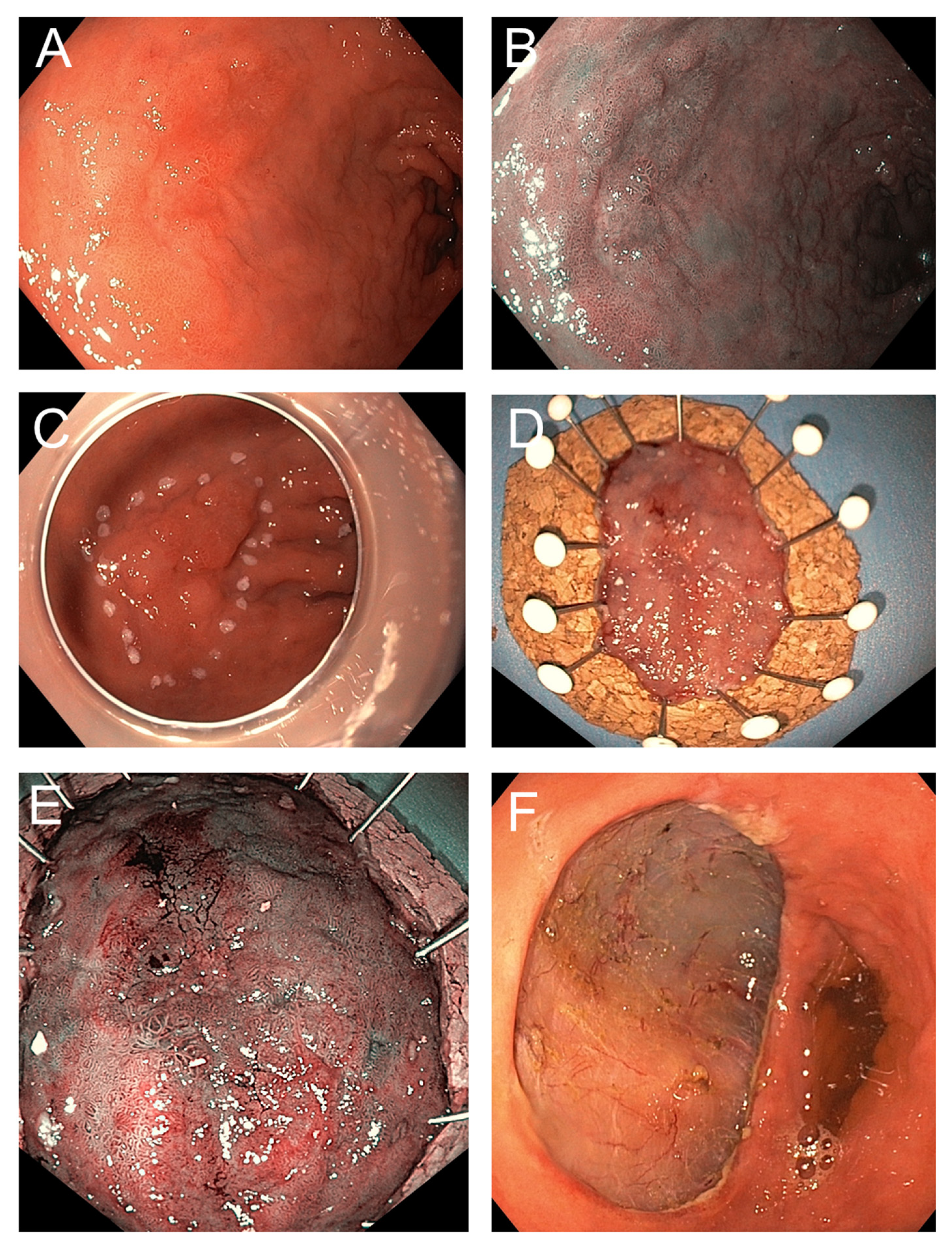
ESD is performed under high-definition endoscopic visualization [
11,
51]. The procedure begins with careful characterization and delineation of the lesion using advanced imaging modalities, including white-light endoscopy, chromoendoscopy, or narrow-band imaging, to define margins with high accuracy. After demarcating the lesion’s periphery, a submucosal injection—typically consisting of a viscous or saline-based solution mixed with epinephrine and a contrast dye—is administered. This creates a safety cushion that elevates the lesion from the muscularis propria, thereby reducing the risk of deep thermal injury and potential perforation [
10,
52] (
Figure 2A,B).
A circumferential mucosal incision is then created along the marked margins using an electrosurgical knife. Subsequent careful submucosal dissection is performed to separate the lesion En bloc from the underlying tissue, proceeding along the relatively avascular plane to preserve adjacent healthy mucosa. During dissection, visible vessels are proactively coagulated with hemostatic forceps to minimize intraprocedural bleeding, and continuous irrigation as well as transparent distal attachments (caps) are employed to optimize visualization of the dissection plane [
12,
15,
53,
54] (
Figure 2C–F).
Potential complications include bleeding, perforation, and stricture formation, which are generally infrequent but require expert management when encountered [
51].
A sign for deeper tumor infiltration is the lack of lifting after submucosal injection. Apart from tumor intrinsic changes caused by tumor infiltration and associated stromal desmoplasia, fibrosis, due to prior biopsies, previous endoscopic resections, or chronic inflammation, can make the submucosal layer tough and less distinct. Marked fibrosis causes poor lesion lifting upon submucosal injection, makes dissection more complex, and heightens the risk of perforation or incomplete removal. In cases of severe fibrosis, ER can become technically unfeasible or unsafe, requiring longer procedure times and increasing adverse event rates [
52].
Once the lesion is resected, the specimen must be immediately retrieved and properly prepared for later histopathology work up (
Figure 2D,E). Ideally the specimen should be pinned, mucosal side up, on a flat board usually made of cork, Styrofoam, or silicone. This step preserves the anatomical orientation, prevents curling, and allows for reproducible spatial correlation between endoscopic and histological findings. Specimen shrinkage can make margin assessment difficult if not stretched properly before fixation. After pinning, the specimen is immersed in 10% neutral-buffered formalin, typically for a duration of 24 to 48 h. A fixative volume of at least 10 times the specimen volume is recommended. Fixation delay or uneven penetration can result in artifacts such as epithelial detachment, obscuring diagnostic features [
22].
7. Macroscopic Work up in Pathology
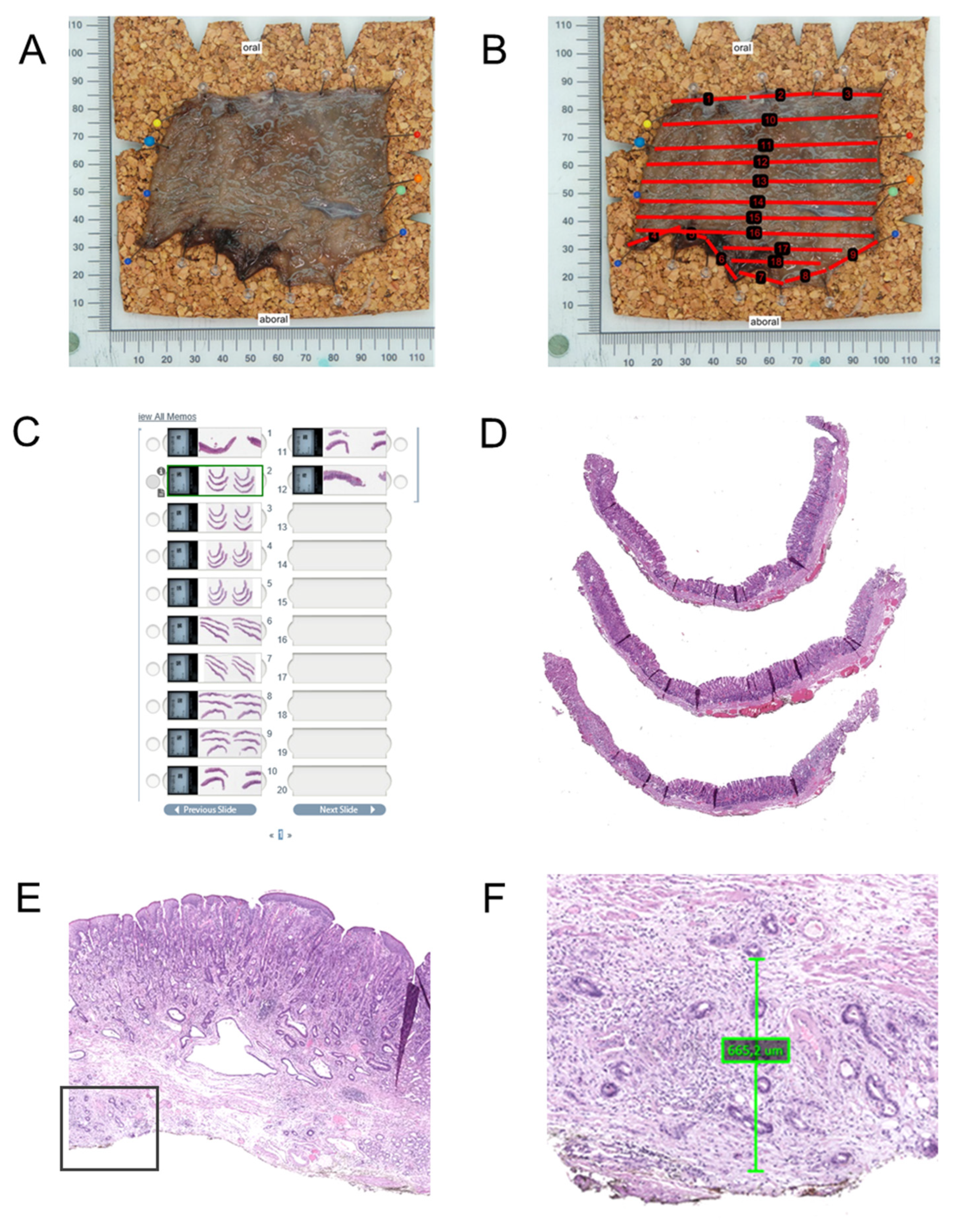
Upon receipt in the pathology laboratory, the specimen is photographed, ideally with a ruler for scale and with annotations to indicate the lesion and margins (
Figure 3). Macroscopic assessment includes documentation of lesion size, ulceration, depth, and proximity to margins if possible. Marking dyes (e.g., India ink or tissue-marking dyes in different colors) are applied to lateral and deep margins, for accurate determination of margins by subsequent histology work up.
The specimen is then serially sectioned perpendicular to the long axis at intervals of 2 to 3 mm. Careful placement and orientation of the sections in the cassettes is of utmost importance for the correct histopathology assessment. In most cases standard tissue cassettes can be used for further histology processing including paraffin embedding and sectioning. For large specimens, the use of macro-cassettes or L-shaped embedding molds may help to optimize tissue handling and reduce the number of blocks, while preserving orientation and diagnostic yield.
8. Microscopic Evaluation
8.1. Work up
Microscopic work up is based on perpendicular sections through the lesion and margins [
55] (
Figure 3A–D). The whole specimen should be histologically analyzed. Usually, standard Hematoxylin-Eosin (HE) staining is sufficient to provide all necessary information for the diagnosis of the lesions and the assessment of risk factors. In selected cases, immunohistochemistry for cytokeratin, particularly AE1/AE3, may be useful for the detection of single infiltrating cells and for demonstration of a subtle infiltrating poorly cohesive carcinoma (particularly in the stomach) or tumor buds. It can be helpful in delineating the extent of the carcinoma and demonstrating submucosal invasion where it is subtle or obscured by inflammatory cell infiltrates. Spindle cell (squamous) carcinoma may express cytokeratin, aiding distinction from primary sarcomas and spindle cell melanoma. In the case of poorly differentiated carcinoma, high-molecular-weight cytokeratin (e.g., CK5/6), p63, and/or p40 (which are all typically positive in squamous cell carcinoma) may help differentiate squamous cell carcinoma from adenocarcinoma and basaloid squamous carcinoma of the esophagus from the rare adenoid cystic carcinoma. Simple mucin stains (alcian blue, periodic acid–Schiff, or mucicarmine) may aid in the differentiation of adenocarcinoma from poorly differentiated squamous cell carcinomas. For precancerous lesions, overexpression of p53 and a high Ki-67 labeling index in atypical cells beyond the basal layers of the epithelium for squamous lesions or at the surface for glandular lesions suggest neoplastic progression. This can help resolve diagnostic uncertainty in areas of inflammation or regenerative atypia [
56,
57]. Immunohistochemistry for smooth muscle markers (desmin or another smooth muscle marker) demonstrates the smooth muscle of vessel walls when venous invasion is suspected. It is also useful for highlighting and delineating the muscularis mucosae in areas with suspected submucosal invasion (particularly in the setting of duplicated muscularis mucosae in Barrett’s esophagus). Alternatively, histochemical stains may be used for highlighting the muscularis mucosae (trichrome and Elastica van Gieson). Vascular markers can be helpful in detecting lymphovascular vessel invasion and may demonstrate this feature when it is not seen on HE-stained sections (e.g., D2-40, CD34, CD31, and ERG) [
55].
8.2. Tumor Diagnosis
Tumor diagnosis and typing should be performed according to the WHO classification [
23]. Other classification systems such as the Laurén classification for gastric cancer and the Japanese classifications for esophageal and gastric cancers do exist and may be applied as well [
21]. Grading is also performed according to the respective guidelines. In the western world, poor differentiation or aggressive histology, such as poorly cohesive or signet-ring cell components may occur only rarely in ER specimens, since they would represent contraindications for local excision if diagnosed before the intervention, in contrast to Asia. Other potentially adverse prognostic morphologic tumor features such as tumor budding may be reported. However, due to lack of data and the observation that tumor budding is rather a phenomenon of advanced carcinomas, reporting is not generally recommended in contrast to the lower gastrointestinal tract.
8.3. Assessment of Invasion Depth
In ER specimens, invasion depth is usually limited to the mucosa or submucosa. For intramucosal carcinomas, two systematic methods of differentiating depth of invasion into the different layers of the mucosa have been introduced (
Figure 4). The first method recognizes three levels (M1–M3) [
58]. M1: carcinoma confined to the intraepithelial layer, without invasion into the underlying lamina propria mucosae; M2: invasion into the lamina propria mucosae, but not reaching the muscularis mucosae; M3: infiltration into or through the muscularis mucosae, but not into the submucosa. This method is generally more appropriate for squamous carcinomas of the esophagus [
59]. The second method, (Vieth and Stolte system [
51]), is more comprehensive and separates intraepithelial lesions from invasive carcinomas clearly. It categorizes M1–M4 levels, taking the duplication of the muscularis mucosae, which is frequently observed in Barrett’s esophagus, into consideration—M1: carcinoma limited to the lamina propria mucosae (no invasion of muscularis mucosae); M2: invasion into the superficial muscularis mucosae; M3: carcinoma infiltrating the layer between the superficial and deep muscularis mucosae, often referred to as the intervening layer or duplicated muscularis mucosae zone; M4: invasion into the deep muscularis mucosae. This system is therefore more appropriate for use in esophageal adenocarcinomas and requires larger specimens for the division between M3 and M4. The method used should be recorded in the report.
Submucosal invasion is measured from the deepest layer of the lamina muscularis mucosae to the deepest point of invasion. Generally, submucosal invasion is divided into three tiers (sm1—superficial one third of submucosa; sm2—intermediate one third of submucosa, and sm3—outer one third of submucosa). This division may be difficult, as it depends on the amount of submucosa included in the specimen (as in ER specimens) [
60]. Since there is no muscularis propria for a landmark, the division is not accurate. For the classification of superficial neoplastic lesions measurements in microns has been recommended as an alternative [
61] and should be considered standard as it provides a more accurate risk stratification for lymph node metastasis. In accordance with the increased risk for lymph node metastases for sm2 carcinomas, the threshold for sm2/sm3 has been defined as 500/1000 µm for adenocarcinomas and 200 µm for the sm2 category of squamous cell carcinomas.
8.4. Biomarker Testing
Biomarker testing may also be performed on ER tissue samples. However, depending on local or national guidelines, testing of early carcinomas may not be mandatory due to a lack of immediate clinical consequences, i.e., systemic treatment as administered for locally advanced or metastasized carcinomas. If upfront testing for therapeutically relevant biomarkers is performed, this should ideally include HER2, Mismatch Repair Deficiency /Microsatellite Instability Status, PD-L1, and Claudin18.2 for adenocarcinomas. For squamous cell carcinomas, only PD-L1 is currently used as a therapeutically relevant biomarker [
62,
63].
8.5. Pathology Reporting According to the International Collaboration on Cancer Reporting
Structured pathology reporting is increasingly recognized as essential for standardization, clarity, and interdisciplinary communication [
55]. There exist reporting guidelines of various national pathology societies such as the CAP (College of American Pathologists (CAP) [
64] or the Royal College of Pathologists (RCPath) [
65]. The International Collaboration on Cancer Reporting (ICCR) aims at integrating pathological and clinical reporting and has developed evidence-based datasets for almost all tumor entities [
18].
Besides datasets for esophagectomies [
55] or gastrectomies, dedicated datasets for reporting local excisions of the esophagus and gastroesophageal junction [
7] and stomach [
17] have also been published. ICCR datasets distinguish between core (mandatory) and non-core (optional but recommended) reporting elements.
For ESD specimens from the esophagus or GEJ, core items include the anatomical site and tumor type (according to WHO classification), histological grade, maximal invasion depth with precise measurement in micrometers (particularly for submucosal invasion), resection margin status (lateral and deep), and the presence or absence of lymphovascular invasion. Tumor size in three dimensions, as well as presence of ulceration or Barrett-associated intestinal metaplasia, are also mandatory in Barrett-related neoplasia. Non-core items include background mucosal pathology (e.g., chronic esophagitis or intestinal metaplasia), degree of host inflammatory response, and presence of tumor budding. While not always required, inclusion of these features can provide additional context for clinical decision-making, especially in early adenocarcinomas of the GEJ.
For gastric cancers, core items comprise: endoscopic procedure (e.g., EMR, ESD, or other), tumor focality, tumor site (anatomical location), tumor dimensions (maximum size in mm), histological tumor type (WHO classification), histological grade (applicable only for tubular and papillary adenocarcinomas; well, moderate, poor, undifferentiated), layers present in specimen (lamina propria, muscularis mucosae, submucosa, etc.), extent of invasion (including depth of submucosal invasion in µm), absence or presence of lymphovascular invasion, and margin status (deep and lateral margins, including distance from closest margin in mm; also involvement of margins by high-grade or low grade dysplasia). Non-core items include clinical information (relevant biopsy results, prior cancer, risk conditions, indication for ER), macroscopic tumor type (e.g., Paris classification: 0-I, 0-IIa/b/c, 0-III), Laurén classification, additional tumor measurements (other than maximum dimension), specimen dimensions (overall, not just tumor), coexistent pathology (e.g., Helicobacter pylori gastritis, autoimmune gastritis, reactive gastropathy, intestinal metaplasia, polyps), previous neoadjuvant therapy details and response, ancillary studies that are not required for clinical management (e.g., neuroendocrine markers and Ki-67 for non-MiNEN/NEC), ancillary studies impacting management (e.g., HER2, MSI/MMR, EBV, as indicated for carcinoma type or context), and additional comments (e.g., tissue orientation issues, underestimation, recommendations for further management) [
17,
18].
9. Risk Factors and Their Clinical Significance
The risk of lymph node metastasis differs between organs and also between squamous cell carcinomas and adenocarcinomas [
66,
67,
68,
69]. For esophageal squamous cell carcinomas, oncological curative resection is defined for tumors showing the following features: pT1b (sm1) with an invasion depth less than 200 µm in the submucosal layer without risk factors (L0 and V0) and tumor differentiation of G1 or G2. For esophageal (Barrett’s) adenocarcinoma or adenocarcinomas of the esophagogastric junction, it is as follows: pT1b (sm1) with an infiltration depth up to 500 µm in the submucosal layer, L0 and V0, and tumor differentiation of G1 or G2 [
70,
71]. For gastric carcinomas ER is considered oncologically safe for differentiated non-ulcerated tumors < 2 cm diameter and L0 and V0 with up to a maximum of one of the expanded criteria: pT1b (sm1) with an infiltration depth up to 500 µm in the submucosal layer, a non-ulcerated lesion independent of size, a differentiated ulcerated lesion < 3 cm diameter, or an undifferentiated lesion < 2 cm diameter [
72].
From a clinical perspective, the pathological findings following ESD determine whether the resection is curative. A complete (R0) resection without high-risk features supports continued endoscopic follow-up. In cases with high-risk histological features or incomplete vertical margin (R1), the multidisciplinary team may recommend additional surgical resection or adjuvant therapy. For incomplete lateral margins, follow-up biopsies for control and/or additional radiofrequency ablation may be sufficient and should be discussed in a multidisciplinary setting. After an R1 resection in dysplastic Barrett’s esophagus without invasive adenocarcinoma (i.e., positive margins after endoscopic submucosal dissection or mucosal resection), the optimal management strategy is determined by the risk profile of the residual neoplasia and patient factors [
11,
70,
71] (
Table 2).
Repeat endoscopy is recommended as first-line management for most patients with Barrett’s neoplasia limited to the mucosa (T1a) or superficial submucosa (sm1, <500 μm), provided there is no lymphovascular invasion and the lesion is well- or moderately differentiated. The American College of Gastroenterology and the American Gastroenterological Association both recommend that visible residual or recurrent lesions should undergo repeat endoscopic resection, while flat residual Barrett’s mucosa should be treated with ablation, most commonly radiofrequency ablation, to achieve complete eradication of intestinal metaplasia and reduce recurrence risk. Endoscopic therapy is preferred over esophagectomy for T1a and select low-risk T1b lesions, given comparable long-term survival and lower morbidity [
54,
56,
59]
Esophagectomy is indicated if high-risk features are present—deep submucosal invasion (>500 μm), poor differentiation, or lymphovascular invasion—or if endoscopic therapy fails to achieve complete eradication after repeat attempts. Multidisciplinary evaluation is recommended in these cases.
Surveillance after endoscopic therapy should be performed at 3, 6, and 12 months, then annually, as suggested by the American Gastroenterological Association, to detect early recurrence [
71]. All management decisions should incorporate shared decision-making, considering patient comorbidities and preferences [
11,
70,
71].
The eCura score is a validated risk stratification system used to estimate the risk of lymph node metastasis (LNM) in patients with early gastric cancer (EGC) who have undergone non-curative endoscopic submucosal dissection (ESD) [
73]. The score is calculated based on five pathological factors: lymphatic invasion (three points), tumor size > 30 mm (one point), positive vertical margin (one point), venous invasion (one point), and submucosal invasion ≥ 500 μm (one point). The total score ranges from 0 to 7, and patients are categorized into three risk groups: low- (0–1), intermediate- (2–4), and high- (5–7) risk for LNM [
73]. This system helps guide post-ESD management decisions, such as whether to recommend additional surgery or observation. Multiple studies have demonstrated that the eCura score reliably predicts LNM risk and cancer-specific outcomes. High-risk patients have an approximately 25% rate of lymph node metastases and benefit most from additional surgery, while low-risk patients show lymph node metastases in only up to 3% and have excellent cancer-specific survival even without further intervention [
74,
75]. For patients in the low-risk eCura tier, endoscopic resection may therefore generally be considered sufficient, and management may consist of endoscopic and clinical surveillance without additional surgery, with a focus on regular follow-up to detect local recurrence or metachronous lesions. For patients in the intermediate-risk tier, management may be individualized, balancing the estimated risk of lymph node metastasis against patient age, comorbidities, and preferences. Additional gastrectomy with lymphadenectomy may be considered, but careful shared decision-making and multidisciplinary discussion are recommended. For patients in the high-risk eCura tier, additional gastrectomy with lymph node dissection may be recommended because of the substantial risk of lymph node metastasis. Observation alone may then generally be reserved for patients who are unfit for surgery or who decline further intervention after counseling. The eCura system is widely used in clinical practice in Japan and has been externally validated in large multicenter cohorts [
76,
77].
10. Pathologic Evaluation of Endoscopic Submucosal Dissection Versus Endoscopic Mucosal Resection Specimens
ESD specimens and EMR differ substantially in their histopathologic evaluability. The principal distinction arises from the method of resection: ESD typically achieves En bloc removal irrespective of lesion size, whereas EMR—particularly for lesions > 20 mm—frequently necessitates piecemeal excision [
11,
78]. En bloc resection in ESD preserves the anatomic relationships between the lesion, adjacent mucosa, and underlying submucosa, enabling precise assessment of both lateral and deep margins. Margin status can therefore be determined with high confidence, and the depth of submucosal invasion can be measured accurately. Furthermore, architectural preservation facilitates reliable identification of lymphovascular invasion (LVI) and characterization of histologic heterogeneity, including focal high-grade dysplasia or submucosal invasive carcinoma that may not be apparent endoscopically [
41].
In contrast, the piecemeal nature of EMR inherently limits margin assessment, as fragmentation disrupts specimen orientation and spatial continuity. Depth of invasion may be underestimated or rendered indeterminate when submucosal tissue is incompletely sampled, and LVI detection is less reliable due to distortion from cautery and crush artifact. These limitations are particularly problematic for large, flat, or laterally spreading tumors, in which individual EMR fragments may fail to capture the deepest or most histologically aggressive areas [
79,
80].
Artifacts differ between the two modalities. EMR specimens more commonly exhibit crush artifact and coagulative distortion from snare excision, which can impair cytologic and stromal interpretation [
11]. Particularly in piecemeal specimens, these artifacts may occur in any margin of any single specimen, causing major challenges for proper orientation, reconstruction, and subsequent determination of a final resection status. ESD specimens may also show localized thermal injury from electrosurgical knives, occasionally obscuring the epithelial–stromal interface at the margin. However, this is observed in a lower frequency and better to interpret when pre-intervention assessment could clearly show the demarcation of the target lesion that allows sufficient security distance to the margin of the specimen. EMR specimens may be generally simpler and faster to process in the pathology laboratory, but ESD specimens yield superior diagnostic details [
16,
59,
80]. In general, ESD generates specimens that allow precise macroscopic work up, proper orientation, and further tissue processing for histology, allowing exact characterization of malignant and premalignant lesions and histological risk factors. Moreover, the precise determination of invasion depth in contrast to some EMR specimens makes ESD the preferred method for the oncologically safe removal of most early upper GI malignancies.
From a pathologist’s perspective, however, understanding the procedural steps and their impact on tissue morphology is essential for accurate evaluation, especially of margins, depth of invasion, and lymphovascular invasion (LVI). Although to a lesser amount compared to EMR, several steps during the procedure itself can cause challenges in the pathological evaluation:
For lesion marking the endoscopist first identifies the lesion and marks the perimeter approximately 5 mm beyond its visible edge using a coagulation device. These markings help guide resection boundaries. However, coagulation marks may appear histologically as small foci of cautery artifact and should not be mistaken for dysplasia or tumor extension [
41].
For lifting the mucosa and creating a safety plane above the muscularis propria, a fluid (e.g., saline, glycerol, or hyaluronic acid, often with epinephrine or dye) is injected beneath the lesion. This submucosal lifting can create artificial tissue planes or spaces, which may mimic lymphatic channels. It is important not to misinterpret these artifacts as evidence of lymphovascular invasion. The injection may also lead to localized edema or stromal changes [
16,
80].
Mucosal incision is performed just outside the marked borders to allow access to the submucosa using an electrosurgical knife. Therefore, incision edges often show thermal injury, which can obscure margin assessment. Cautery artifacts at these points may mimic high-grade dysplasia or carcinoma in situ [
41].
Finally, submucosal dissection continues in the submucosal plane using the same electrosurgical tools. Hemostasis is maintained using coagulation forceps or the knife itself. The goal is to remove the lesion in a single piece (En bloc) while preserving surrounding tissue. Thermal artifacts are therefore common, especially along the deep margin and at bleeding sites. Crush artifacts may occur due to manipulation with forceps, potentially distorting cellular architecture and complicating evaluation of dysplasia or invasion. In some cases, especially after prior biopsies, submucosal fibrosis is seen and can be misinterpreted as desmoplastic reaction or tumor-associated fibrosis [
16].
11. Summary: Integrating Clinical and Pathological Perspectives in Endoscopic Resections for Upper GI Tumors
The integration of clinical and pathological perspectives is critical to maximizing the safety, efficacy, and long-term outcomes of endoscopic resection, particularly submucosal dissection in patients with upper gastrointestinal tumors (
Table 3). This is important at different stages of diagnostics and therapy:
First, enhanced patient selection is achieved by clinical assessment through high-resolution endoscopy and imaging. This helps to identify which neoplastic lesions are best suited for ESD, focusing on those with superficial invasion and low risk of lymph node metastasis. The pathological evaluation of the diagnostic biopsy provides confirmation of malignancy, histologic type, and differentiation, ensuring that candidates for ESD meet evidence-based curability criteria [
11,
16,
30,
32,
33,
70].
Subsequently, accurate resection and histological assessment for risk stratification is a basis for tailored management. Here, ESD aims clinically for En-bloc resection, minimizing recurrence risk and preserving organ function. Pathologically, thorough examination of the resected specimen allows for confirmation and more detailed characterization of the tumor, including precise measurement of invasion depth, margin status, and detection of lymphovascular invasion. Additional biomarker testing can be performed if required. Additional findings, such as underlying intestinal metaplasia with or without dysplasia, inflammation, etc., should also be reported [
8,
11,
16,
32,
33,
70].
In summary, clinical–pathological correlation facilitates intra-procedural decisions, such as determining resection extent or halting the procedure if malignancy is more advanced than expected. This approach supports multidisciplinary collaboration, reduces adverse event rates, and aligns care with the most current evidence. Academically, integrating both perspectives supports the generation of robust data on ESD effectiveness and safety, which will offer clinicians and pathologists feedback loops that help refine criteria for ESD indications and post-procedure surveillance. This may particularly helpful in non-curative situations where the risk of surgery needs to be balanced against the risk of lymph node metastases.
Author Contributions
Conceptualization, A.Z. and R.L.; data curation, A.Z., M.W., D.H.W.L., P.P., and R.L.; writing—original draft preparation, A.Z., M.W., and R.L.; writing—review and editing, A.Z., M.W., D.H.W.L., P.P., and R.L.; visualization, A.Z. and R.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. Open access publication was supported by the Österreichische Krebshilfe Oberösterreich.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
During the preparation and the revision of this manuscript, the authors used Perplexity Pro
https://www.perplexity.ai for the purposes of research, text generation, and language editing. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chiarello, M.M.; Fico, V.; Pepe, G.; Tropeano, G.; Adams, N.J.; Altieri, G.; Brisinda, G. Early gastric cancer: A challenge in Western countries. World J. Gastroenterol. 2022, 28, 693–703. [Google Scholar] [CrossRef]
- Bird-Lieberman, E.L.; Fitzgerald, R.C. Early diagnosis of oesophageal cancer. Br. J. Cancer 2009, 101, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sepesi, B.; Watson, T.J.; Zhou, D.; Polomsky, M.; Litle, V.R.; Jones, C.E.; Raymond, D.P.; Hu, R.; Qiu, X.; Peters, J.H. Are endoscopic therapies appropriate for superficial submucosal esophageal adenocarcinoma? An analysis of esophagectomy specimens. J. Am. Coll. Surg. 2010, 210, 418–427. [Google Scholar] [CrossRef]
- Bennett, C.; Wang, Y.; Pan, T. Endoscopic mucosal resection for early gastric cancer. Cochrane Database Syst. Rev. 2009, 2009, Cd004276. [Google Scholar] [CrossRef]
- Gertler, R.; Stein, H.J.; Langer, R.; Nettelmann, M.; Schuster, T.; Hoefler, H.; Siewert, J.R.; Feith, M. Long-term outcome of 2920 patients with cancers of the esophagus and esophagogastric junction: Evaluation of the New Union Internationale Contre le Cancer/American Joint Cancer Committee staging system. Ann. Surg. 2011, 253, 689–698. [Google Scholar] [CrossRef]
- Verheij, E.P.D.; van Munster, S.N.; Bergman, J.J.G.H.M.; Pouw, R.E. Advancing Approaches for Superficial Esophageal Adenocarcinoma: Shifting Toward More Patient-tailored Therapy. Tech. Innov. Gastrointest. Endosc. 2023, 25, 177–185. [Google Scholar] [CrossRef]
- Pech, O.; May, A.; Manner, H.; Behrens, A.; Pohl, J.; Weferling, M.; Hartmann, U.; Manner, N.; Huijsmans, J.; Gossner, L.; et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology 2014, 146, 652–660.e651. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, R.; Katada, C. History of endoscopic diagnosis and treatment for esophageal and pharyngeal squamous cell carcinoma. Dig. Endosc. 2022, 34, 23–26. [Google Scholar] [CrossRef]
- Soetikno, R.; Kaltenbach, T.; Yeh, R.; Gotoda, T. Endoscopic mucosal resection for early cancers of the upper gastrointestinal tract. J. Clin. Oncol. 2005, 23, 4490–4498. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Takizawa, K.; Suzuki, S.; Koike, Y.; Nonaka, S.; Yamasaki, Y.; Minagawa, T.; Sato, C.; Takeuchi, C.; Watanabe, K.; et al. Conventional versus traction-assisted endoscopic submucosal dissection for gastric neoplasms: A multicenter, randomized controlled trial (with video). Gastrointest. Endosc. 2018, 87, 1231–1240. [Google Scholar] [CrossRef]
- Pimentel-Nunes, P.; Libânio, D.; Bastiaansen, B.A.J.; Bhandari, P.; Bisschops, R.; Bourke, M.J.; Esposito, G.; Lemmers, A.; Maselli, R.; Messmann, H.; et al. Endoscopic submucosal dissection for superficial gastrointestinal lesions: European Society of Gastrointestinal Endoscopy (ESGE) Guideline–Update 2022. Endoscopy 2022, 54, 591–622. [Google Scholar] [CrossRef]
- Esaki, M.; Hayashi, Y.; Ikehara, H.; Ihara, E.; Horii, T.; Tamura, Y.; Ichijima, R.; Yamakawa, S.; Irie, A.; Shibuya, H.; et al. The effect of scissor-type versus non-scissor-type knives on the technical outcomes in endoscopic submucosal dissection for superficial esophageal cancer: A multi-center retrospective study. Dis. Esophagus 2020, 33, doz077. [Google Scholar] [CrossRef] [PubMed]
- Marino, K.A.; Sullivan, J.L.; Weksler, B. Esophagectomy versus endoscopic resection for patients with early-stage esophageal adenocarcinoma: A National Cancer Database propensity-matched study. J. Thorac. Cardiovasc. Surg. 2018, 155, 2211–2218.e2211. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, C.; Probst, A.; Ebigbo, A.; Faiss, S.; Schumacher, B.; Allgaier, H.P.; Dumoulin, F.L.; Steinbrueck, I.; Anzinger, M.; Marienhagen, J.; et al. Endoscopic Submucosal Dissection in Europe: Results of 1000 Neoplastic Lesions From the German Endoscopic Submucosal Dissection Registry. Gastroenterology 2021, 161, 1168–1178. [Google Scholar] [CrossRef]
- Draganov, P.V.; Aihara, H.; Karasik, M.S.; Ngamruengphong, S.; Aadam, A.A.; Othman, M.O.; Sharma, N.; Grimm, I.S.; Rostom, A.; Elmunzer, B.J.; et al. Endoscopic Submucosal Dissection in North America: A Large Prospective Multicenter Study. Gastroenterology 2021, 160, 2317–2327.e2312. [Google Scholar] [CrossRef] [PubMed]
- Kumarasinghe, M.P.; Bourke, M.J.; Brown, I.; Draganov, P.V.; McLeod, D.; Streutker, C.; Raftopoulos, S.; Ushiku, T.; Lauwers, G.Y. Pathological assessment of endoscopic resections of the gastrointestinal tract: A comprehensive clinicopathologic review. Mod. Pathol. 2020, 33, 986–1006. [Google Scholar] [CrossRef]
- Shi, C.; Webster, F.; Nagtegaal, I.D. Pathology Reporting of Gastric Endoscopic Resections: Recommendations From the International Collaboration on Cancer Reporting. Gastroenterology 2023, 164, 1039–1043. [Google Scholar] [CrossRef]
- Lam, A.K.; Nagtegaal, I.D. Pathology Reporting of Esophagus Endoscopic Resections: Recommendations From the International Collaboration on Cancer Reporting. Gastroenterology 2022, 162, 373–378. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef]
- Grillo, F.; Mastracci, L.; Saragoni, L.; Vanoli, A.; Limarzi, F.; Gullo, I.; Ferro, J.; Paudice, M.; Parente, P.; Fassan, M. Neoplastic and pre-neoplastic lesions of the oesophagus and gastro-oesophageal junction. Pathologica 2020, 112, 138–152. [Google Scholar] [CrossRef]
- Al-Nattah, S.; Matkovic, E.; Schwalbe, M.; Matkowskyj, K.A. Pathologic Features of Esophageal and Gastric Malignancies. In Gastrointestinal Malignancies; Bentrem, D., Benson, A.B., Eds.; Springer: Cham, Switzerland, 2024; Volume 192, pp. 19–48. [Google Scholar]
- Sung, J.; Lin, D.; Fitzgerald, R.; Sharma, P.; Law, S.; Ilson, D. Carcinoma of Esophagus. J. Gastroenterol. Hepatol. 2025, 40, 1861–1875. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. WHO Classification of Tumors: Digestive System Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2019. [Google Scholar]
- Waldum, H.L.; Fossmark, R. Types of Gastric Carcinomas. Int. J. Mol. Sci. 2018, 19, 4109. [Google Scholar] [CrossRef]
- Laurén, P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. Acta Pathol. Microbiol. Scand. 1965, 64, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011, 14, 101–112. [CrossRef] [PubMed]
- Mastracci, L.; Rindi, G.; Grillo, F.; Solcia, E.; Campora, M.; Fassan, M.; Parente, P.; Vanoli, A.; La Rosa, S. Neuroendocrine neoplasms of the esophagus and stomach. Pathologica 2021, 113, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Papke, D.J., Jr.; Hornick, J.L. Recent developments in gastroesophageal mesenchymal tumours. Histopathology 2021, 78, 171–186. [Google Scholar] [CrossRef]
- Obermannová, R.L.; Leong, T. ESMO Clinical Practice Guideline interim update on the treatment of locally advanced oesophageal and oesophagogastric junction adenocarcinoma and metastatic squamous-cell carcinoma. ESMO Open 2025, 10, 104134. [Google Scholar] [CrossRef]
- Lordick, F.; Carneiro, F.; Cascinu, S.; Fleitas, T.; Haustermans, K.; Piessen, G.; Vogel, A.; Smyth, E.C. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1005–1020. [Google Scholar] [CrossRef]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Farjah, F.; Gerdes, H.; et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 2023, 21, 393–422. [Google Scholar] [CrossRef]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Corvera, C.U.; Das, P.; Enzinger, P.C.; Enzler, T.; Gerdes, H.; Gibson, M.K.; Grierson, P.; et al. Gastric Cancer, Version 2.2025, NCCN Clinical Practice Guidelines In Oncology. J. Natl. Compr. Canc Netw. 2025, 23, 169–191. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Ishihara, R.; Ishikawa, H.; Ito, Y.; Oyama, T.; Oyama, T.; Kato, K.; Kato, H.; Kawakubo, H.; Kawachi, H.; et al. Esophageal cancer practice guidelines 2022 edited by the Japan esophageal society: Part 1. Esophagus 2023, 20, 343–372. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Ishihara, R.; Ishikawa, H.; Ito, Y.; Oyama, T.; Oyama, T.; Kato, K.; Kato, H.; Kawakubo, H.; Kawachi, H.; et al. Esophageal cancer practice guidelines 2022 edited by the Japan Esophageal Society: Part 2. Esophagus 2023, 20, 373–389. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer 2023, 26, 1–25. [Google Scholar] [CrossRef]
- Jiang, K.Y.; Huang, H.; Chen, W.Y.; Yan, H.J.; Wei, Z.T.; Wang, X.W.; Li, H.X.; Zheng, X.Y.; Tian, D. Risk factors for lymph node metastasis in T1 esophageal squamous cell carcinoma: A systematic review and meta-analysis. World J. Gastroenterol. 2021, 27, 737–750. [Google Scholar] [CrossRef]
- Nam, M.J.; Oh, S.J.; Oh, C.A.; Kim, D.H.; Bae, Y.S.; Choi, M.G.; Noh, J.H.; Sohn, T.S.; Bae, J.M.; Kim, S. Frequency and predictive factors of lymph node metastasis in mucosal cancer. J. Gastric Cancer 2010, 10, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Kook, M.C. Risk Factors for Lymph Node Metastasis in Undifferentiated-Type Gastric Carcinoma. Clin. Endosc. 2019, 52, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Banks, M.; Graham, D.; Jansen, M.; Gotoda, T.; Coda, S.; di Pietro, M.; Uedo, N.; Bhandari, P.; Pritchard, D.M.; Kuipers, E.J.; et al. British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut 2019, 68, 1545–1575. [Google Scholar] [CrossRef]
- Chung, C.-S.; Wang, H.-P. Screening for Precancerous Lesions of Upper Gastrointestinal Tract: From the Endoscopists′ Viewpoint. Gastroenterol. Res. Pract. 2013, 2013, 681439. [Google Scholar] [CrossRef]
- Horiuchi, Y.; Fujisaki, J.; Yamamoto, N.; Shimizu, T.; Omae, M.; Ishiyama, A.; Yoshio, T.; Hirasawa, T.; Yamamoto, Y.; Tsuchida, T.; et al. Accuracy of diagnostic demarcation of undifferentiated-type early gastric cancer for magnifying endoscopy with narrow-band imaging: Surgical cases. Surg. Endosc. 2017, 31, 1906–1913. [Google Scholar] [CrossRef]
- Kurumi, H.; Nonaka, K.; Ikebuchi, Y.; Yoshida, A.; Kawaguchi, K.; Yashima, K.; Isomoto, H. Fundamentals, Diagnostic Capabilities and Perspective of Narrow Band Imaging for Early Gastric Cancer. J. Clin. Med. 2021, 10, 2918. [Google Scholar] [CrossRef] [PubMed]
- Ok, K.-S.; Kim, G.H.; Park, D.Y.; Lee, H.J.; Jeon, H.K.; Baek, D.H.; Lee, B.E.; Song, G.A. Magnifying Endoscopy with Narrow Band Imaging of Early Gastric Cancer: Correlation with Histopathology and Mucin Phenotype. Gut Liver 2016, 10, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Vincze, Á. Endoscopic diagnosis and treatment in gastric cancer: Current evidence and new perspectives. Front. Surg. 2023, 10, 1122454. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Sun, S.; Guo, J.; Ge, N.; Wang, S.; Liu, X.; Wang, G.; Hu, J.; Wang, S. Is endoscopic ultrasonography useful for endoscopic submucosal dissection? Endosc. Ultrasound 2016, 5, 284–290. [Google Scholar] [CrossRef]
- Fairweather, M.; Jajoo, K.; Sainani, N.; Bertagnolli, M.M.; Wang, J. Accuracy of EUS and CT imaging in preoperative gastric cancer staging. J. Surg. Oncol. 2015, 111, 1016–1020. [Google Scholar] [CrossRef]
- Baretton, G.B.; Lordick, F.; Gaiser, T.; Hofheinz, R.; Horst, D.; Lorenzen, S.; Moehler, M.; Röcken, C.; Schirmacher, P.; Stahl, M.; et al. Standardized and quality-assured predictive PD-L1 testing in the upper gastrointestinal tract. J. Cancer Res. Clin. Oncol. 2023, 149, 16231–16238. [Google Scholar] [CrossRef]
- Lordick, F.; Al-Batran, S.E.; Dietel, M.; Gaiser, T.; Hofheinz, R.D.; Kirchner, T.; Kreipe, H.H.; Lorenzen, S.; Möhler, M.; Quaas, A.; et al. HER2 testing in gastric cancer: Results of a German expert meeting. J. Cancer Res. Clin. Oncol. 2017, 143, 835–841. [Google Scholar] [CrossRef]
- Schlemper, R.J.; Riddell, R.H.; Kato, Y.; Borchard, F.; Cooper, H.S.; Dawsey, S.M.; Dixon, M.F.; Fenoglio-Preiser, C.M.; Fléjou, J.F.; Geboes, K.; et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut 2000, 47, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Draganov, P.V.; Wang, A.Y.; Othman, M.O.; Fukami, N. AGA Institute Clinical Practice Update: Endoscopic Submucosal Dissection in the United States. Clin. Gastroenterol. Hepatol. 2019, 17, 16–25.e11. [Google Scholar] [CrossRef]
- Zeki, S.S.; Bergman, J.J.; Dunn, J.M. Endoscopic management of dysplasia and early oesophageal cancer. Best Pract. Res. Clin. Gastroenterol. 2018, 36–37, 27–36. [Google Scholar] [CrossRef]
- Esaki, M.; Ihara, E.; Gotoda, T. Endoscopic instruments and techniques in endoscopic submucosal dissection for early gastric cancer. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 1009–1020. [Google Scholar] [CrossRef]
- Esaki, M.; Yoshida, M.; Takizawa, K.; Notsu, A.; Nonaka, S.; Shichijo, S.; Suzuki, S.; Sato, C.; Komori, H.; Minagawa, T.; et al. Comparison of treatment outcomes between endoscopic submucosal dissection with the needle-type knife and insulated-tip knife for superficial esophageal neoplasms. Dis. Esophagus 2023, 36, doac067. [Google Scholar] [CrossRef]
- Yoshida, N.; Dohi, O.; Inoue, K.; Yasuda, R.; Ishida, T.; Hirose, R.; Naito, Y.; Ogiso, K.; Murakami, T.; Morinaga, Y.; et al. Efficacy of scissor-type knives for endoscopic mucosal dissection of superficial gastrointestinal neoplasms. Dig. Endosc. 2020, 32, 4–15. [Google Scholar] [CrossRef]
- Kumarasinghe, M.P.; Armstrong, M.; Foo, J.; Raftopoulos, S.C. The modern management of Barrett’s oesophagus and related neoplasia: Role of pathology. Histopathology 2021, 78, 18–38. [Google Scholar] [CrossRef]
- Snyder, P.; Dunbar, K.; Cipher, D.J.; Souza, R.F.; Spechler, S.J.; Konda, V.J.A. Aberrant p53 Immunostaining in Barrett’s Esophagus Predicts Neoplastic Progression: Systematic Review and Meta-Analyses. Dig. Dis. Sci. 2019, 64, 1089–1097. [Google Scholar] [CrossRef]
- Januszewicz, W.; Pilonis, N.D.; Sawas, T.; Phillips, R.; O’Donovan, M.; Miremadi, A.; Malhotra, S.; Tripathi, M.; Blasko, A.; Katzka, D.A.; et al. The utility of P53 immunohistochemistry in the diagnosis of Barrett’s oesophagus with indefinite for dysplasia. Histopathology 2022, 80, 1081–1090. [Google Scholar] [CrossRef]
- Westerterp, M.; Koppert, L.B.; Buskens, C.J.; Tilanus, H.W.; ten Kate, F.J.; Bergman, J.J.; Siersema, P.D.; van Dekken, H.; van Lanschot, J.J. Outcome of surgical treatment for early adenocarcinoma of the esophagus or gastro-esophageal junction. Virchows Arch. 2005, 446, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Kodama, M.; Kakegawa, T. Treatment of superficial cancer of the esophagus: A summary of responses to a questionnaire on superficial cancer of the esophagus in Japan. Surgery 1998, 123, 432–439. [Google Scholar] [CrossRef]
- Gotink, A.W.; Ten Kate, F.J.; Doukas, M.; Wijnhoven, B.P.; Bruno, M.J.; Looijenga, L.H.; Koch, A.D.; Biermann, K. Do pathologists agree with each other on the histological assessment of pT1b oesophageal adenocarcinoma? United Eur. Gastroenterol. J. 2019, 7, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Manner, H.; Pech, O. Measurement of the tumor invasion depth into the submucosa in early adenocarcinoma of the esophagus (pT1b): Can microns be the new standard for the endoscopist? United Eur. Gastroenterol. J. 2015, 3, 501–504. [Google Scholar] [CrossRef]
- Alsina Maqueda, M.; Teijo Quintáns, A.; Cuatrecasas, M.; Fernández Aceñero, M.J.; Fernández Montes, A.; Gómez Martín, C.; Jiménez Fonseca, P.; Martínez Ciarpaglini, C.; Rivera Herrero, F.; Iglesias Coma, M. Biomarkers in gastroesophageal cancer 2025: An updated consensus statement by the Spanish Society of Medical Oncology (SEOM) and the Spanish Society of Pathology (SEAP). Clin. Transl. Oncol. 2025, 9, 3580–3594. [Google Scholar] [CrossRef] [PubMed]
- Businello, G.; Angerilli, V.; Lonardi, S.; Bergamo, F.; Valmasoni, M.; Farinati, F.; Savarino, E.; Spolverato, G.; Fassan, M. Current molecular biomarkers evaluation in gastric/gastroesophageal junction adenocarcinoma: Pathologist does matter. Updates Surg. 2023, 75, 291–303. [Google Scholar] [CrossRef]
- College of American Pathologists (CAP). Available online: https://documents.cap.org/protocols/cp-esophagus-17protocol-4000.pdf (accessed on 20 October 2025).
- Royal College of Pathologists (RCPath). Available online: https://www.rcpath.org/static/f8b1ea3d-5529-4f85-984c8d4d8556e0b7/068e9093-0aea-4316-bdd49771564784b9/g006-dataset-for-histopathological-reporting-of-oesophageal-and-gastric-carcinoma.pdf (accessed on 20 October 2025).
- Xu, W.; Liu, X.B.; Li, S.B.; Yang, Z.H.; Tong, Q. Prediction of lymph node metastasis in superficial esophageal squamous cell carcinoma in Asia: A systematic review and meta-analysis. Dis. Esophagus 2020, 33, doaa032. [Google Scholar] [CrossRef]
- Gotoda, T.; Yanagisawa, A.; Sasako, M.; Ono, H.; Nakanishi, Y.; Shimoda, T.; Kato, Y. Incidence of lymph node metastasis from early gastric cancer: Estimation with a large number of cases at two large centers. Gastric Cancer 2000, 3, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Alvarez Herrero, L.; Pouw, R.E.; van Vilsteren, F.G.; ten Kate, F.J.; Visser, M.; van Berge Henegouwen, M.I.; Weusten, B.L.; Bergman, J.J. Risk of lymph node metastasis associated with deeper invasion by early adenocarcinoma of the esophagus and cardia: Study based on endoscopic resection specimens. Endoscopy 2010, 42, 1030–1036. [Google Scholar] [CrossRef]
- Abdelfatah, M.M.; Barakat, M.; Othman, M.O.; Grimm, I.S.; Uedo, N. The incidence of lymph node metastasis in submucosal early gastric cancer according to the expanded criteria: A systematic review. Surg. Endosc. 2019, 33, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, J.H.; Sawas, T.; Wani, S.; Eluri, S.; Singh, S.; Chandar, A.K.; Perumpail, R.B.; Inadomi, J.M.; Thrift, A.P.; Piscoya, A.; et al. AGA Clinical Practice Guideline on Endoscopic Eradication Therapy of Barrett’s Esophagus and Related Neoplasia. Gastroenterology 2024, 166, 1020–1055. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Shaheen, N.J.; Katzka, D.; Bergman, J.J.G.H.M. AGA Clinical Practice Update on Endoscopic Treatment of Barrett’s Esophagus with Dysplasia and/or Early Cancer: Expert Review. Gastroenterology 2020, 158, 760–769. [Google Scholar] [CrossRef]
- Forbes, N.; Elhanafi, S.E.; Al-Haddad, M.A.; Thosani, N.C.; Draganov, P.V.; Othman, M.O.; Ceppa, E.P.; Kaul, V.; Feely, M.M.; Sahin, I.; et al. American Society for Gastrointestinal Endoscopy guideline on endoscopic submucosal dissection for the management of early esophageal and gastric cancers: Summary and recommendations. Gastrointest. Endosc. 2023, 98, 271–284. [Google Scholar] [CrossRef]
- Hatta, W.; Gotoda, T.; Oyama, T.; Kawata, N.; Takahashi, A.; Yoshifuku, Y.; Hoteya, S.; Nakagawa, M.; Hirano, M.; Esaki, M.; et al. A Scoring System to Stratify Curability after Endoscopic Submucosal Dissection for Early Gastric Cancer: “eCura system”. Am. J. Gastroenterol. 2017, 112, 874–881. [Google Scholar] [CrossRef]
- Kim, T.W.; Yang, H.J.; Lee, G.; Park, S.K.; Jung, Y.S.; Park, J.H.; Park, D.I.; Sohn, C.I. Stratifying Risk of Lymph Node Metastasis After Non-Curative Endoscopic Submucosal Dissection of Early Gastric Cancer: Comparison of the eCura System and Elderly Criteria. J. Gastric Cancer 2025, 25, 370–381. [Google Scholar] [CrossRef]
- Niwa, H.; Ozawa, R.; Kurahashi, Y.; Kumamoto, T.; Nakanishi, Y.; Okumura, K.; Matsuda, I.; Ishida, Y.; Hirota, S.; Shinohara, H. The eCura system as a novel indicator for the necessity of salvage surgery after non-curative ESD for gastric cancer: A case-control study. PLoS ONE 2018, 13, e0204039. [Google Scholar] [CrossRef]
- Mao, S.; Li, W.; Pan, Y.; Wu, H.; Xiang, Y.; Liu, M.; Zhao, T.; Tao, H.; Wang, L.; Xu, G. Long-term outcomes of additional surgery vs. observation after noncurative endoscopic submucosal dissection for early gastric cancer and application value of the eCura scoring system: A propensity score-matched study. J. Gastrointest. Surg. 2025, 29, 102030. [Google Scholar] [CrossRef]
- Yabuuchi, Y.; Masui, Y.; Kumagai, K.; Iwagami, H.; Murai, K.; Setoyama, T.; Tochio, T.; Utsumi, T.; Yoshikawa, T.; Araki, O.; et al. External validation of the eCura system and comparison with the W-eCura score for predicting lymph node metastasis after non-curative endoscopic submucosal dissection for early gastric cancer: A multicenter retrospective cohort study. J. Gastroenterol. 2025, 60, 829–837. [Google Scholar] [CrossRef]
- Gotoda, T.; Yamamoto, H.; Soetikno, R.M. Endoscopic submucosal dissection of early gastric cancer. J. Gastroenterol. 2006, 41, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Villanacci, V.; Cengia, G.; Cestari, R.; Bassotti, G. Is it possible to improve the histological yield of oesophageal endoscopic mucosectomies? Dig. Liver Dis. 2012, 44, 179–180. [Google Scholar] [CrossRef] [PubMed]
- Reggiani Bonetti, L.; Manta, R.; Manno, M.; Conigliaro, R.; Missale, G.; Bassotti, G.; Villanacci, V. Optimal processing of ESD specimens to avoid pathological artifacts. Tech. Coloproctol. 2018, 22, 857–866. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).