Real-Life Characteristics of Patients with COPD and Discordant CAT-mMRC Questionnaires
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Study Population
2.3. GOLD Classification
2.4. Statistical Analysis
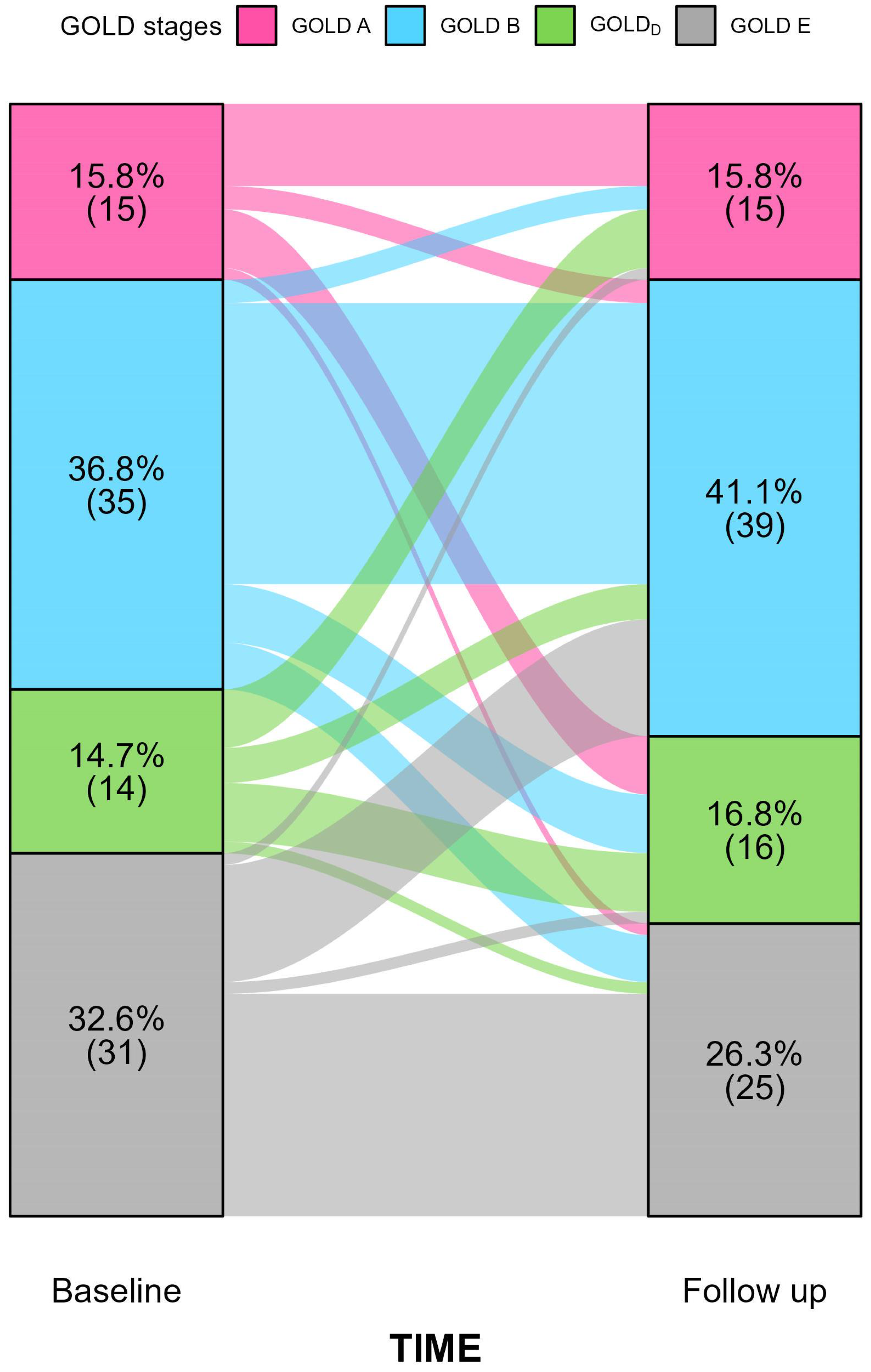
3. Results
Baseline Features
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABC | Arterial blood gas analysis |
| AUC | area under the curve |
| CAT | COPD Assessment Test |
| COPD | Chronic obstructive pulmonary disease |
| GOLD | Global Initiative for Chronic Obstructive Lung Disease |
| GOLDD | GOLDDiscordant |
| HRCT | high-resolution computed tomography |
| IQR | interquartile ranges |
| mMRC | modified Medical Research Council |
| 6MWT | 6 min walking test |
| OR | odds ratios |
| PROs | patient-reported outcomes |
| ROC | receiver operating characteristic |
| SD | standard deviations |
References
- Global Initiative for Chronic Obstructive Lung Disease-GOLD. 2024 GOLD Report. Internet. 2024. Available online: https://goldcopd.org/2024-gold-report/ (accessed on 20 March 2024).
- Miravitlles, M.; Koblizek, V.; Esquinas, C.; Milenkovic, B.; Barczyk, A.; Tkacova, R.; Zatloukal, J.; Plutinsky, M.; Kopecky, M.; Svoboda, M.; et al. Determinants of CAT (COPD Assessment Test) scores in a population of patients with COPD in central and Eastern Europe: The POPE study. Respir. Med. 2019, 150, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Pinto, L.; Morogan, A.; Bourbeau, J. The COPD assessment test: A systematic review. Eur. Respir. J. 2014, 44, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Munari, A.; Gulart, A.; Dos Santos, K.; Venâncio, R.; Karloh, M.; Mayer, A. Modified medical research council dyspnea scale in GOLD classification better reflects physical activities of daily living. Respir. Care 2018, 63, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Oh, J.; Kim, Y.I.; Ban, H.; Kwon, Y.; Oh, I.; Kim, C.; Jung, K.; Lee, S.; Kim, T.; et al. Differences in classification of COPD group using COPD assessment test (CAT) or modified Medical Research Council (mMRC) dyspnea scores: A cross-sectional analyses. BMC Pulm. Med. 2013, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Stanojevic, S.; Kaminsky, D.; Miller, M.; Thompson, B.; Aliverti, A.; Barjaktarevic, I.; Cooper, B.; Culver, B.; Ederle, J.; Hall, G.; et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur. Respir. J. 2021, 60, 2101499. [Google Scholar] [CrossRef] [PubMed]
- Houben-Wilke, S.; Augustin, I.; Vercoulen, J.; van Ranst, D.; de Vaate, E.; Wempe, J.; Spruit, M.; Wouters, E.; Janssen, D.; Franssen, F.; et al. COPD stands for complex obstructive pulmonary disease. Eur. Respir. Rev. 2018, 27, 180027. [Google Scholar] [CrossRef] [PubMed]
- Agusti, A.; Barnes, N.; Cruz, A.; Gibson, P.; Heaney, L.; Inoue, H.; Kocks, J.; Lee, A.; Patalano, F.; Ryan, D.; et al. Moving towards a Treatable Traits model of care for the management of obstructive airways diseases. Respir. Med. 2021, 187, 106572. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Chhabra, S. GOLD Classification of COPD: Discordance in Criteria for Symptoms and Exacerbation Risk Assessment. COPD J. Chronic Obstr. Pulm. Dis. 2017, 14, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Adamek, L.; Nadeau, G.; Banik, N. Comparisons of health status scores with MRC grades in COPD: Implications for the GOLD 2011 classification. Eur. Respir. J. 2013, 42, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Holt, S.; Sheahan, D.; Helm, C.; Tofield, C.; Corin, A.; Kocks, J. Little agreement in GOLD category using CAT and mMRC in 450 primary care COPD patients in New Zealand. NPJ Prim. Care Respir. Med. 2014, 24, 14003. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, W.; Wu, M.; Chen, H.; Hsu, J.; Tsai, Y.; Tao, C.; Kuo, H.; Huang, C.; Hwang, J.; Hsu, W.; et al. Features of COPD patients by comparing CAT with mMRC: A retrospective, cross-sectional study. NPJ Prim. Care Respir. Med. 2015, 25, 15068. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alqahtani, J.; Njoku, C.; Bereznicki, B.; Wimmer, B.; Peterson, G.; Kinsman, L.; Aldabayan, Y.; Alrajeh, A.; Mandal, S.; Hurst, J.; et al. Risk factors for all-cause hospital readmission following exacerbation of COPD: A systematic review and meta-analysis. Eur. Respir. Rev. 2020, 29, 190166. [Google Scholar] [CrossRef] [PubMed]
- Sunjaya, A.; Poulos, L.; Reddel, H.; Jenkins, C. Qualitative validation of the modified Medical Research Council (mMRC) dyspnoea scale as a patient-reported measure of breathlessness severity. Respir. Med. 2022, 203, 106996. [Google Scholar] [CrossRef] [PubMed]
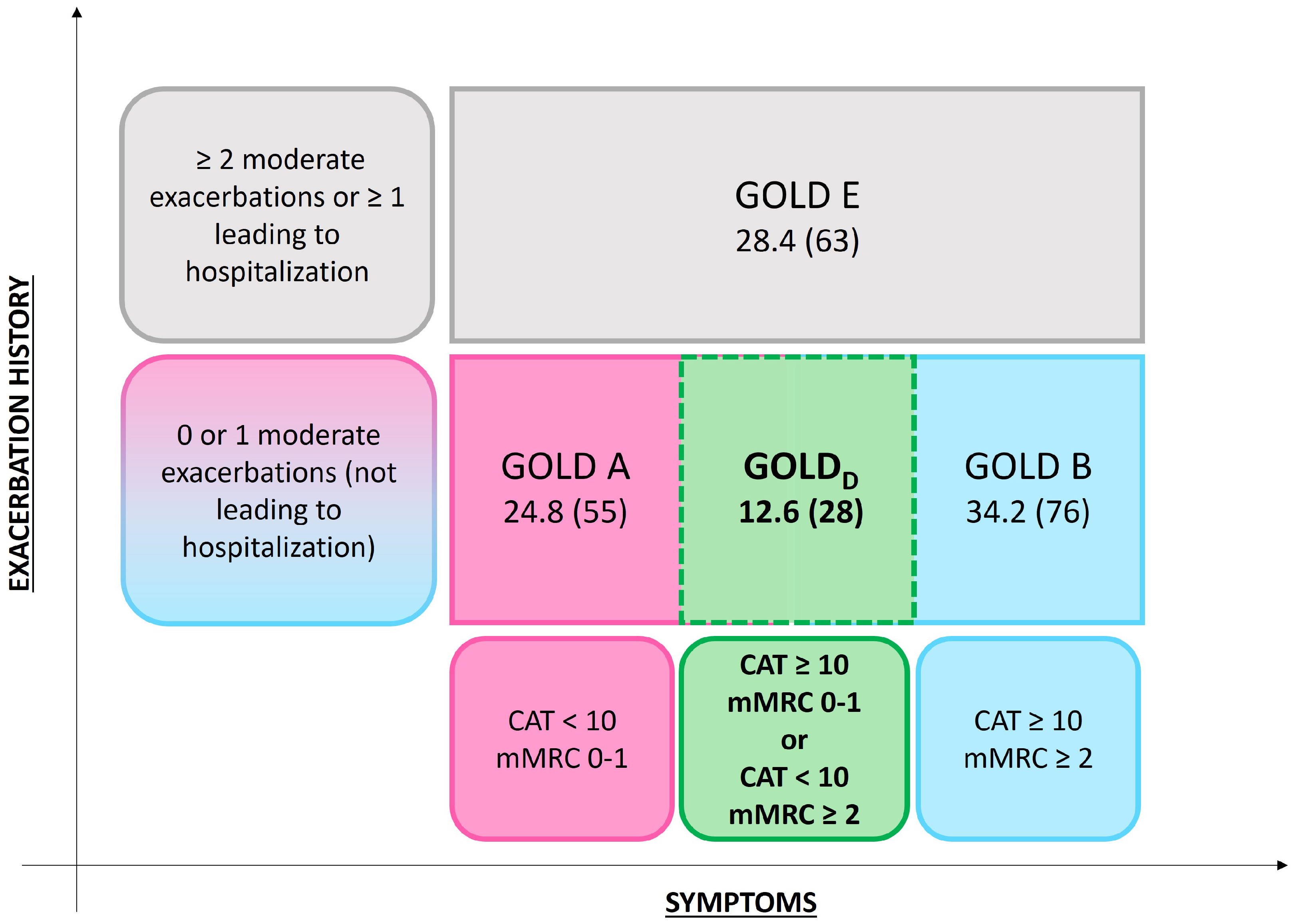
| Group | Criteria |
|---|---|
| GOLD A | CAT < 10 and mMRC < 1 |
| GOLD B | CAT ≥ 10 and mMRC ≥ 2 |
| GOLD E | ≥2 moderate exacerbations or ≥1 severe exacerbation |
| GOLDD | CAT ≥ 10 and mMRC < 2 |
| CAT < 10 and mMRC ≥ 2 |
| GOLDD | GOLD A | vs. p-Value | GOLD B | vs. p-Value | |
|---|---|---|---|---|---|
| Patients (n, %) | 12.6 (28) | 24.8 (55) | 34.2 (76) | ||
| Age (Years, Median, IQR) | 69.5 [63.7–77.2] | 68 [61–74] | 0.12 | 72 [63.2–79] | 0.72 |
| Gender (Male/Female, %) | 92.9/7.1 | 78.2/21.8 | 0.12 | 80.3/19.7 | 0.15 |
| BMI (kg/m2 Median, IQR) | 25 [22.5–27.3] | 26 [23–29] | 0.25 | 29 [25–33.7] | 0.001 |
| Smoke habits (n, %) | 0.35 | 0.32 | |||
| Current smoker | 28.6 (8) | 43.6 (24) | 44.7 (34) | ||
| Former smoker | 64.3 (18) | 47.3 (26) | 48.7 (37) | ||
| No smoker | 7.1 (2) | 9.1 (5) | 6.6 (5) | ||
| Atopy (n, %) | 21.4 (6) | 20 (11) | 0.99 | 18.4 (14) | 0.78 |
| Familiarity (n, %) | 10.7 (3) | 23.6 (13) | 0.24 | 11.8 (9) | 0.99 |
| Work exposure (n, %) | 3.6 (1) | 32.7 (18) | 0.002 | 38.2 (29) | <0.0001 |
| Comorbidities (n, %) | |||||
| Arterial hypertension | 50 (14) | 70.9 (39) | 0.09 | 64.5 (49) | 0.26 |
| Ischemic cardiomyopathy | 25 (7) | 12.7 (7) | 0.22 | 31.6 (24) | 0.63 |
| Atrial fibrillation | 17.9 (5) | 9.1 (5) | 0.29 | 15.8 (12) | 0.77 |
| Pulmonary hypertension | 3.6 (1) | 1.8 (1) | 0.99 | 2.6 (2) | 0.99 |
| Asthma | 0 | 0 | / | 5.3 (4) | 0.57 |
| GERD | 3.6 (1) | 12.7 (7) | 0.26 | 10.5 (8) | 0.44 |
| OSAS | 7.1 (2) | 9.1 (5) | 0.99 | 21.1 (16) | 0.14 |
| Cancer | 21.6 (6) | 23.6 (13) | 0.99 | 15.8 (12) | 0.56 |
| CKD | 3.6 (1) | 5.5 (3) | 0.99 | 11.8 (9) | 0.28 |
| Type2 Diabetes | 28.6 (8) | 20 (11) | 0.42 | 19.7 (15) | 0.42 |
| Dyslipidemia | 25 (7) | 41.8 (23) | 0.15 | 32.9 (25) | 0.48 |
| Thyroid disease | 14.3 (4) | 18.2 (10) | 0.76 | 18.4 (14) | 0.77 |
| Depression | 3.6 (1) | 7.3 (4) | 0.66 | 7.9 (6) | 0.67 |
| Total comorbidities (Median, IQR) | 2 [1–3.7] | 3 [1–4] | 0.47 | 3 [1.2–5] | 0.17 |
| Symptoms at first visit (n, %) | |||||
| Dyspnea | 82.1 (23) | 81.8 (45) | 0.99 | 91.6 (73) | 0.03 |
| Cough | 50 (14) | 52.7 (29) | 0.82 | 52.6 (40) | 0.83 |
| Phlegm | 46.4 (13) | 50.9 (28) | 0.82 | 51.3 (39) | 0.82 |
| Wheezing | 0 | 1.8 (1) | 0.99 | 0 | / |
| Weight loss | 0 | 0 | / | 3.9 (3) | 0.56 |
| CAT (Median, IQR) | 11 [8.2–15.7] | 6 [4–8] | <0.0001 | 16 [13–23] | <0.0001 |
| mMRC (Median, IQR) | 1 [1–2] | 1 [1–1] | <0.0001 | 3 [2–3] | <0.0001 |
| Exacerbations (n, %) | 17.9 (5) | 16.4 (9) | 0.99 | 26.3 (20) | 0.44 |
| Lung function | |||||
| FEV1 (%, Mean, SD) | 67.9 ± 18 | 75.5 ± 17.9 | 0.08 | 61.3 ± 18.1 | 0.01 |
| FVC (%, Mean, SD) | 88.2 ± 18 | 94 ± 17.9 | 0.19 | 77.9 ± 20.9 | 0.01 |
| FEV1/FVC (%, Mean, SD) | 55.3 ± 12 | 60.2 ± 6.9 | 0.06 | 58.4 ± 12.7 | 0.21 |
| RV/TLC (%, Median, IQR) | 127 [110–145.5] | 122 [112–133] | 0.29 | 135 [116–156.8] | 0.07 |
| Treatments (%, n) | |||||
| LAMA | 14.3 (4) | 34.5 (19) | 0.007 | 7.9 (6) | 0.45 |
| LAMA + LABA | 31.1 (9) | 31.7 (18) | 0.99 | 28.9 (22) | 0.81 |
| LAMA + LABA + ICS | 42.9 (12) | 18.2 (10) | 0.02 | 44.7 (34) | 0.99 |
| OCS | 3.6 (1) | 1.8 (1) | 0.99 | 2.6 (2) | 0.99 |
| LTOT | 10.7 (3) | 1.8 (1) | 0.11 | 34.2 (6) | 0.02 |
| N-Acetylcysteine | 3.6 (1) | 5.5 (3) | 0.99 | 9.2 (7) | 0.68 |
| GOLDD | GOLD A | vs.p-Value | GOLD B | vs. p-Value | |
| Patients (n, %) | 12.6 (28) | 24.8 (55) | 34.2 (76) | ||
| Age (Years, Median, IQR) | 69.5 [63.7–77.2] | 68 [61–74] | 0.12 | 72 [63.2–79] | 0.72 |
| Gender (Male/Female, %) | 92.9/7.1 | 78.2/21.8 | 0.12 | 80.3/19.7 | 0.15 |
| BMI (kg/m2 Median, IQR) | 25 [22.5–27.3] | 26 [23–29] | 0.25 | 29 [25–33.7] | 0.001 |
| Smoke habits (n, %) | 0.35 | 0.32 | |||
| Current smoker | 28.6 (8) | 43.6 (24) | 44.7 (34) | ||
| Former smoker | 64.3 (18) | 47.3 (26) | 48.7 (37) | ||
| No smoker | 7.1 (2) | 9.1 (5) | 6.6 (5) | ||
| Atopy (n, %) | 21.4 (6) | 20 (11) | 0.99 | 18.4 (14) | 0.78 |
| Familiarity (n, %) | 10.7 (3) | 23.6 (13) | 0.24 | 11.8 (9) | 0.99 |
| Work exposure (n, %) | 3.6 (1) | 32.7 (18) | 0.002 | 38.2 (29) | <0.0001 |
| Comorbidities (n, %) | |||||
| Arterial hypertension | 50 (14) | 70.9 (39) | 0.09 | 64.5 (49) | 0.26 |
| Ischemic cardiomyopathy | 25 (7) | 12.7 (7) | 0.22 | 31.6 (24) | 0.63 |
| Atrial fibrillation | 17.9 (5) | 9.1 (5) | 0.29 | 15.8 (12) | 0.77 |
| Pulmonary hypertension | 3.6 (1) | 1.8 (1) | 0.99 | 2.6 (2) | 0.99 |
| Asthma | 0 | 0 | / | 5.3 (4) | 0.57 |
| GERD | 3.6 (1) | 12.7 (7) | 0.26 | 10.5 (8) | 0.44 |
| OSAS | 7.1 (2) | 9.1 (5) | 0.99 | 21.1 (16) | 0.14 |
| Cancer | 21.6 (6) | 23.6 (13) | 0.99 | 15.8 (12) | 0.56 |
| CKD | 3.6 (1) | 5.5 (3) | 0.99 | 11.8 (9) | 0.28 |
| Type2 Diabetes | 28.6 (8) | 20 (11) | 0.42 | 19.7 (15) | 0.42 |
| Dyslipidemia | 25 (7) | 41.8 (23) | 0.15 | 32.9 (25) | 0.48 |
| Thyroid disease | 14.3 (4) | 18.2 (10) | 0.76 | 18.4 (14) | 0.77 |
| Depression | 3.6 (1) | 7.3 (4) | 0.66 | 7.9 (6) | 0.67 |
| Total comorbidities (Median, IQR) | 2 [1–3.7] | 3 [1–4] | 0.47 | 3 [1.2–5] | 0.17 |
| Symptoms at first visit (n, %) | |||||
| Dyspnea | 82.1 (23) | 81.8 (45) | 0.99 | 91.6 (73) | 0.03 |
| Cough | 50 (14) | 52.7 (29) | 0.82 | 52.6 (40) | 0.83 |
| Phlegm | 46.4 (13) | 50.9 (28) | 0.82 | 51.3 (39) | 0.82 |
| Wheezing | 0 | 1.8 (1) | 0.99 | 0 | / |
| Weight loss | 0 | 0 | / | 3.9 (3) | 0.56 |
| CAT (Median, IQR) | 11 [8.2–15.7] | 6 [4–8] | <0.0001 | 16 [13–23] | <0.0001 |
| mMRC (Median, IQR) | 1 [1–2] | 1 [1–1] | <0.0001 | 3 [2–3] | <0.0001 |
| Exacerbations (n, %) | 17.9 (5) | 16.4 (9) | 0.99 | 26.3 (20) | 0.44 |
| Lung function | |||||
| FEV1 (%, Mean, SD) | 67.9 ± 18 | 75.5 ± 17.9 | 0.08 | 61.3 ± 18.1 | 0.01 |
| FVC (%, Mean, SD) | 88.2 ± 18 | 94 ± 17.9 | 0.19 | 77.9 ± 20.9 | 0.01 |
| FEV1/FVC (%, Mean, SD) | 55.3 ± 12 | 60.2 ± 6.9 | 0.06 | 58.4 ± 12.7 | 0.21 |
| RV/TLC (%, Median, IQR) | 127 [110–145.5] | 122 [112–133] | 0.29 | 135 [116–156.8] | 0.07 |
| Treatments (%, n) | |||||
| LAMA | 14.3 (4) | 34.5 (19) | 0.007 | 7.9 (6) | 0.45 |
| LAMA + LABA | 31.1 (9) | 31.7 (18) | 0.99 | 28.9 (22) | 0.81 |
| LAMA + LABA + ICS | 42.9 (12) | 18.2 (10) | 0.02 | 44.7 (34) | 0.99 |
| OCS | 3.6 (1) | 1.8 (1) | 0.99 | 2.6 (2) | 0.99 |
| LTOT | 10.7 (3) | 1.8 (1) | 0.11 | 34.2 (6) | 0.02 |
| N-Acetylcysteine | 3.6 (1) | 5.5 (3) | 0.99 | 9.2 (7) | 0.68 |
| GOLDD | GOLD A | vs. p-Value | GOLD B | vs. p-Value | |
|---|---|---|---|---|---|
| HRCT assessed (%, n) | 78.6 (22) | 63.6 (35) | 0.21 | 64 (48) | 0.23 |
| HRCT findings (%, n) | |||||
| Emphysema | 39.3 (11) | 36.4 (20) | 0.81 | 40 (30) | 0.99 |
| GGO | 10.7 (3) | 3.6 (2) | 0.33 | 8 (6) | 0.7 |
| Consolidations | 21.4 (6) | 3.6 (2) | 0.02 | 4 (3) | 0.01 |
| Septal thickening | 10.7 (3) | 14.5 (8) | 0.74 | 13.3 (10) | 0.99 |
| Lung scars | 7.1 (2) | 21.8 (12) | 0.12 | 18.7 (14) | 0.22 |
| Bronchiectasis | 14.3 (4) | 12.7 (7) | 0.99 | 13.3 (10) | 0.99 |
| Mosaic attenuation | 3.6 (1) | 1.8 (1) | 0.99 | 4 (3) | 0.99 |
| Multiple nodules | 32.1 (9) | 10.9 (9) | 0.03 | 10.7 (8) | 0.01 |
| Solitary nodules | 14.3 (4) | 14.5 (8) | 0.99 | 16 (12) | 0.99 |
| Pleural effusion | 0 | 0 | / | 1.3 (1) | 0.99 |
| GOLDD | GOLD A | vs. p-Value | GOLD B | vs. p-Value | |
|---|---|---|---|---|---|
| ABG | |||||
| pH (median, IQR) | 7.43 [7.41–7.44] | 7.43 [7.42–7.44] | 0.43 | 7.43 [7.41–7.45] | 0.83 |
| PaO2 (mmHg, mean, SD) | 0.06 | 0.45 | |||
| PaCO2 (mmHg, median, IQR) | 39 [37.5–43] | 39 [36–41.6] | 0.39 | 40 [37–43.2] | 0.93 |
| HCO3− (mEq/L, median, IQR) | 26 [25–27.5] | 25.7 [24.2–27.9] | 0.6 | 26.8 [24.5–28.1] | 0.78 |
| 6MWT | |||||
| Pre-test Borg scale (median, IQR) | 0 [0–1.75] | 0 [0–0] | 0.22 | 0.25 [0–1.25] | 0.55 |
| Post-test Borg scale (median, IQR) | 3 [2–4.75] | 2 [1–3] | 0.15 | 4 [2–7] | 0.36 |
| Pre-test SpO2 (%, median, IQR) | 97 [95.2–98] | 97 [95–97] | 0.23 | 95.5 [94–97] | 0.006 |
| Post-test SpO2 (%, median, IQR) | 94 [91–95] | 93 [95–92] | 0.87 | 92 [89–95] | 0.27 |
| Pre-test HR (bpm, mean, SD) | 0.67 | 0.21 | |||
| Post-test HR (bpm, mean, SD) | 0.94 | 0.66 | |||
| 6MWD (m, median, IQR) | 445 [352.5–485] | 460 [391.5–501] | 0.37 | 310 [212–440] | 0.002 |
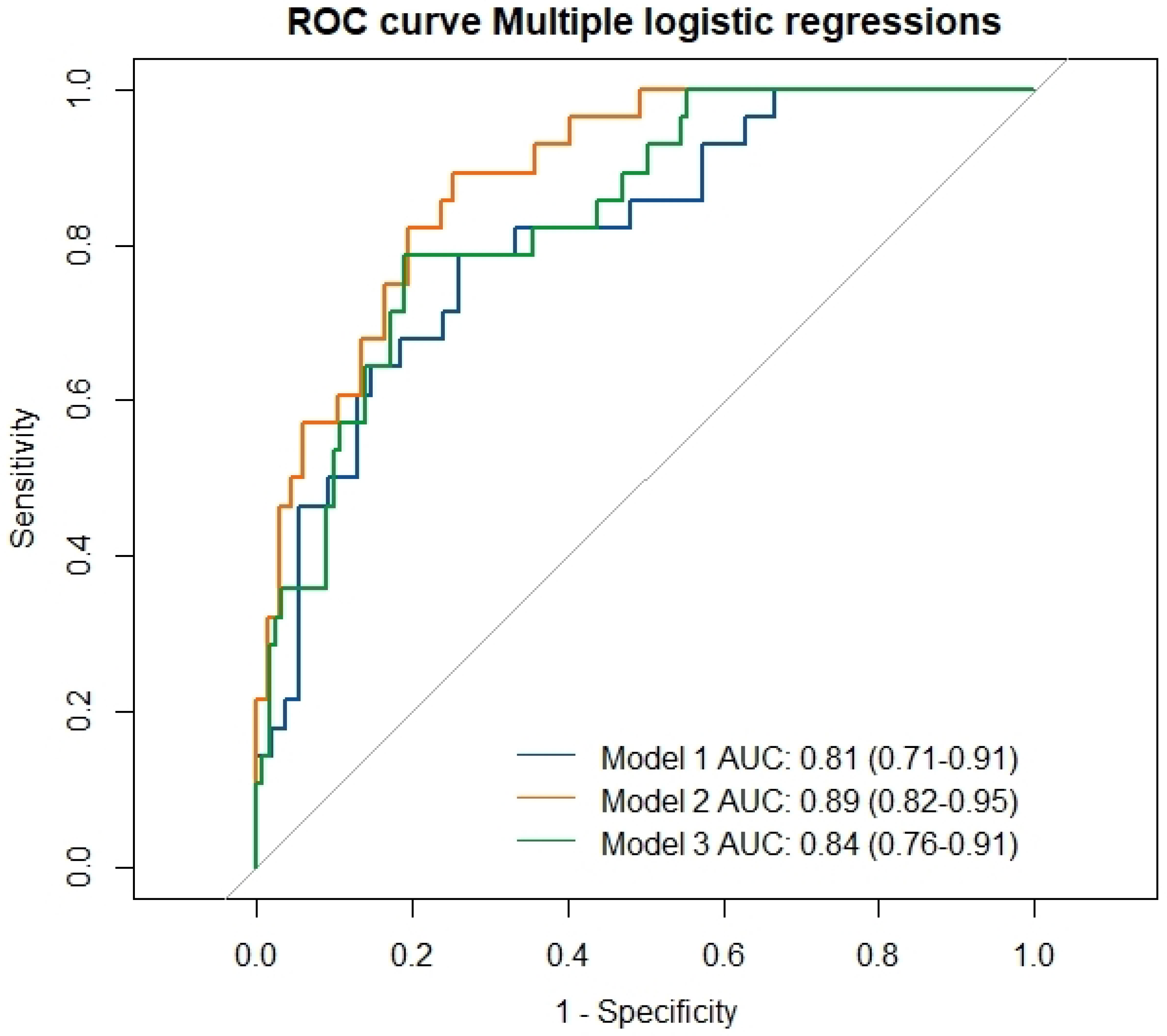
| AUC [95% CI] | OR [95% CI] | p Value | |
|---|---|---|---|
| Model 1 | 0.81 [0.71–0.91] | <0.0001 | |
| Work exposure | 0.03 [0.001–0.28] | 0.009 | |
| Chest HRCT | |||
| Consolidations | 7.8 [1.3–74.7] | 0.04 | |
| Centrilobular nodules | 4.4 [1.2–18.2] | 0.03 | |
| FVC | 2.1 [0.96–4.9] | 0.08 | |
| 6MWD | 0.99 [0.99–1] | 0.1 | |
| Model 2 | 0.89 [0.82–0.95] | <0.0001 | |
| Work exposure | 0.02 [0.0006–0.2] | 0.002 | |
| BMI | 0.9 [0.77–0.99] | 0.05 | |
| FEV1 | 8.7 [2.7–37.2] | 0.001 | |
| Chest HRCT | |||
| Consolidations | 13.7 [1.8–249.3] | 0.02 | |
| Model 3 | 0.84 [0.76–0.91] | <0.0001 | |
| Work exposure | 0.03 [0.001–0.2] | 0.005 | |
| BMI | 0.92 [0.004–7.7] | 0.37 | |
| FVC | 2.3 [1.2–4.6] | 0.01 | |
| Chest HRCT | |||
| Consolidations | 5.9 [1.3–32.7] | 0.03 | |
| Centrilobular nodules | 3.5 [1.1–11.3] | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portacci, A.; Quaranta, V.N.; Marinelli, A.; Capuano, A.; Vulpi, M.R.; Diaferia, F.; Santomasi, C.; Grimaldi, M.; Sanasi, G.; Cicchetti, M.; et al. Real-Life Characteristics of Patients with COPD and Discordant CAT-mMRC Questionnaires. J. Clin. Med. 2025, 14, 8771. https://doi.org/10.3390/jcm14248771
Portacci A, Quaranta VN, Marinelli A, Capuano A, Vulpi MR, Diaferia F, Santomasi C, Grimaldi M, Sanasi G, Cicchetti M, et al. Real-Life Characteristics of Patients with COPD and Discordant CAT-mMRC Questionnaires. Journal of Clinical Medicine. 2025; 14(24):8771. https://doi.org/10.3390/jcm14248771
Chicago/Turabian StylePortacci, Andrea, Vitaliano Nicola Quaranta, Alessio Marinelli, Alessandro Capuano, Maria Rosaria Vulpi, Fabrizio Diaferia, Carla Santomasi, Mariafrancesca Grimaldi, Giovanni Sanasi, Marianna Cicchetti, and et al. 2025. "Real-Life Characteristics of Patients with COPD and Discordant CAT-mMRC Questionnaires" Journal of Clinical Medicine 14, no. 24: 8771. https://doi.org/10.3390/jcm14248771
APA StylePortacci, A., Quaranta, V. N., Marinelli, A., Capuano, A., Vulpi, M. R., Diaferia, F., Santomasi, C., Grimaldi, M., Sanasi, G., Cicchetti, M., Ricciardi, E., Amoruso, G., Vozza, A., Dragonieri, S., & Carpagnano, G. E. (2025). Real-Life Characteristics of Patients with COPD and Discordant CAT-mMRC Questionnaires. Journal of Clinical Medicine, 14(24), 8771. https://doi.org/10.3390/jcm14248771








