Management of Calcified Coronary Lesions—A Review of Plaque Modification Methods
Abstract
1. Introduction
2. Lesion Assessment
3. Modification Methods for Calcified Plaques
3.1. SC and NC Balloons
3.2. Super-High-Pressure Non-Compliant Balloons
3.3. Scoring and Cutting Balloons
3.4. Coronary Atherectomy
3.4.1. Rotational Atherectomy
3.4.2. Orbital Atherectomy
3.4.3. Laser Atherectomy
3.5. Intravascular Lithotripsy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Cesare, M.; Perel, P.; Taylor, S.; Kabudula, C.; Bixby, H.; Gaziano, T.A.; Vaca McGhie, D.; Mwangi, J.; Pervan, B.; Narula, J.; et al. The Heart of the World. Glob. Heart 2024, 19, 11. [Google Scholar] [CrossRef]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Rekondo Olaetxea, J.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef]
- Onnis, C.; Virmani, R.; Kawai, K.; Nardi, V.; Lerman, A.; Cademartiri, F.; Scicolone, R.; Boi, A.; Congiu, T.; Faa, G.; et al. Coronary Artery Calcification: Current Concepts and Clinical Implications. Circulation 2024, 149, 251–266. [Google Scholar] [CrossRef]
- Budoff, M.J.; Young, R.; Lopez, V.A.; Kronmal, R.A.; Nasir, K.; Blumenthal, R.S.; Detrano, R.C.; Bild, D.E.; Guerci, A.D.; Liu, K.; et al. Progression of Coronary Calcium and Incident Coronary Heart Disease Events: MESA (Multi-Ethnic Study of Atherosclerosis). J. Am. Coll. Cardiol. 2013, 61, 1231–1239. [Google Scholar] [CrossRef]
- Pugliese, G.; Iacobini, C.; Blasetti Fantauzzi, C.; Menini, S. The Dark and Bright Side of Atherosclerotic Calcification. Atherosclerosis 2015, 238, 220–230. [Google Scholar] [CrossRef]
- Mitsis, A.; Khattab, E.; Christodoulou, E.; Myrianthopoulos, K.; Myrianthefs, M.; Tzikas, S.; Ziakas, A.; Fragakis, N.; Kassimis, G. From Cells to Plaques: The Molecular Pathways of Coronary Artery Calcification and Disease. J. Clin. Med. 2024, 13, 6352. [Google Scholar] [CrossRef]
- Petousis, S.; Skalidis, E.; Zacharis, E.; Kochiadakis, G.; Hamilos, M. The Role of Intracoronary Imaging for the Management of Calcified Lesions. J. Clin. Med. 2023, 12, 4622. [Google Scholar] [CrossRef]
- Caiazzo, G.; Di Mario, C.; Kedhi, E.; De Luca, G. Current Management of Highly Calcified Coronary Lesions: An Overview of the Current Status. J. Clin. Med. 2023, 12, 4844. [Google Scholar] [CrossRef] [PubMed]
- Ikari, Y.; Saito, S.; Nakamura, S.; Shibata, Y.; Yamazaki, S.; Tanaka, Y.; Ako, J.; Yokoi, H.; Kobayashi, Y.; Kozuma, K. Device Indication for Calcified Coronary Lesions Based on Coronary Imaging Findings. Cardiovasc. Interv. Ther. 2023, 38, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.F.; Patel, M.P.; Abbott, J.D.; Bangalore, S.; Brilakis, E.S.; Croce, K.J.; Doshi, D.; Kaul, P.; Kearney, K.E.; Kerrigan, J.L.; et al. SCAI Expert Consensus Statement on the Management of Calcified Coronary Lesions. J. Soc. Cardiovasc. Angiogr. Interv. 2024, 3, 101259. [Google Scholar] [CrossRef] [PubMed]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the Management of Chronic Coronary Syndromes: Developed by the Task Force for the Management of Chronic Coronary Syndromes of the European Society of Cardiology (ESC) Endorsed by the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar] [CrossRef] [PubMed]
- Osborne-Grinter, M.; Ali, A.; Williams, M.C. Prevalence and Clinical Implications of Coronary Artery Calcium Scoring on Non-Gated Thoracic Computed Tomography: A Systematic Review and Meta-Analysis. Eur. Radiol. 2024, 34, 4459–4474. [Google Scholar] [CrossRef] [PubMed]
- Dzaye, O.; Razavi, A.C.; Jelwan, Y.A.; Peng, A.W.; Grant, J.K.; Blaha, M.J. Coronary Artery Calcium Scoring on Dedicated Cardiac CT and Noncardiac CT Scans. Radiol. Cardiothorac. Imaging 2025, 7, e240548. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.R.; Lopes, P.M.; Ferreira, A.M. Use of Coronary Artery Calcium Score and Coronary CT Angiography to Guide Cardiovascular Prevention and Treatment. Ther. Adv. Cardiovasc. Dis. 2024, 18, 17539447241249650. [Google Scholar] [CrossRef]
- Bienstock, S.; Lin, F.; Blankstein, R.; Leipsic, J.; Cardoso, R.; Ahmadi, A.; Gelijns, A.; Patel, K.; Baldassarre, L.A.; Hadley, M.; et al. Advances in Coronary Computed Tomographic Angiographic Imaging of Atherosclerosis for Risk Stratification and Preventive Care. JACC Cardiovasc. Imaging 2023, 16, 1099–1115. [Google Scholar] [CrossRef]
- Mickley, H.; Veien, K.T.; Gerke, O.; Lambrechtsen, J.; Rohold, A.; Steffensen, F.H.; Husic, M.; Akkan, D.; Busk, M.; Jessen, L.B.; et al. Diagnostic and Clinical Value of FFRCT in Stable Chest Pain Patients with Extensive Coronary Calcification. JACC Cardiovasc. Imaging 2022, 15, 1046–1058. [Google Scholar] [CrossRef]
- van der Werf, N.R.; Si-Mohamed, S.; Rodesch, P.A.; van Hamersvelt, R.W.; Greuter, M.J.W.; Boccalini, S.; Greffier, J.; Leiner, T.; Boussel, L.; Willemink, M.J.; et al. Coronary Calcium Scoring Potential of Large Field-of-View Spectral Photon-Counting CT: A Phantom Study. Eur. Radiol. 2022, 32, 152–162. [Google Scholar] [CrossRef]
- Hagar, M.T.; Soschynski, M.; Saffar, R.; Rau, A.; Taron, J.; Weiss, J.; Stein, T.; Faby, S.; von Zur Muehlen, C.; Ruile, P.; et al. Accuracy of Ultrahigh-Resolution Photon-Counting CT for Detecting Coronary Artery Disease in a High-Risk Population. Radiology 2023, 307, e223305. [Google Scholar] [CrossRef]
- Halfmann, M.C.; Bockius, S.; Emrich, T.; Hell, M.; Schoepf, U.J.; Laux, G.S.; Kavermann, L.; Graafen, D.; Gori, T.; Yang, Y.; et al. Ultrahigh-Spatial-Resolution Photon-Counting Detector CT Angiography of Coronary Artery Disease for Stenosis Assessment. Radiology 2024, 310, e231956. [Google Scholar] [CrossRef]
- Dawood, M.; Elwany, M.; Abdelgawad, H.; Sanhoury, M.; Zaki, M.; Elsharkawy, E.; Nawar, M. Coronary Calcifications, the Achilles Heel in Coronary Interventions. Adv. Interv. Cardiol. 2024, 20, 1–17. [Google Scholar] [CrossRef]
- Wu, W.; Zhao, S.; Banga, A.; Trivedi, Y.V.; Dasari, V.S.; Munjal, P.; Bhat, R.R.; Tapia-Orihuela, R.K.A.; Oguz, U.M.; Zafar, H.; et al. High-Definition Intravascular Ultrasound Versus Optical Coherence Tomography: Lumen Size and Plaque Morphology. J. Soc. Cardiovasc. Angiogr. Interv. 2025, 4, 102520. [Google Scholar] [CrossRef]
- Jurado-Román, A.; Gómez-Menchero, A.; Gonzalo, N.; Martín-Moreiras, J.; Ocaranza, R.; Ojeda, S.; Palazuelos, J.; Rodríguez-Leor, O.; Salinas, P.; Vaquerizo, B.; et al. Plaque Modification Techniques to Treat Calcified Coronary Lesions: Position Paper from the ACI-SEC. REC Interv. Cardiol. (Engl. Ed.) 2023, 5, 46–61. [Google Scholar] [CrossRef]
- Sato, T.; Matsumura, M.; Yamamoto, K.; Sugizaki, Y.; Shlofmitz, E.; Moses, J.W.; Khalique, O.K.; Thomas, S.V.; Malik, S.; Dakroub, A.; et al. A Revised Optical Coherence Tomography–Derived Calcium Score to Predict Stent Underexpansion in Severely Calcified Lesions. JACC Cardiovasc. Interv. 2025, 18, 622–633. [Google Scholar] [CrossRef]
- Shlofmitz, E.; Croce, K.; Bezerra, H.; Sheth, T.; Chehab, B.; West, N.E.J.; Shlofmitz, R.; Ali, Z.A. The MLD MAX OCT Algorithm: An Imaging-Based Workflow for Percutaneous Coronary Intervention. Catheter. Cardiovasc. Interv. 2022, 100, S7–S13. [Google Scholar] [CrossRef] [PubMed]
- Koganti, S.; Kotecha, T.; Rakhit, R.D. Choice of Intracoronary Imaging: When to Use Intravascular Ultrasound or Optical Coherence Tomography. Interv. Cardiol. 2016, 11, 11–16. [Google Scholar] [CrossRef]
- Mori, H.; Torii, S.; Kutyna, M.; Sakamoto, A.; Finn, A.V.; Virmani, R. Coronary Artery Calcification and Its Progression: What Does It Really Mean? JACC Cardiovasc. Imaging 2018, 11, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Fujino, A.; Mintz, G.S.; Matsumura, M.; Lee, T.; Kim, S.-Y.; Hoshino, M.; Usui, E.; Yonetsu, T.; Haag, E.S.; Shlofmitz, R.A.; et al. A New Optical Coherence Tomography-Based Calcium Scoring System to Predict Stent Underexpansion. EuroIntervention 2018, 13, e2182–e2189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Matsumura, M.; Usui, E.; Noguchi, M.; Fujimura, T.; Fall, K.N.; Zhang, Z.; Nazif, T.M.; Parikh, S.A.; Rabbani, L.E.; et al. Intravascular Ultrasound-Derived Calcium Score to Predict Stent Expansion in Severely Calcified Lesions. Circ. Cardiovasc. Interv. 2021, 14, e010296. [Google Scholar] [CrossRef]
- Shin, D.; Karimi Galougahi, K.; Spratt, J.C.; Maehara, A.; Collet, C.; Barbato, E.; Ribichini, F.L.; Gonzalo, N.; Sakai, K.; Mintz, G.S.; et al. Calcified Nodule in Percutaneous Coronary Intervention: Therapeutic Challenges. JACC Cardiovasc. Interv. 2024, 17, 1187–1199. [Google Scholar] [CrossRef]
- Lee, T.; Mintz, G.S.; Matsumura, M.; Zhang, W.; Cao, Y.; Usui, E.; Kanaji, Y.; Murai, T.; Yonetsu, T.; Kakuta, T.; et al. Prevalence, Predictors, and Clinical Presentation of a Calcified Nodule as Assessed by Optical Coherence Tomography. JACC Cardiovasc. Imaging 2017, 10, 883–891. [Google Scholar] [CrossRef]
- Sato, T.; Matsumura, M.; Yamamoto, K.; Shlofmitz, E.; Moses, J.W.; Khalique, O.K.; Thomas, S.V.; Tsoulios, A.; Cohen, D.J.; Mintz, G.S.; et al. Impact of Eruptive vs Noneruptive Calcified Nodule Morphology on Acute and Long-Term Outcomes After Stenting. JACC Cardiovasc. Interv. 2023, 16, 1024–1035. [Google Scholar] [CrossRef]
- Maffey, M.W.; Bagur, R. Dedicated Balloon Techniques for Coronary Calcium Modification. Interv. Cardiol. 2024, 19, e13. [Google Scholar] [CrossRef] [PubMed]
- Cuculi, F.; Bossard, M.; Zasada, W.; Moccetti, F.; Voskuil, M.; Wolfrum, M.; Malinowski, K.P.; Toggweiler, S.; Kobza, R. Performing Percutaneous Coronary Interventions with Predilatation Using Non-Compliant Balloons at High-Pressure versus Conventional Semi-Compliant Balloons: Insights from Two Randomised Studies Using Optical Coherence Tomography. Open Heart 2020, 7, e001204. [Google Scholar] [CrossRef] [PubMed]
- Bulluck, H.; McEntegart, M. Contemporary Tools and Devices for Coronary Calcium Modification. JRSM Cardiovasc. Dis. 2022, 11, 20480040221089760. [Google Scholar] [CrossRef]
- Secco, G.G.; Ghione, M.; Mattesini, A.; Dall’Ara, G.; Ghilencea, L.; Kilickesmez, K.; De Luca, G.; Fattori, R.; Parisi, R.; Marino, P.N.; et al. Very High-Pressure Dilatation for Undilatable Coronary Lesions: Indications and Results with a New Dedicated Balloon. EuroIntervention 2016, 12, 359–365. [Google Scholar] [CrossRef]
- Perfetti, M.; Fulgenzi, F.; Radico, F.; Toro, A.; Procopio, A.; Maddestra, N.; Zimarino, M. Calcific Lesion Preparation for Coronary Bifurcation Stenting. Cardiol. J. 2019, 26, 429–437. [Google Scholar] [CrossRef]
- Scalamogna, M.; Kuna, C.; Voll, F.; Aytekin, A.; Lahu, S.; Kessler, T.; Kufner, S.; Rheude, T.; Sager, H.B.; Xhepa, E.; et al. Modified Balloons to Prepare Severely Calcified Coronary Lesions before Stent Implantation: A Systematic Review and Meta-Analysis of Randomized Trials. Clin. Res. Cardiol. 2024, 113, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Najam, O.; Bhindi, R.; De Silva, K. Calcium Modification Techniques in Complex Percutaneous Coronary Intervention. Circ. Cardiovasc. Interv. 2021, 14, e009870. [Google Scholar] [CrossRef]
- Mangieri, A.; Nerla, R.; Castriota, F.; Reimers, B.; Regazzoli, D.; Leone, P.P.; Gasparini, G.L.; Khokhar, A.A.; Laricchia, A.; Giannini, F.; et al. Cutting Balloon to Optimize Predilation for Stent Implantation: The COPS Randomized Trial. Catheter. Cardiovasc. Interv. 2023, 101, 798–805. [Google Scholar] [CrossRef]
- Ishihara, T.; Iida, O.; Takahara, M.; Tsujimura, T.; Okuno, S.; Kurata, N.; Asai, M.; Okamoto, S.; Nanto, K.; Mano, T. Improved Crossability with Novel Cutting Balloon versus Scoring Balloon in the Treatment of Calcified Lesion. Cardiovasc. Interv. Ther. 2021, 36, 198–207. [Google Scholar] [CrossRef]
- Nowak, A.; Ratajczak, J.; Kasprzak, M.; Sukiennik, A.; Fabiszak, T.; Wojakowski, W.; Ochała, A.; Wańha, W.; Kuczmik, W.; Navarese, E.P.; et al. Long-Term Outcome of Rotational Atherectomy According to Burr-to-Artery Ratio and Changes in Coronary Artery Blood Flow: Observational Analysis. Cardiol. J. 2023, 30, 361–368. [Google Scholar] [CrossRef]
- Barbato, E.; Carrié, D.; Dardas, P.; Fajadet, J.; Gaul, G.; Haude, M.; Khashaba, A.; Koch, K.; Meyer-Gessner, M.; Palazuelos, J.; et al. European Expert Consensus on Rotational Atherectomy. EuroIntervention 2015, 11, A6. [Google Scholar] [CrossRef]
- Sakakura, K.; Ito, Y.; Shibata, Y.; Okamura, A.; Kashima, Y.; Nakamura, S.; Hamazaki, Y.; Ako, J.; Yokoi, H.; Kobayashi, Y.; et al. Clinical Expert Consensus Document on Rotational Atherectomy from the Japanese Association of Cardiovascular Intervention and Therapeutics: Update 2023. Cardiovasc. Interv. Ther. 2023, 38, 141–162. [Google Scholar] [CrossRef] [PubMed]
- Sakakura, K.; Taniguchi, Y.; Yamamoto, K.; Tsukui, T.; Jinnouchi, H.; Seguchi, M.; Wada, H.; Fujita, H. Modifiable and Unmodifiable Factors Associated with Slow Flow Following Rotational Atherectomy. PLoS ONE 2021, 16, e0250757. [Google Scholar] [CrossRef] [PubMed]
- Mattaroccia, G.; Redivo, M.; Cianca, A.; Dell’Aquila, F.; Casenghi, M.; Giovannelli, F.; Rigattieri, S.; Berni, A.; Tommasino, A.; Barbato, E. The New Era of Coronary Angioplasty: How Cutting-Edge Technologies Are Redefining Complex Interventions. Heart Surg. Forum 2025, 28, E107–E119. [Google Scholar] [CrossRef]
- Kini, A.; Marmur, J.D.; Duvvuri, S.; Dangas, G.; Choudhary, S.; Sharma, S.K. Rotational Atherectomy: Improved Procedural Outcome with Evolution of Technique and Equipment. Single-Center Results of First 1,000 Patients. Catheter. Cardiovasc. Interv. 1999, 46, 305–311. [Google Scholar] [CrossRef]
- Januszek, R.; Siudak, Z.; Dziewierz, A.; Dudek, D.; Bartuś, S. Predictors of In-Hospital Effectiveness and Complications of Rotational Atherectomy (from the ORPKI Polish National Registry 2014–2016). Catheter. Cardiovasc. Interv. 2018, 92, E278–E287. [Google Scholar] [CrossRef]
- Mechery, A.; Jordan, P.J.; Doshi, S.N.; Khan, S.Q. Retrieval of a Stuck Rotablator Burr (“Kokeshi Phenomenon”) and Successful Percutaneous Coronary Intervention. J. Cardiol. Cases 2015, 13, 90–92. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, P.; Zheng, Z.; Shi, Y.; Liu, J. Comparison of Intravascular Lithotripsy versus Rotational Atherectomy for the Treatment of Severe Coronary Artery Calcification. BMC Cardiovasc. Disord. 2024, 24, 311. [Google Scholar] [CrossRef]
- MicroPort. MicroPort® RotaPace Receives Approval for FireRaptor® in China. MicroPort News. 2025. Available online: https://microport.com/news/microport-rotapace-receives-approval-for-fireraptor-in-china (accessed on 28 October 2025).
- Shlofmitz, E.; Jeremias, A.; Shlofmitz, R.; Ali, Z.A. Lesion Preparation with Orbital Atherectomy. Interv. Cardiol. Rev. 2019, 14, 169–173. [Google Scholar] [CrossRef]
- Khattak, S.; Sharma, H.; Khan, S.Q. Atherectomy Techniques: Rotablation, Orbital and Laser. Interv. Cardiol. 2024, 19, e21. [Google Scholar] [CrossRef]
- Shipman, J.N.; Agasthi, P. Orbital Atherectomy. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK563144/ (accessed on 28 November 2025).
- Goel, S.; Pasam, R.T.; Chava, S.; Gotesman, J.; Sharma, A.; Malik, B.A.; Frankel, R.; Shani, J.; Gidwani, U.; Latib, A. Orbital Atherectomy versus Rotational Atherectomy: A Systematic Review and Meta-Analysis. Int. J. Cardiol. 2020, 303, 16–21. [Google Scholar] [CrossRef]
- Rawlins, J.; Din, J.N.; Talwar, S.; O’Kane, P. Coronary Intervention with the Excimer Laser: Review of the Technology and Outcome Data. Interv. Cardiol. Rev. 2016, 11, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Jawad-Ul-Qamar, M.; Sharma, H.; Vetrugno, V.; Sandhu, K.; Ludman, P.F.; Doshi, S.N.; Townend, J.N.; Osheiba, M.; Zaphiriou, A.; Khan, S.Q. Contemporary Use of Excimer Laser in Percutaneous Coronary Intervention with Indications, Procedural Characteristics, Complications and Outcomes in a University Teaching Hospital. Open Heart 2021, 8, e001522. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.P.; Hobson, A.R.; McKenzie, D.; Shah, N.; Sinha, M.K.; Wells, T.A.; Levy, T.M.; Swallow, R.A.; Talwar, S.; O’Kane, P.D. Beyond the Balloon: Excimer Coronary Laser Atherectomy Used Alone or in Combination with Rotational Atherectomy in the Treatment of Chronic Total Occlusions, Non-Crossable and Non-Expansible Coronary Lesions. EuroIntervention 2013, 9, 243–250. [Google Scholar] [CrossRef]
- Li, H.; Ai, H.; Li, L.; Zheng, N.; Tang, G.; Yang, G.; Zhao, Y.; Sun, F.; Zhang, H. The Therapeutic Effects of Excimer Laser Coronary Atherectomy Therapy for In-Stent Restenosis Chronic Total Occlusions. BMC Cardiovasc. Disord. 2021, 21, 399. [Google Scholar] [CrossRef]
- Giri, S.; Ito, S.; Lansky, A.J.; Mehran, R.; Margolis, J.; Gilmore, P.; Garratt, K.N.; Cummins, F.; Moses, J.; Rentrop, P.; et al. Clinical and Angiographic Outcome in the Laser Angioplasty for Restenotic Stents (LARS) Multicenter Registry. Catheter. Cardiovasc. Interv. 2001, 52, 24–34. [Google Scholar] [CrossRef]
- Protty, M.B.; Gallagher, S.; Farooq, V.; Sharp, A.S.P.; Egred, M.; O’Kane, P.; Kinnaird, T. Combined Use of Rotational and Excimer Laser Coronary Atherectomy (RASER) during Complex Coronary Angioplasty—An Analysis of Cases (2006–2016) from the British Cardiovascular Intervention Society Database. Catheter. Cardiovasc. Interv. 2021, 97, E911–E918. [Google Scholar] [CrossRef]
- Hesse, K.; Mehta, S.; Tam, C.; Ahmed, F.; Shahid, F.; Mintz, G.S.; Ahmed, J.M. Combined Rotational Excimer Laser Coronary Atherectomy (RASER) in Non-Crossable, Non-Dilatable Coronary Artery Disease: Observations from a Single Center. J. Invasive Cardiol. 2024, 36. [Google Scholar] [CrossRef] [PubMed]
- Kereiakes, D.J.; Virmani, R.; Hokama, J.Y.; Illindala, U.; Mena-Hurtado, C.; Holden, A.; Hill, J.M.; Lyden, S.P.; Ali, Z.A. Principles of Intravascular Lithotripsy for Calcific Plaque Modification. JACC Cardiovasc. Interv. 2021, 14, 1275–1292. [Google Scholar] [CrossRef]
- Oliveira, C.; Vilela, M.; Nobre Menezes, M.; Silva Marques, J.; Moreira Jorge, C.; Rodrigues, T.; Almeida Duarte, J.; Marques da Costa, J.; Carrilho Ferreira, P.; Francisco, A.R.; et al. Coronary Intravascular Lithotripsy Effectiveness and Safety in a Real-World Cohort. J. Pers. Med. 2024, 14, 438. [Google Scholar] [CrossRef]
- Honton, B.; Monsegu, J. Best Practice in Intravascular Lithotripsy. Interv. Cardiol. 2022, 17, e02. [Google Scholar] [CrossRef]
- Tovar Forero, M.N.; Sardella, G.; Salvi, N.; Cortese, B.; di Palma, G.; Werner, N.; Aksoy, A.; Escaned, J.; Salazar, C.H.; Gonzalo, N.; et al. Coronary Lithotripsy for the Treatment of Underexpanded Stents: The International Multicentre CRUNCH Registry. EuroIntervention 2022, 18, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Kaul, A.; Dhalla, P.S.; Bapatla, A.; Khalid, R.; Garcia, J.; Armenta-Quiroga, A.S.; Khan, S. Current Treatment Modalities for Calcified Coronary Artery Disease: A Review Article Comparing Novel Intravascular Lithotripsy and Traditional Rotational Atherectomy. Cureus 2020, 12, e10922. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.M.; Kereiakes, D.J.; Shlofmitz, R.A.; Klein, A.J.; Riley, R.F.; Price, M.J.; Herrmann, H.C.; Bachinsky, W.; Waksman, R.; Stone, G.W. Intravascular Lithotripsy for Treatment of Severely Calcified Coronary Artery Disease. J. Am. Coll. Cardiol. 2020, 76, 2635–2646. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Yang, H.; Xu, G.; Li, Q.; Zhu, L.; Yang, Y.; Yang, T. The Safety and Efficacy of Intravascular Lithotripsy in the Treatment of Severe Coronary Artery Calcification in 261 Cases: A Retrospective Study. Health Sci. Rep. 2025, 8, e70474. [Google Scholar] [CrossRef]
- van Oort, M.J.H.; Al Amri, I.; Bingen, B.O.; Oliveri, F.; Claessen, B.E.P.M.; Dimitriu-Leen, A.C.; Vossenberg, T.N.; Kefer, J.; Girgis, H.; van der Kley, F.; et al. Evolving Use and Clinical Outcomes of Coronary Intravascular Lithotripsy: Insights from an International, Multicentre Registry. Heart 2024, 111, 62–68. [Google Scholar] [CrossRef]
- Shockwave Medical Website. Shockwave Medical Enrolls First Patient in FORWARD CAD Pivotal Study of Novel Forward Intravascular Lithotripsy Platform. Available online: https://shockwavemedical.com/de/news/shockwave-medical-enrolls-first-patient-in-forward-cad-pivotal-study-of-novel-forward-intravascular-lithotripsy-platform/ (accessed on 28 November 2025).
- Russo, D.; Massaro, G.; Benedetto, D.; Russo, G.; Canova, P.; Pescetelli, I.; Fiocca, L.; Romano, M.; Lettieri, C.; Chiricolo, G.; et al. Contemporary Approach to Heavily Calcified Lesions: Tools of the Trade, Challenges, and Pitfalls. Vessel Plus 2024, 8, 9. [Google Scholar] [CrossRef]
- Sasi, V.; Szántó, G.; Achim, A.; Ungi, I.; Varga, A.; Ruzsa, Z. Combination of Laser Atherectomy and Super High-Pressure Non-Compliant Balloon to Treat Stent Under-Expansion in Cases of Failed Interventional Options. Interv. Cardiol. 2023, 18, e23. [Google Scholar] [CrossRef]
- Yeung, J.Y.K.; Chiang, M. Current Role of Excimer Laser Coronary Angioplasty Atherectomy in Calcified Coronary Artery Disease Management. JACC Case Rep. 2025, 30, 103025. [Google Scholar] [CrossRef]
- Elixir Medical Website. Elixir Medical Announces Launch of LithiX™ Hertz Contact (HC) Intravascular Lithotripsy System (IVL) in Europe. Novel IVL Technology Designed to Deliver Calcium Fragmentation for Treatment of Moderate to Severely Calcified Coronary Artery Lesions Without Requiring an Energy Source. Available online: https://elixirmedical.com/elixir-medical-announces-launch-of-lithix-hertz-contact-hc-intravascular-lithotripsy-system-ivl-in-europe/ (accessed on 28 November 2025).
- PCR. EuroPCR 2024: Primary Outcomes from PINNACLE I Clinical Trial Establish Safety and Effectiveness of Elixir Medical’s Hertz Contact Intravascular Lithotripsy System for Calcium Fragmentation in Moderate to Severe Calcified Coronary Artery Lesions. 2024. Press Release, PCR Online. Available online: https://www.pcronline.com/News/Press-releases/2024/EuroPCR-2024-Primary-outcomes-from-PINNACLE-I-clinical-trial-establish-safety-and-effectiveness-of-Elixir-Medical-s-Hertz-Contact-Intravascular-Lithotripsy-System-for-calcium-fragmentation-in-moderate-to-severe-calcified-coronary-artery-lesions (accessed on 28 November 2025).
- Bennett, J.; Hamer, B.; Paradies, V.; Tonino, P.; Bataille, Y.; Verheye, S. TCT-381 Safety and Effectiveness of a Novel Intravascular Lithotripsy Device Using the Hertz Contact Stress Mechanism for Calcium Fragmentation: Six-Month Outcomes of the PINNACLE I Clinical Trial. J. Am. Coll. Cardiol. 2024, 84 (Suppl. 18), B104. [Google Scholar] [CrossRef]
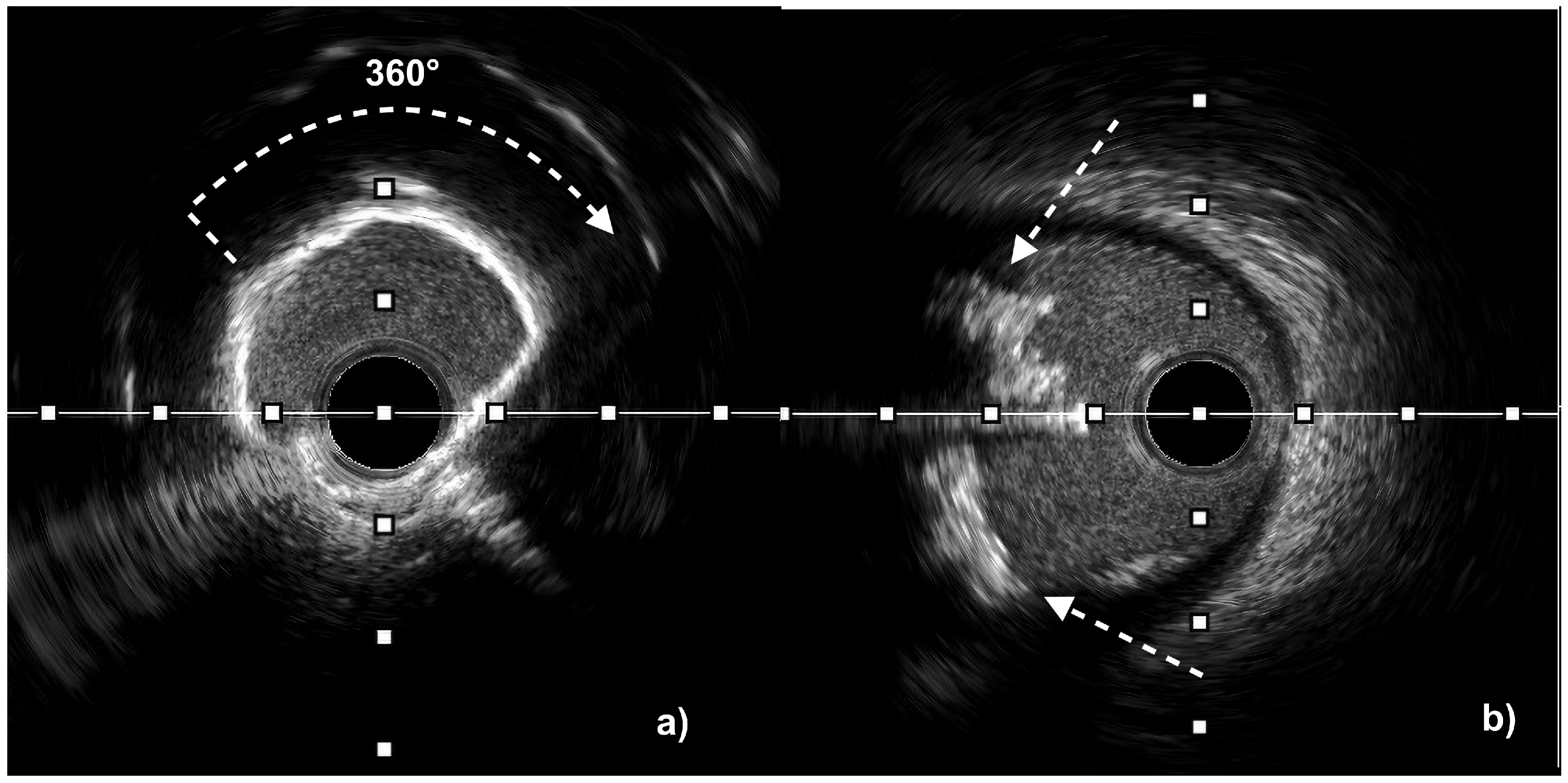
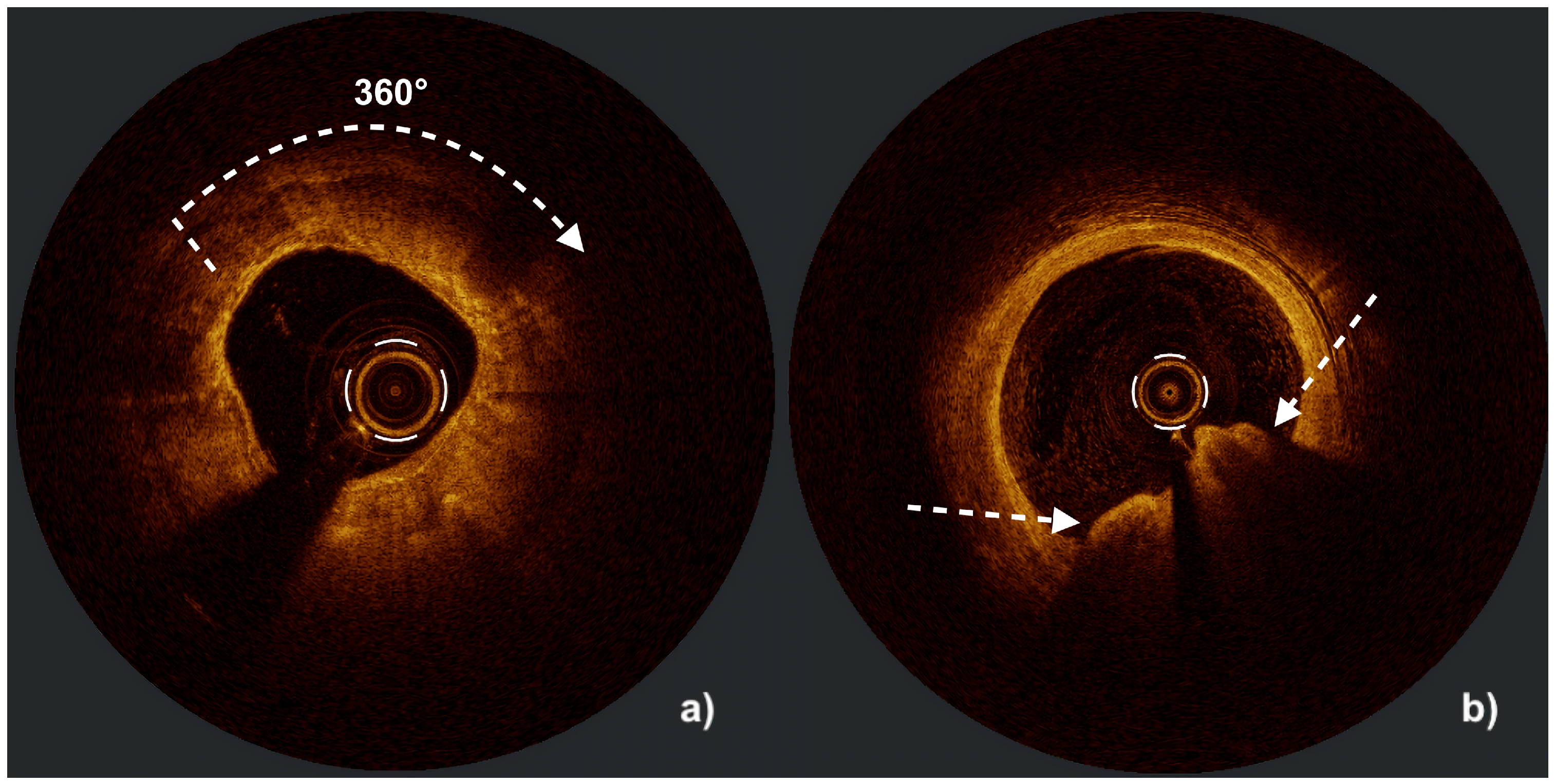
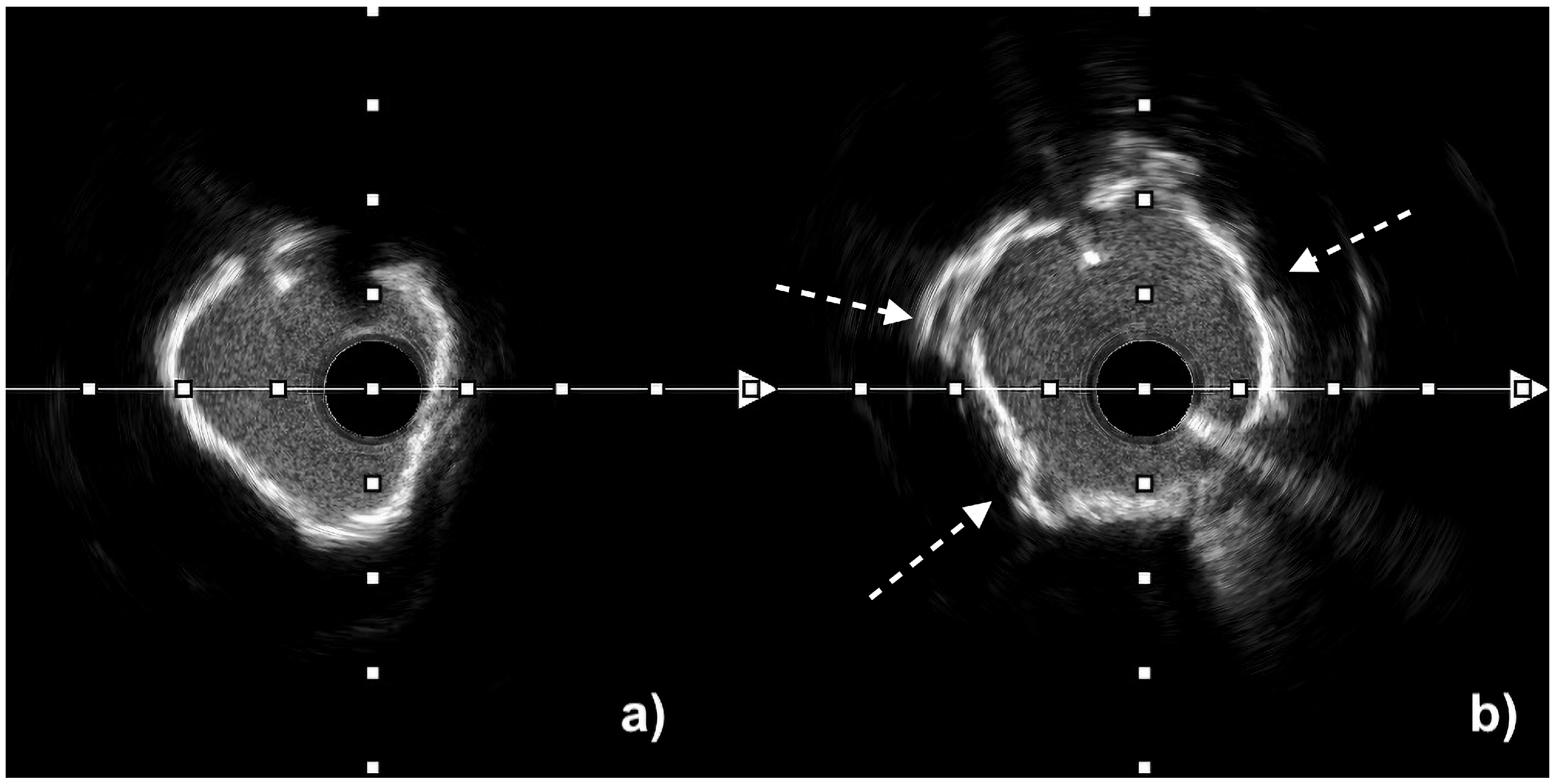
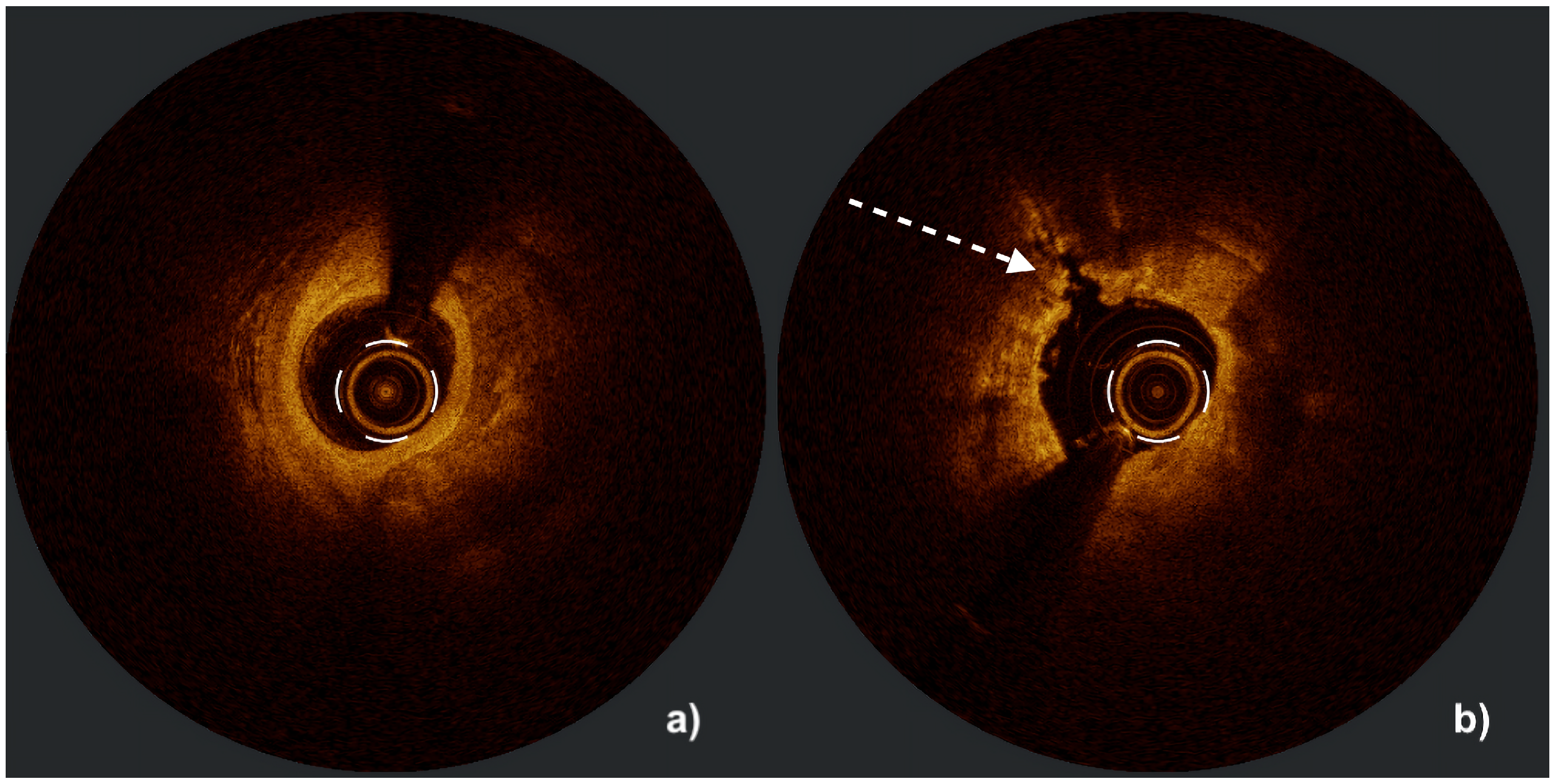
| Aspect | IVUS | OCT |
|---|---|---|
| Imaging modality | Ultrasound | Near-infrared light |
| Axial resolution | 100–150 m (20–40 MHz IVUS probes) 20–60 m (60 MHz HD-IVUS probes) | 10–20 m |
| Lateral resolution | 200 m | 20 m |
| Tissue penetration depth | >5 mm | 1–2 mm |
| Need for blood clearance | No | Yes |
| Need for contrast injection | No | Yes (may be replaced by dextran) |
| Catheter size | Up to 3.2 Fr | 2.7 Fr |
| Assessment of plaque morphology | Moderate resolution for plaque assessment | Superior fine detail (fibrous cap, lipid core, thrombus) |
| Calcium assessment | Semi-quantitative (measures calcium length and arc but only estimates thickness) Microcalcifications not assessable Difficulty in differentiating between eruptive and non-eruptive CNs | Quantitative (measures calcium length, arc, and thickness) Microcalcifications assessable Accurate differentiation between eruptive and non-eruptive CNs Medial calcifications difficult to assess |
| Evaluation of vessel remodeling | Yes | Limited |
| Utility in aorto-ostial lesions | Preferred (guideline-recommended) | Difficult, less preferred |
| Stent expansion assessment | Reliable, deeper wall visualization | Highly precise but limited by EEM 1 visibility |
| Detection of malapposition/dissection | Possible, less sensitive | Superior due to resolution |
| OCT | IVUS | ||||
|---|---|---|---|---|---|
| Calcium arc | <360° | 0 | Calcium arc | ≤270° | 0 |
| 360° | 1 | >270° and >5 mm length | 1 | ||
| Calcium thickness | ≤0.3 mm | 0 | Calcified nodule | No | 0 |
| >0.3 mm | 1 | Yes | 1 | ||
| Length of calcium > 270° | ≤3 mm | 0 | Coronary artery diameter | ≥3.5 mm | 0 |
| >3 mm | 1 | <3.5 mm | 1 | ||
| Aspect | Semi-Compliant Balloon | Non-Compliant Balloon | Super-High-Pressure NC Balloon | Scoring Balloon | Cutting Balloon |
|---|---|---|---|---|---|
| Device design | Thin, extensible single-layer balloon | Thick, non-extensible single-layer balloon | Double-layer, reinforced non-compliant balloon | SC or NC balloon with nitinol scoring elements | NC balloon with longitudinal microblades |
| Primary mechanism of action | Uniform plaque compression and vessel stretching at low pressures | Focal plaque compression, predictable expansion | Modification of fibro-calcific lesions with barostatic force | Controlled scores created by nitinol elements | Tissue microincisions from microblades |
| Typical pressure range (NP-RBP) | ∼6–14 atm | ∼12–22 atm | ∼10–35 atm | ∼8–20 atm | ∼6–12 atm |
| Main indications | Narrow lesions, predilatation in mild disease | Predilatation, postdilatation, moderately calcified lesions | Heavily calcified lesions, resistant underexpanded stents | Fibrotic/moderately calcified lesions, ISR | Focal fibro-calcific lesions, ISR |
| Advantages | Flexibility, deliverability, low crossing profile | Predictable diameter; good stent expansion | Effective for resistant lesions; strong radial force | Reduced slippage, controlled plaque modification | Precise plaque incision; low risk of arterial wall barotrauma |
| Limitations | Ineffective in severe calcification, risk of over-dilatation | Low efficiency in severe calcifications, risk of dissections | Bulky, higher perforation risk if oversized | Bulky, difficult to deliver, costly | Bulky, difficult to deliver, costly |
| Deliverability | + | + | - - | - | - - |
| Calcified lesions | - | + | ++ | ++ | ++ |
| Calcific nodules | - | - | - - | + | + |
| Fibrotic lesions | - | +/- | - | + | ++ |
| Stent underexpansion | - | + | ++ | + | + |
| ISR | - | + | + | ++ | ++ |
| Aspect | IVL | RA | OA | ELCA |
|---|---|---|---|---|
| Mechanism of action | Lithotripsy via acoustic pressure waves | Atheroablation via front abrasion | Atheroablation via sanding | Photoablation (light, acoustic pressure waves, cavitation microbubbles) |
| Guidewire | Elective 0.014″ wire | Dedicated 0.009″/0.014″ tip wire | Dedicated 0.012″/0.014″ tip wire | Elective 0.014″ wire |
| Device size | 2.5–4.0 mm × 12 mm | 1.25–2.5 mm (5–8 Fr) | One crown size 1.25 mm (6 Fr) | 0.9–2.0 mm with concentric and eccentric tip designs |
| Course of action | Forward and backward On the balloon’s adhesion surface | Forward only Outside curve only | Forward and backward Outside and inside curve | Forward only |
| Effect of wire bias | Independent | Dependent | Less dependent | Limited by vessel curvature (UV light does not deflect) |
| Side branch protection | Yes | No | No | Yes |
| Distal embolization | No or very low risk of no/slow reflow | Higher risk of no/slow reflow | Medium risk of no/slow reflow | Very low risk of no/slow reflow |
| Perforation | Low <1% | Up to 1.5% | Up to 1.8% | 1.5–2% |
| Effect on calcium | Affects superficial and deep calcium | Affects only superficial calcium | Affects only superficial calcium | Different effects on superficial and deep calcium |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kałmucki, P.; Skonieczna, P.; Baszko, A.; Siminiak, T. Management of Calcified Coronary Lesions—A Review of Plaque Modification Methods. J. Clin. Med. 2025, 14, 8566. https://doi.org/10.3390/jcm14238566
Kałmucki P, Skonieczna P, Baszko A, Siminiak T. Management of Calcified Coronary Lesions—A Review of Plaque Modification Methods. Journal of Clinical Medicine. 2025; 14(23):8566. https://doi.org/10.3390/jcm14238566
Chicago/Turabian StyleKałmucki, Piotr, Paulina Skonieczna, Artur Baszko, and Tomasz Siminiak. 2025. "Management of Calcified Coronary Lesions—A Review of Plaque Modification Methods" Journal of Clinical Medicine 14, no. 23: 8566. https://doi.org/10.3390/jcm14238566
APA StyleKałmucki, P., Skonieczna, P., Baszko, A., & Siminiak, T. (2025). Management of Calcified Coronary Lesions—A Review of Plaque Modification Methods. Journal of Clinical Medicine, 14(23), 8566. https://doi.org/10.3390/jcm14238566





