Emerging Techniques and Treatment Outcomes in Pulmonary Arteriovenous Malformations Embolisation: A Narrative Review
Abstract
1. Introduction
1.1. Definition and Clinical Significance
1.2. Etiology
1.3. Historical Perspective of Embolisation as a Treatment
2. Pathophysiology and Clinical Presentation of PAVMs
2.1. Right-to-Left Shunting and Hypoxaemia
2.2. Loss of Capillary Filter Function and Paradoxical Emboli
2.3. Cardiac Compensation and Heart Failure
2.4. Diagnostic Pathways
3. Conventional Embolisation Techniques
3.1. Indications and Patient Selection
3.2. Overview of Traditional Materials
3.3. Embolisation Procedure Technique
3.4. Short-Term and Long-Term Success Rate
4. Innovations in Embolisation Materials
4.1. Newer Coil Design
4.2. Microvascular Plugs
5. Imaging Advances Supporting Embolisation
5.1. High-Resolution CTA and 3D Reconstruction
5.2. Cone Beam CT for Intra-Procedural Guidance
5.3. Role of 4D Flow MRI in Assessing Haemodynamics Pre- and Post-Embolisation
5.4. AI-Assisted Image Analysis and Segmentation
6. Technique Modifications and Adjuncts
6.1. Embolisation of Feeding Artery vs. Nidus Targeting
6.2. Dual Catheters Technique
6.3. Balloon-Occlusion Embolisation
6.4. Navigation Tools (Robotic or Augmented Reality Platforms)
7. Clinical Outcomes and Complications
8. Special Populations and Considerations
8.1. Pregnancy
8.2. Management in HHT
9. Future Directions and Research Gaps
9.1. Role of Computational Fluid Dynamics and Personalised Modelling
9.2. Predictive Modelling for Recanalisation Risk
10. Drug-Eluting Embolic Materials
Long-Term Registry and Future Research
Author Contributions
Funding
Conflicts of Interest
References
- Danyalian, A.; Hernandez, F. Pulmonary Arteriovenous Malformation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560696/ (accessed on 22 June 2025).
- Yuranga Weerakkody D’Souza, D. Pulmonary Arteriovenous Malformation. Radiopaedia.org. 2 May 2008. Available online: https://radiopaedia.org/articles/pulmonary-arteriovenous-malformation?lang=gb (accessed on 22 June 2025).
- Shovlin, C.L.; Condliffe, R.; Donaldson, J.W.; Kiely, D.G.; Wort, S.J. British Thoracic Society Clinical Statement on Pulmonary Arteriovenous Malformations. Thorax 2017, 72, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Udan, R.S.; Culver, J.C.; Dickinson, M.E. Understanding vascular development. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 327–346. [Google Scholar] [CrossRef] [PubMed]
- Locke, T.; Gollamudi, J.; Chen, P. Hereditary Hemorrhagic Telangiectasia (HHT). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK578186/ (accessed on 22 June 2025).
- Cullivan, S.; Kevane, B.; McCullagh, B.; O’Connor, T.M.; Condliffe, R.; Gaine, S. Pulmonary vascular manifestations of hereditary haemorrhagic telangiectasia. Pulm. Circ. 2024, 14, e70007. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, J.W.; McKeever, T.M.; Hall, I.P.; Hubbard, R.B.; Fogarty, A.W. The UK prevalence of hereditary haemorrhagic telangiectasia and its association with sex, socioeconomic status and region of residence: A population-based study. Thorax 2014, 69, 161–167. [Google Scholar] [CrossRef]
- McDonald, J.; Pyeritz, R.E. Hereditary Hemorrhagic Telangiectasia. In Adam MP; Nih.gov; University of Washington: Seattle, WA, USA, 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1351/ (accessed on 22 June 2025).
- Bansal, K.; Gore, M.; Mittal, S. Hepatopulmonary Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK562169/ (accessed on 22 June 2025).
- Kavarana, M.N.; Jones, J.A.; Stroud, R.E.; Bradley, S.M.; Ikonomidis, J.S.; Mukherjee, R. Pulmonary arteriovenous malformations after the superior cavopulmonary shunt: Mechanisms and clinical implications. Expert Rev. Cardiovasc. Ther. 2014, 12, 703–713. [Google Scholar] [CrossRef]
- Bakhos, C.T.; Wang, S.C.; Rosen, J.M. Contemporary role of minimally invasive thoracic surgery in the management of pulmonary arteriovenous malformations: Report of two cases and review of the literature. J. Thorac. Dis. 2016, 8, 195–197. [Google Scholar]
- Meek, M.E.; Meek, J.C.; Beheshti, M.V. Management of Pulmonary Arteriovenous Malformations. Semin. Interv. Radiol. 2011, 28, 24–31. [Google Scholar] [CrossRef]
- Yuan, Y. Vascularized lung tissue engineering. In Encyclopedia of Tissue Engineering and Regenerative Medicine; Academic Press: Cambridge, MA, USA, 2019; pp. 179–187. [Google Scholar]
- Si-Mohamed, S.A.; Cierco, A.; Gamondes, D.; Restier, L.M.; Delagrange, L.; Cottin, V.; Dupuis-Girod, S.; Revel, D. Embolization of Recurrent Pulmonary Arteriovenous Malformations by Ethylene Vinyl Alcohol Copolymer (Onyx®) in Hereditary Hemorrhagic Telangiectasia: Safety and Efficacy. J. Pers. Med. 2022, 12, 1091. [Google Scholar] [CrossRef]
- Shovlin, C.L. Pulmonary Arteriovenous Malformations. Am. J. Respir. Crit. Care Med. 2014, 190, 1217–1228. [Google Scholar] [CrossRef]
- Liao, Y.; Chen, K.H.; Huang, G.Y.; Song, W. Pulmonary arteriovenous malformations presenting as refractory heart failure. J. Thorac. Dis. 2014, 6, E169–E172. [Google Scholar]
- Cho, D.; Kim, S.; Kim, M.; Seo, Y.H.; Kim, W.; Kang, S.H.; Park, S.-M.; Shim, W. Two Cases of High Output Heart Failure Caused by Hereditary Hemorrhagic Telangiectasia. Korean Circ. J. 2012, 42, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Saboo, S.S.; Chamarthy, M.; Bhalla, S.; Park, H.; Sutphin, P.; Kay, F.; Battaile, J.; Kalva, S.P. Pulmonary arteriovenous malformations: Diagnosis. Cardiovasc. Diagn. Ther. 2018, 8, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, P.; Lacout, A.; Marcy, P.Y.; Binsse, S.; Sellier, J.; Bensalah, M.; Chinet, T.; Bourgault-Villada, I.; Blivet, S.; Roume, J.; et al. Diagnosis and treatment of pulmonary arteriovenous malformations in hereditary hemorrhagic telangiectasia: An overview. Diagn. Interv. Imaging 2013, 94, 835–848. [Google Scholar] [CrossRef]
- Shimohira, M.; Kawai, T.; Ohta, K. An Update on Embolization for Pulmonary Arteriovenous Malformations. Interv. Radiol. 2023, 8, 56–63. [Google Scholar] [CrossRef]
- Pollak, J.S.; Saluja, S.; Thabet, A.; Henderson, K.J.; Denbow, N.; White, R.I. Clinical and Anatomic Outcomes after Embolotherapy of Pulmonary Arteriovenous Malformations. J. Vasc. Interv. Radiol. 2006, 17, 35–45. [Google Scholar] [CrossRef]
- Hartnell, G.G.; Jackson, J.E.; Allison, D.J. Coil embolization of pulmonary arteriovenous malformations. Cardiovasc. Interv. Radiol. 1990, 13, 347–350. [Google Scholar] [CrossRef]
- Baba, Y. Embolisation coils and microcoils. In Radiopaediaorg; Radiopaedia Australia Pty Ltd.: Melbourne, Australia, 2023. [Google Scholar]
- Liebig, T.; Henkes, H.; Fischer, S.; Weber, W.; Miloslavski, E.; Mariushi, W.; Brew, S.; Kühne, D. Fibered Electrolytically Detachable Platinum Coils Used for the Endovascular Treatment of Intracranial Aneurysms: Initial Experiences and Mid-Term Results in 474 Aneurysms. Interv. Neuroradiol. 2004, 10, 5–26. [Google Scholar] [CrossRef]
- Hong, J.; Lee, S.Y.; Cha, J.G.; Lim, J.K.; Park, J.; Lee, J.; Cha, S.-I.; Kim, C.-H.; Seo, H. Pulmonary arteriovenous malformation (PAVM) embolization: Prediction of angiographically-confirmed recanalization according to PAVM Diameter changes on CT. CVIR Endovasc. 2021, 4, 16. [Google Scholar] [CrossRef]
- Sue, M.J.; Luong, T.T.; Park, J.; Ding, P.-X.; Hao, F.; Eghbalieh, N.; Lee, E.W. A Multicenter, Retrospective, Matched, Comparison Study of Clinical Efficacy and Cost-Effectiveness of Caterpillar Arterial Embolization Device versus Fibered Coils in Arterial Embolization. Appl. Sci. 2022, 12, 5262. [Google Scholar] [CrossRef]
- Wang, W.; Li, H.; Tam, M.K.; Zhou, D.Y.; Wang, D.; Spain, J. The Amplatzer Vascular Plug: A Review of the Device and its Clinical Applications. Cardiovasc. Interv. Radiol. 2012, 35, 725–740. [Google Scholar] [CrossRef]
- Loffroy, R.; Chevallier, O.; Mazit, A.; Malakhia, A.; Midulla, M. AmplatzerTM Vascular Plugs for Embolisation: A 10-Year Single-Centre Retrospective Study. J. Clin. Med. 2023, 12, 6790. [Google Scholar] [CrossRef]
- How I Do It: Pulmonary Arteriovenous Malformations: A Summary of Interventional Management. Endovascular Today. 2022. Available online: https://evtoday.com/articles/2022-apr/how-i-do-it-pulmonary-arteriovenous-malformations-a-summary-of-interventional-management (accessed on 26 June 2025).
- Prasad, S.N.; Sharma, S.; Singh, V.; Phadke, R.V. Endovascular management of pulmonary arteriovenous malformations presenting as multiple brain abscesses. BMJ Case Rep. 2022, 15, e251593. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, G.P.; Reed, R.A.; Larsen, D.; Lee, R.K.; Pentecost, M.J.; Finck, E.J.; Katz, M.D. Microcatheter Embolization of Non-neurologic Traumatic Vascular Lesions. J. Vasc. Interv. Radiol. 1993, 4, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Guneyli, S.; Cinar, C.; Bozkaya, H.; Parildar, M.; Oran, I. Applications of the Amplatzer Vascular Plug to various vascular lesions. Diagn. Interv. Radiol. 2014, 20, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.; Kohno, S.; Arizono, S.; Onishi, Y.; Fumimoto, M.; Yoshida, A.; Ishikura, R.; Ando, K. Enhancing precision in vascular embolization: Evaluating the effectiveness of the intentional early detachment technique with detachable coils in complex cases. CVIR Endovasc. 2024, 7, 40. [Google Scholar] [CrossRef]
- Xiao, N.; Lewandowski, R.J. Embolic Agents: Coils. Semin. Interv. Radiol. 2022, 39, 113–118. [Google Scholar] [CrossRef]
- OpenAthens. Sign in Oclc.org. 2025. Available online: https://www-sciencedirect-com.knowledge.idm.oclc.org/science/article/pii/S1089251608000085 (accessed on 26 June 2025).
- Botsford, A.; Tradi, F.; Loubet, A.; Tantawi, S.; Soulez, G.; Giroux, M.-F.; Faughnan, M.E.; Gauthier, A.; Perreault, P.; Bouchard, L.; et al. Transarterial Embolization of Simple Pulmonary Arteriovenous Malformations: Long-Term Outcomes of 0.018-Inch Coils versus Vascular Plugs. J. Vasc. Interv. Radiol. 2024, 35, 349–360. [Google Scholar] [CrossRef]
- Ferral, H. Hydrogel-Coated Coils: Product Description and Clinical Applications. Semin. Interv. Radiol. 2015, 32, 343–348. [Google Scholar] [CrossRef]
- Iguchi, T.; Hiraki, T.; Matsui, Y.; Fujiwara, H.; Sakurai, J.; Baba, K.; Toyooka, S.; Gobara, H.; Kanazawa, S. Embolization using hydrogel-coated coils for pulmonary arteriovenous malformations. Diagn. Interv. Imaging 2020, 101, 129–135. [Google Scholar] [CrossRef]
- Embolization of a Large Pulmonary Arteriovenous Malformation. Endovascular Today. 2025. Available online: https://evtoday.com/articles/2014-apr-supplement/embolization-of-a-large-pulmonary-arteriovenous-malformation (accessed on 26 June 2025).
- Mathevosian, S.; Sparks, H.; Cusumano, L.; Roberts, D.; Majumdar, S.; McWilliams, J. Embolization of De Novo Pulmonary Arteriovenous Malformations Using High-Volume Detachable Non-Fibered Coils: Propensity-Matched Comparison to Traditional Coils. J. Clin. Med. 2024, 13, 648. [Google Scholar] [CrossRef]
- BIBA Publishing. MVP Microvascular Plug for Peripheral Embolization Gets the CE Mark. Interventional News. 2013. Available online: https://interventionalnews.com/mvp-microvascular-plug-for-peripheral-embolization-gets-the-ce-mark/ (accessed on 26 June 2025).
- The MVPTM Micro Vascular Plug: A New Paradigm in Peripheral Embolization. Endovascular Today. 2015. Available online: https://evtoday.com/articles/2015-apr/the-mvp-micro-vascular-plug-a-new-paradigm-in-peripheral-embolization (accessed on 26 June 2025).
- Mailli, R.; Chevallier, O.; Mazit, A.; Malakhia, A.; Falvo, N.; Loffroy, R. Embolisation Using Microvascular Plugs for Peripheral Applications: Technical Results and Mid-Term Outcomes. Biomedicines 2023, 11, 2172. [Google Scholar] [CrossRef] [PubMed]
- Conrad, M.B.; Ishaque, B.M.; Surman, A.M.; Kerlan RKJr Hope, M.D.; Dickey, M.A.; Hetts, S.W.; Wilson, M.W. Intraprocedural safety and technical success of the MVP Micro Vascular Plug for embolization of pulmonary arteriovenous malformations. J. Vasc. Interv. Radiol. 2015, 26, 1735–1739. [Google Scholar] [CrossRef] [PubMed]
- Ratnani, R.; Sutphin, P.D.; Koshti, V.; Park, H.; Chamarthy, M.; Battaile, J.; Kalva, S.P. Retrospective comparison of pulmonary arteriovenous malformation embolization with the polytetrafluoroethylene-covered nitinol microvascular plug, AMPLATZER plug, and coils in patients with hereditary hemorrhagic telangiectasia. J. Vasc. Interv. Radiol. 2019, 30, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Latif, M.A.; Bailey, C.R.; Motaghi, M.; Areda, M.A.; Galiatsatos, P.; Mitchell, S.E.; Weiss, C.R. Postembolization Persistence of Pulmonary Arteriovenous Malformations: A Retrospective Comparison of Coils and Amplatzer and Micro Vascular Plugs Using Propensity Score Weighting. Am. J. Roentgenol. 2023, 220, 95–103. [Google Scholar] [CrossRef]
- Shahin, Y.; Gill, A.; Lejawka, A.; Willis, R.; Vijayakumar, C.; Abbas, M.; Kusumawidjaja, D. Embolization Outcomes of Pulmonary Arteriovenous Malformations: A 10-Year Experience from a Tertiary Referral Center. Arab. J. Interv. Radiol. 2024, 8, A89. [Google Scholar] [CrossRef]
- Giurazza, F.; Ierardi, A.M.; Contegiacomo, A.; Corvino, F.; Carrafiello, G.; Niola, R. Embolization with MVP (Micro Vascular Plug®): Experience on 104 patients in emergent and elective scenarios. CVIR Endovasc. 2021, 4, 59. [Google Scholar] [CrossRef]
- Shin, S.M.; Kim, H.K.; Crotty, E.J.; Hammill, A.M.; Wusik, K.; Kim, D.H. CT Angiography Findings of Pulmonary Arteriovenous Malformations in Children and Young Adults With Hereditary Hemorrhagic Telangiectasia. Am. J. Roentgenol. 2020, 214, 1369–1376. [Google Scholar] [CrossRef]
- Kato, Y.; Sano, H.; Katada, K.; Ogura, Y.; Hayakawa, M.; Kanaoka, N.; Kanno, T. Application of three-dimensional CT angiography (3D-CTA) to cerebral aneurysms. Surg. Neurol. 1999, 52, 113–122. [Google Scholar] [CrossRef]
- Barral, M.; Chevallier, O.; Cornelis, F.H. Perspectives of Cone-Beam Computed Tomography in Interventional Radiology: Techniques for Planning, Guidance, and Monitoring. Tech. Vasc. Interv. Radiol. 2023, 26, 100912. [Google Scholar] [CrossRef]
- Park, S.J.; Cho, Y.; Lee, H.N.; Lee, S.; Chung, H.H.; Park, C.H. Enhancing procedural decision making with cone beam CT in renal artery embolization. Sci. Rep. 2024, 14, 18198. [Google Scholar] [CrossRef]
- Raz, E.; Nossek, E.; Sahlein, D.H.; Sharashidze, V.; Narayan, V.; Ali, A.; Esparza, R.; Peschillo, S.; Chung, C.; Diana, F.; et al. Principles, techniques and applications of high resolution cone beam CT angiography in the neuroangio suite. J. Neurointerv. Surg. 2023, 15, 600–607. [Google Scholar] [CrossRef]
- Hyodo, R.; Takehara, Y.; Mizuno, T.; Ichikawa, K.; Horiguchi, R.; Kawakatsu, S.; Ebata, T.; Naganawa, S.; Jin, N.; Ichiba, Y. Four-dimensional Flow MRI Assessment of Portal Hemodynamics and Hepatic Regeneration after Portal Vein Embolization. Radiology 2023, 308, e230709. [Google Scholar] [CrossRef]
- Yamashita, R.; Nishio, M.; Do, R.K.G.; Togashi, K. Convolutional neural networks: An overview and application in radiology. Insights Imaging 2018, 9, 611–629. [Google Scholar] [CrossRef] [PubMed]
- Langius-Wiffen, E.; de Jong, P.A.; Hoesein, F.A.M.; Dekker, L.; van den Hoven, A.F.; Nijholt, I.M.; Boomsma, M.F.; Veldhuis, W.B. Retrospective batch analysis to evaluate the diagnostic accuracy of a clinically deployed AI algorithm for the detection of acute pulmonary embolism on CTPA. Insights Imaging 2023, 14, 102. [Google Scholar] [CrossRef] [PubMed]
- Mank, Q.J.; Thabit, A.; Maat, A.P.W.M.; Siregar, S.; van Walsum, T.; Kluin, J.; Sadeghi, A.H. Artificial intelligence-based pulmonary vessel segmentation: An opportunity for automated three-dimensional planning of lung segmentectomy. Interdiscip. Cardiovasc. Thorac. Surg. 2025, 40, ivaf101. [Google Scholar] [CrossRef] [PubMed]
- Müller-Hülsbeck, S.; Marques, L.; Maleux, G.; Osuga, K.; Pelage, J.P.; Wohlgemuth, W.A.; Andersen, P.E. CIRSE Standards of Practice on Diagnosis and Treatment of Pulmonary Arteriovenous Malformations. Cardiovasc. Interv. Radiol. 2020, 43, 353–361. [Google Scholar] [CrossRef]
- Hayashi, S.; Baba, Y.; Senokuchi, T.; Nakajo, M. Efficacy of Venous Sac Embolization for Pulmonary Arteriovenous Malformations: Comparison with Feeding Artery Embolization. J. Vasc. Interv. Radiol. 2012, 23, 1566–1577. [Google Scholar] [CrossRef]
- Srinivas, S.; Roberts, D.G.; McWilliams, J.P.; Cusumano, L.R. Feeding-Artery Microvascular Plug Embolization Versus Nidus-Plus-Feeding-Artery Coil Embolization of Pulmonary Arteriovenous Malformations. J. Clin. Med. 2025, 14, 2980. [Google Scholar] [CrossRef]
- Hirano, Y.; Koizumi, S.; Shojima, M.; Ishikawa, O.; Kiyofuji, S.; Umekawa, M.; Saito, N. Double-catheter technique for the embolization of recurrent cerebral aneurysms: A single-center experience. Surg. Neurol. Int. 2023, 14, 273. [Google Scholar] [CrossRef]
- Greben, C.R.; Setton, A.; Putterman, D.; Caplin, D.; Lenner, R.; Gandras, E.J. Pulmonary Arteriovenous Malformation Embolization: How We Do It. Tech. Vasc. Interv. Radiol. 2013, 16, 39–44. [Google Scholar] [CrossRef]
- Abecassis, I.J.; Nerva, J.D.; Ghodke, B.V.; Sekhar, L.N.; Levitt, M.R.; Kim, L.J. The dual microcatheter technique for transvenous embolization of dural arteriovenous fistulae. J. Neurointerv. Surg. 2017, 9, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Baxter, B.; Rosso, D.; Lownie, S. Double microcatheter technique for detachable coil treatment of large, wide-necked intracranial aneurysms. Am. J. Neuroradiol. 1998, 19, 1176–1178. [Google Scholar] [PubMed]
- Kanematsu, M.; Kondo, H.; Goshima, S.; Tsuge, Y.; Watanabe, H.; Moriyama, N. Giant High-Flow Type Pulmonary Arteriovenous Malformation: Coil Embolization with Flow Control by Balloon Occlusion and an Anchored Detachable Coil. Korean J. Radiol. 2012, 13, 111–114. [Google Scholar] [CrossRef]
- Cil, B.E.; Erdogan, C.; Akmangit, I.; Cekirge, S.; Balkanci, F. Use of the TriSpan Coil to Facilitate the Transcatheter Occlusion of Pulmonary Arteriovenous Malformation. Cardiovasc. Interv. Radiol. 2004, 27, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Shiigai, M.; Saida, T.; Anno, I.; Wada, M.; Minami, M. A Modified Metallic Coil Embolization Technique for Pulmonary Arteriovenous Malformations Using Coil Anchors and Occlusion Balloon Catheters. Cardiovasc. Interv. Radiol. 2008, 31, 638–642. [Google Scholar] [CrossRef]
- Chu, H.H.; Kim, G.H.; Gwon, D.I. An Alternative Endovascular Technique for Treatment of Pulmonary Arteriovenous Malformation: Microballoon-occluded Transcatheter Embolization Using n-butyl-2-cyanoacrylate. Cardiovasc. Interv. Radiol. 2024, 47, 1109–1116. [Google Scholar] [CrossRef]
- Mendes Pereira, V.; Rice, H.; De Villiers, L.; Sourour, N.; Clarencon, F.; Spears, J.; Tomasello, A.; Hernandez, D.; Cancelliere, N.M.; Liu, X.Y.E.; et al. Evaluation of effectiveness and safety of the CorPath GRX robotic system in endovascular embolization procedures of cerebral aneurysms. J. Neurointerv. Surg. 2024, 16, 405–411. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, X.; Chan, K.F.; Song, X.; Zhang, L. Robotic–Assisted Endovascular Embolization: Progress and Future Perspectives. SmartBot 2025, 1, e12009. [Google Scholar] [CrossRef]
- Letourneau-Guillon, L.; Faughnan, M.E.; Soulez, G.; Giroux, M.F.; Oliva, V.L.; Boucher, L.M.; Dubois, J.; Prabhudesai, V.; Therasse, E. Embolization of Pulmonary Arteriovenous Malformations with Amplatzer Vascular Plugs: Safety and Midterm Effectiveness. J. Vasc. Interv. Radiol. 2010, 21, 649–656. [Google Scholar] [CrossRef]
- Chamarthy, M.R.; Park, H.; Sutphin, P.; Kumar, G.; Lamus, D.; Saboo, S.; Anderson, M.; Kalva, S.P. Pulmonary arteriovenous malformations: Endovascular therapy. Cardiovasc. Diagn. Ther. 2018, 8, 338–349. [Google Scholar] [CrossRef]
- Lanza, E.; Gennaro, N.; Poretti, D.; Straffi, L.; Marcheselli, S.; Tramarin, M.; Pedicini, V. Full recovery after non-target cerebral embolization of n-butyl-cyanoacrylate occurred during emergency treatment of a facial arteriovenous malformation. CVIR Endovasc. 2019, 2, 20. [Google Scholar] [CrossRef]
- Haitjerm, T.; ten Berg, J.M.; Overtoom, T.T.C.; Ernst, J.M.P.G.; Westermann, C.J.J. Unusual Complications After Embolization of a Pulmonary Arteriovenous Malformation. Chest 1996, 109, 1401–1404. [Google Scholar] [CrossRef]
- Di Guardo, F.; Presti, V.L.; Costanzo, G.; Zambrotta, E.; Di Gregorio, L.M.; Basile, A.; Palumbo, M. Pulmonary Arteriovenous Malformations (PAVMs) and Pregnancy: A Rare Case of Hemothorax and Review of the Literature. Case Rep. Obstet. Gynecol. 2019, 2019, 8165791. [Google Scholar] [CrossRef] [PubMed]
- Redirect Notice. Google.com. 2025. Available online: https://curehht.org/guidelines/pregnancy/ (accessed on 17 November 2025).
- Majumdar, S.; McWilliams, J.P. Approach to Pulmonary Arteriovenous Malformations: A Comprehensive Update. J. Clin. Med. 2020, 9, 1927. [Google Scholar] [CrossRef] [PubMed]
- Narsinh, K.H.; Ramaswamy, R.; Kinney, T.B. Management of Pulmonary Arteriovenous Malformations in Hereditary Hemorrhagic Telangiectasia Patients. Semin. Interv. Radiol. 2013, 30, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Computational Fluid Dynamics. An overview|ScienceDirect Topics. Sciencedirect.com. 2012. Available online: https://www.sciencedirect.com/topics/materials-science/computational-fluid-dynamics (accessed on 22 June 2025).
- Reid, L.; Rea, P.M. An Introduction to Biomedical Computational Fluid Dynamics. In Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2021; pp. 205–222. [Google Scholar]
- Wang, Q.; Guo, X.; Stäb, D.; Jin, N.; Poon, E.K.W.; Lim, R.P.; Ooi, A. Computational fluid dynamic simulations informed by CT and 4D flow MRI for post-surgery aortic dissection—A case study. Int. J. Heat Fluid Flow 2022, 96, 108986. [Google Scholar] [CrossRef]
- Wiśniewski, K.; Tomasik, B.; Tyfa, Z.; Reorowicz, P.; Bobeff, E.J.; Stefańczyk, L.; Posmyk, B.J.; Jóźwik, K.; Jaskólski, D.J. Porous Media Computational Fluid Dynamics and the Role of the First Coil in the Embolization of Ruptured Intracranial Aneurysms. J. Clin. Med. 2021, 10, 1348. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, X.; Zhang, X.; Ding, G.; Ge, L.; Wang, S. Computational modeling and simulation for endovascular embolization of cerebral arteriovenous malformations with liquid embolic agents. Acta Mech. Sin. 2024, 40, 623042. [Google Scholar] [CrossRef]
- Aoki, K.; Nagashima, H.; Murayama, Y. Risk factors for recanalization after coil embolization for cerebral aneurysms: Importance of the first coil and prediction model. J. Stroke Cerebrovasc. Dis. 2025, 34, 108333. [Google Scholar] [CrossRef]
- Ogilvy, C.S.; Chua, M.H.; Fusco, M.R.; Reddy, A.S.; Thomas, A.J. Stratification of Recanalization for Patients With Endovascular Treatment of Intracranial Aneurysms. Neurosurgery 2015, 76, 390–395. [Google Scholar] [CrossRef]
- He, T.; Chen, K.; Chen, R.D. A predictive model for the recurrence of intracranial aneurysms following coil embolization. Front. Neurol. 2023, 14, 1248603. [Google Scholar] [CrossRef]
- Park, K.B.; Do, Y.S.; Kim, D.I.; Kim, Y.W.; Park, H.S.; Shin, S.W.; Cho, S.K.; Hyun, D.-H.; Choo, S.W. Endovascular treatment results and risk factors for complications of body and extremity arteriovenous malformations. J. Vasc. Surg. 2019, 69, 1207–1218. [Google Scholar] [CrossRef]
- Mikhail, A.S.; Negussie, A.H.; Mauda-Havakuk, M.; Owen, J.W.; Pritchard, W.F.; Lewis, A.L.; Wood, B.J. Drug-eluting embolic microspheres: State-of-the-art and emerging clinical applications. Expert Opin. Drug Deliv. 2021, 18, 383–398. [Google Scholar] [CrossRef]
- Shahin, Y.; Vijayakumar, C.; Gill, A.; Lejawka, A.; Bennett, S.; Willis, R.; Abbas, M.; Kusumawidjaja, D. A Meta-Analysis and Meta-Regression of Embolisation Outcomes of Pulmonary Arteriovenous Malformations. Cardiovasc. Interv. Radiol. 2025, 48, 167–179. [Google Scholar] [CrossRef]
- Trotter, J.P. Patient registries: A new gold standard for “real world” research. Ochsner J. 2002, 4, 211–214. [Google Scholar]
- Pisa, F.; Arias, A.; Bratton, E.; Salas, M.; Sultana, J. Real world data for rare diseases research: The beginner’s guide to registries. Expert Opin. Orphan Drugs 2023, 11, 9–15. [Google Scholar] [CrossRef]
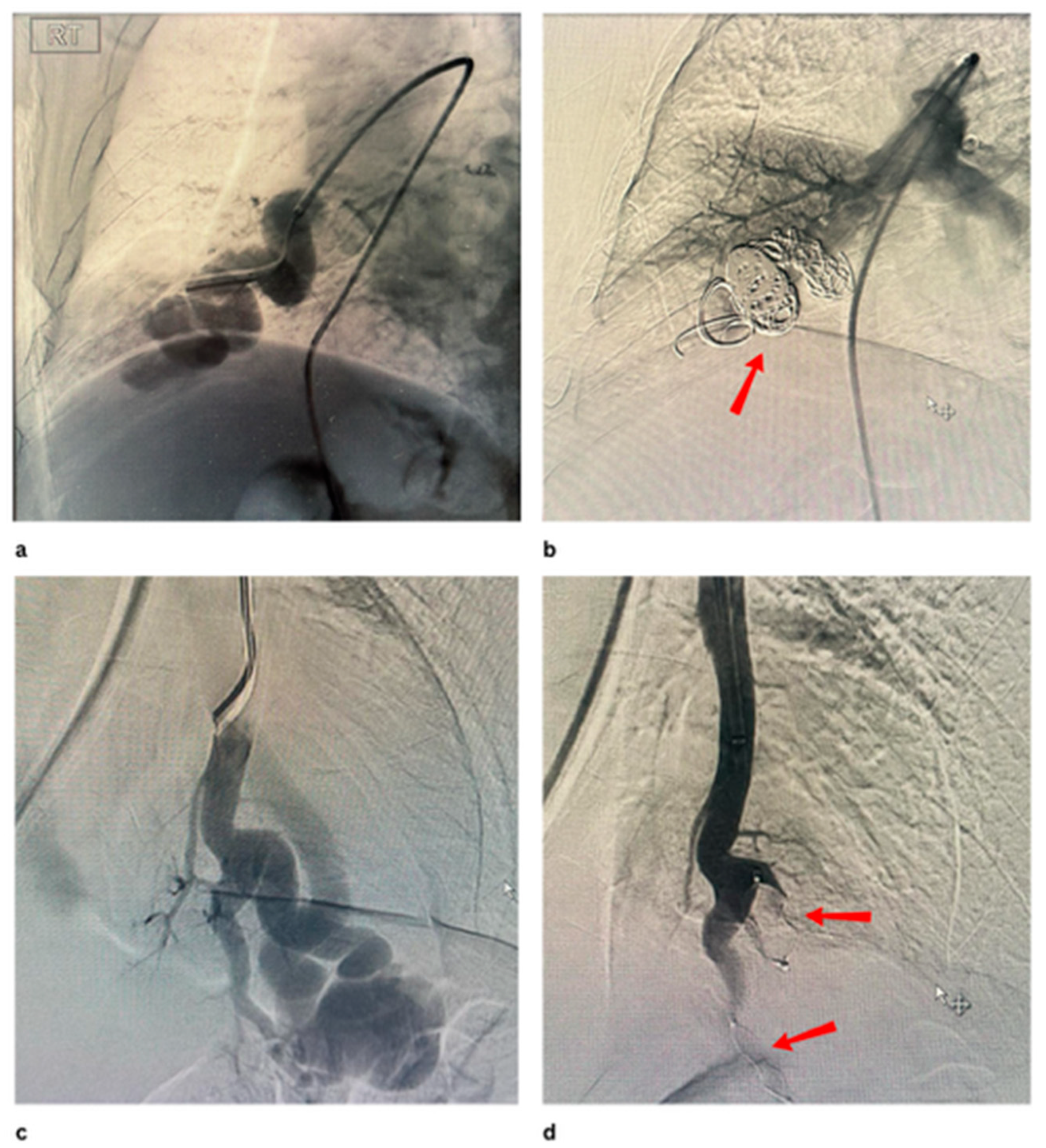
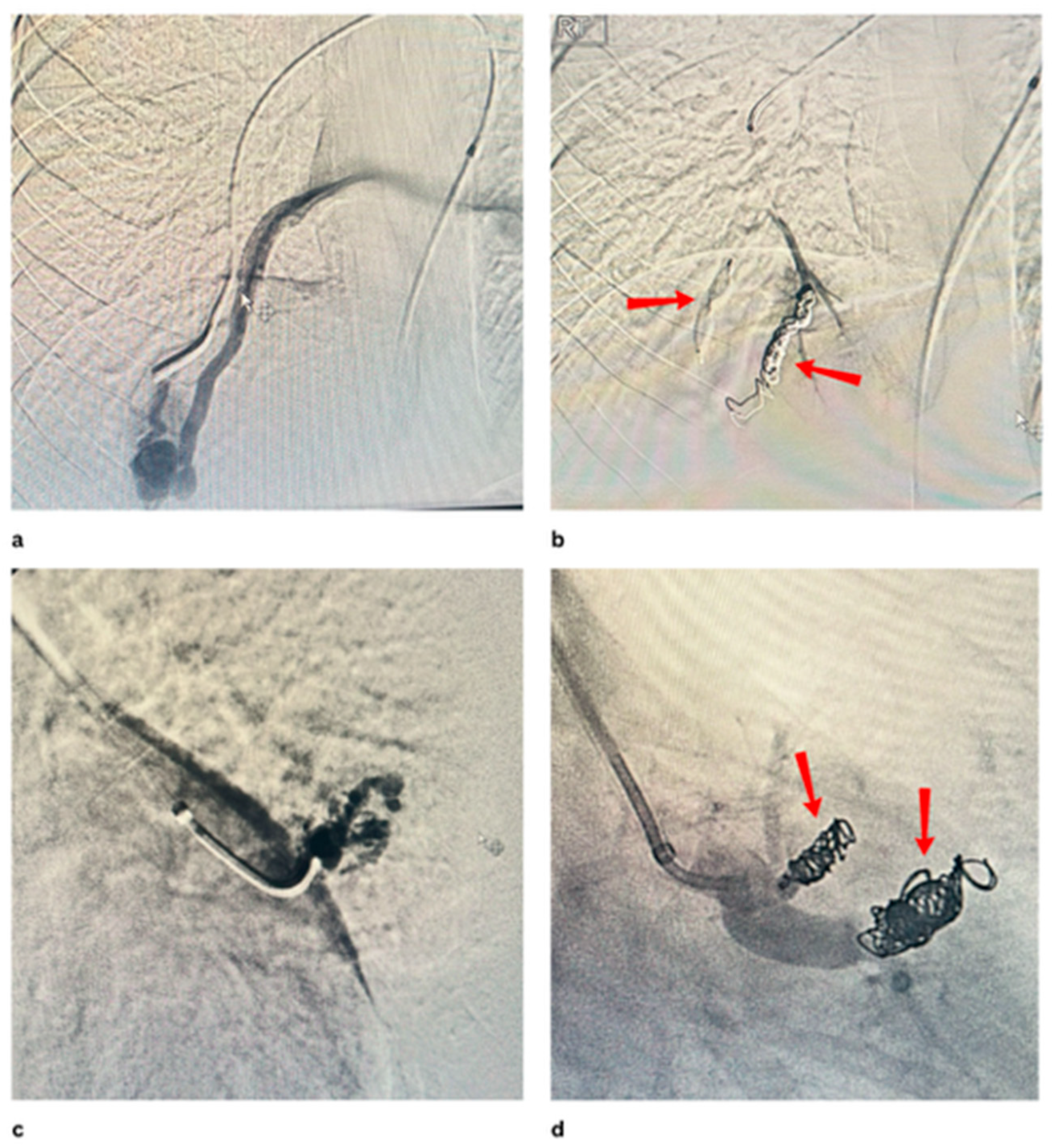
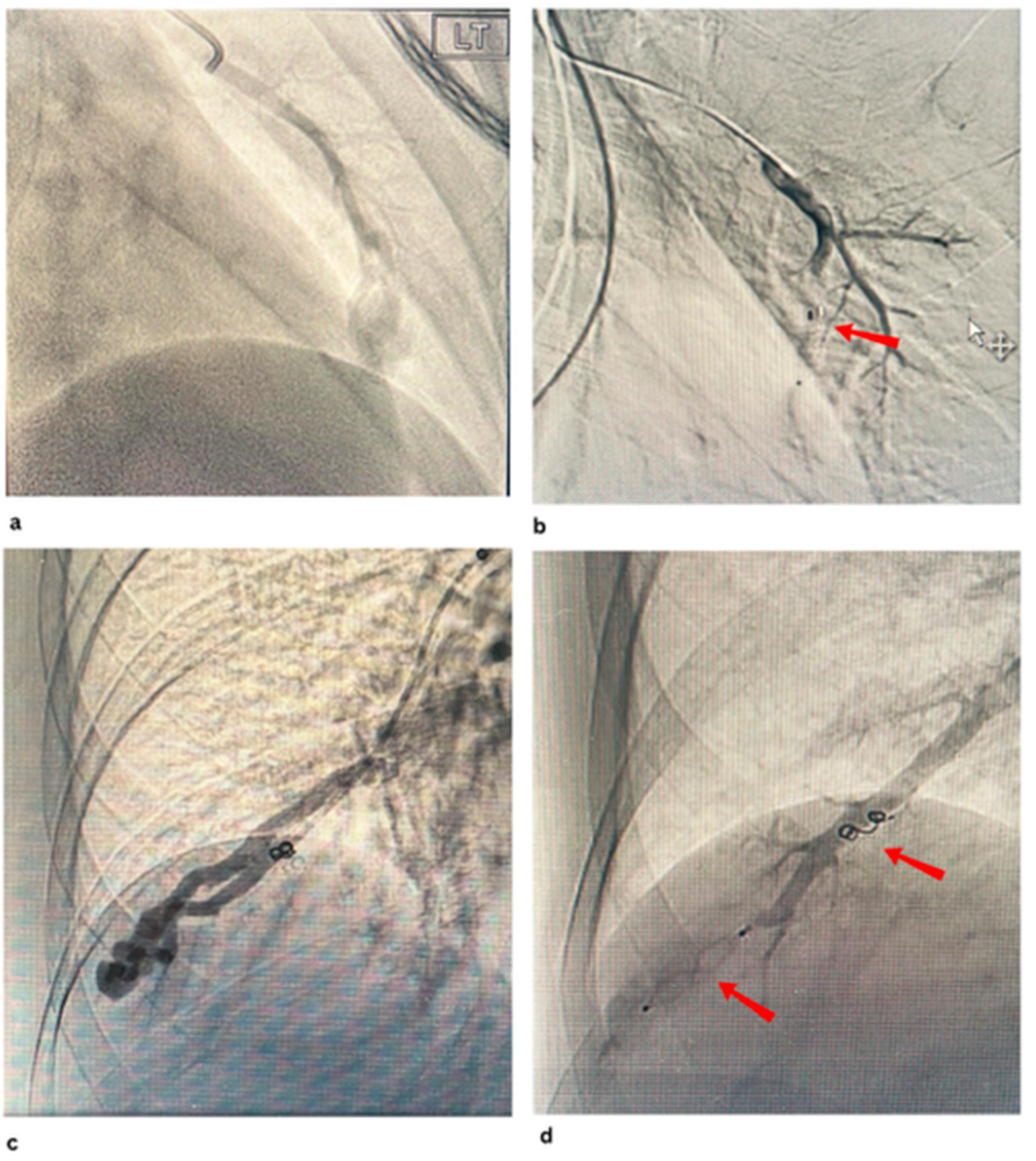
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, C.J.; Shahin, Y. Emerging Techniques and Treatment Outcomes in Pulmonary Arteriovenous Malformations Embolisation: A Narrative Review. J. Clin. Med. 2025, 14, 8455. https://doi.org/10.3390/jcm14238455
Lim CJ, Shahin Y. Emerging Techniques and Treatment Outcomes in Pulmonary Arteriovenous Malformations Embolisation: A Narrative Review. Journal of Clinical Medicine. 2025; 14(23):8455. https://doi.org/10.3390/jcm14238455
Chicago/Turabian StyleLim, Chai Jin, and Yousef Shahin. 2025. "Emerging Techniques and Treatment Outcomes in Pulmonary Arteriovenous Malformations Embolisation: A Narrative Review" Journal of Clinical Medicine 14, no. 23: 8455. https://doi.org/10.3390/jcm14238455
APA StyleLim, C. J., & Shahin, Y. (2025). Emerging Techniques and Treatment Outcomes in Pulmonary Arteriovenous Malformations Embolisation: A Narrative Review. Journal of Clinical Medicine, 14(23), 8455. https://doi.org/10.3390/jcm14238455




