Cytokine Changes in Gingival Crevicular Fluid and Serum After Non-Surgical Periodontal Therapy in Patients with Periodontitis: A Systematic Review and Narrative Synthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Focus Question
2.3. Population
2.4. Comparator
2.5. Outcome
2.6. Search Strategy
2.7. Selection Criteria
2.8. Intervention
2.9. Methodological Appraisal (Risk of Bias) and Certainty of Evidence
2.10. Data Synthesis (Non-Meta)
3. Results
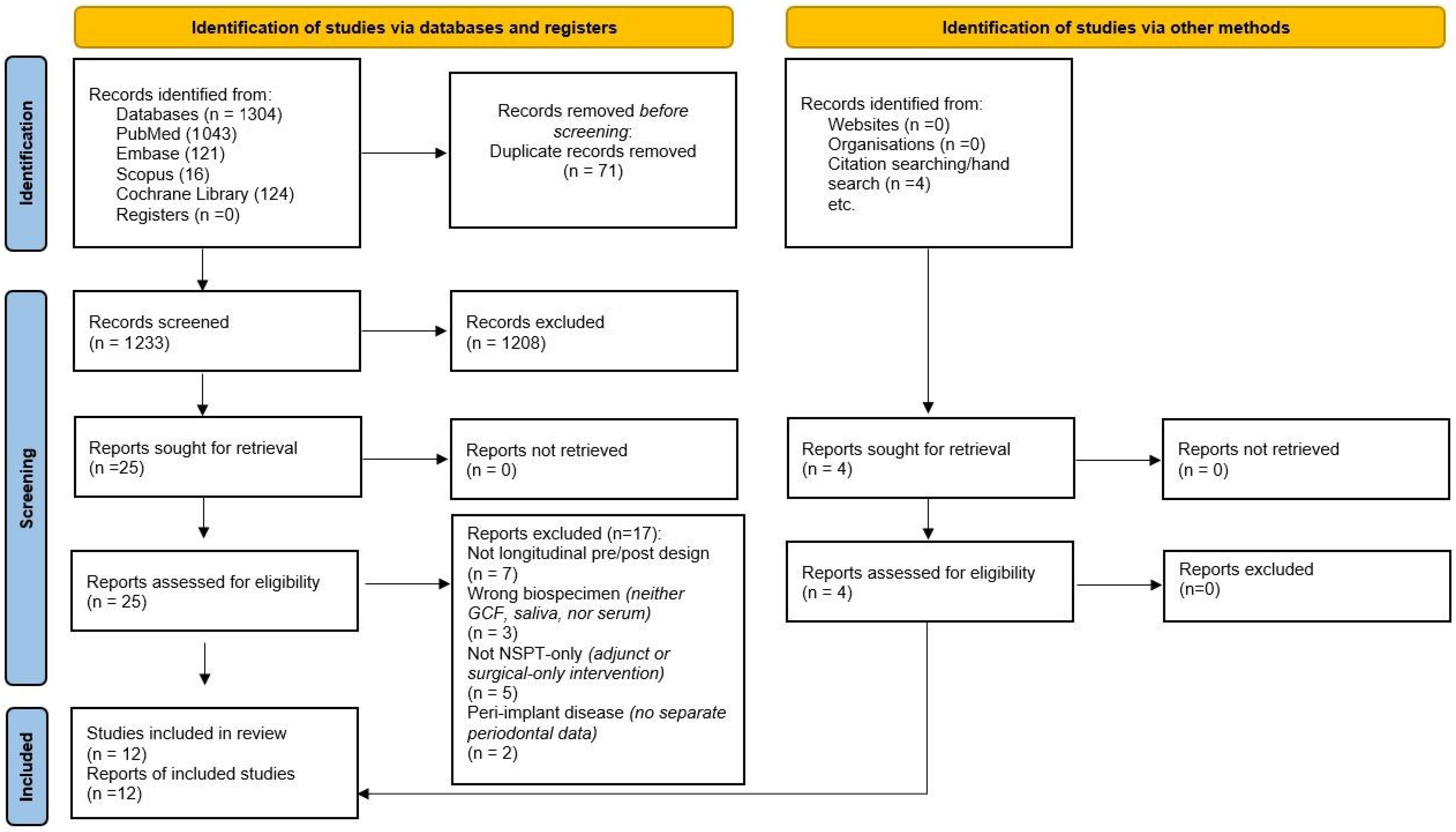
3.1. Study Selection:
3.2. Study Characteristics
3.3. Results Structure
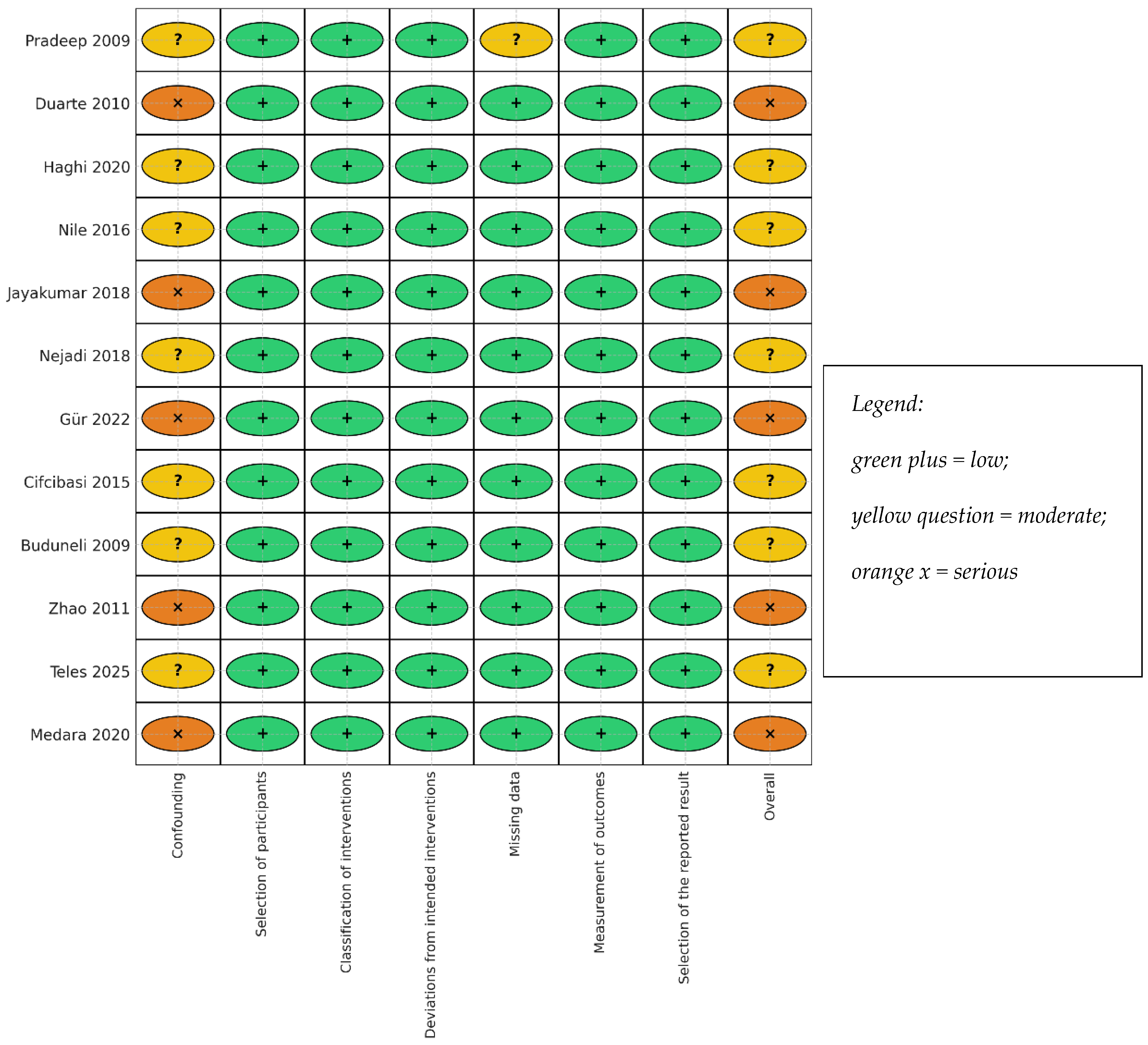
3.4. Risk of Bias
3.5. Local (GCF) Cytokine Kinetics After NSPT
3.5.1. IL-17A
3.5.2. IL-23
3.5.3. IL-21
3.5.4. IL-22
3.5.5. IL-17A and IL-23
3.5.6. Counter-Regulation (e.g., IL-17E/IL-25 Ratio)
3.5.7. Bone Coupling Mediators (sRANKL/OPG)
3.5.8. Clinical–Biochemical Coupling (PD, CAL, BOP vs. Cytokines)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Lamont, R.J. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Mol. Med. 2012, 18, 477–485. [Google Scholar]
- Socransky, S.S.; Haffajee, A.D. Periodontal microbial complexes. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Darveau, R.P. Periodontitis: A polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 2010, 8, 481–490. [Google Scholar] [CrossRef]
- Hajishengallis, G. Immunomicrobial pathogenesis of periodontitis. Trends Immunol. 2014, 35, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Hughes, A.; Taams, L. Presence, function and regulation of IL17 and Th17 cells in periodontitis. J. Clin. Periodontol. 2014, 41, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Zenobia, E.; Hajishengallis, G. Basic biology and role of IL17 in mucosal immunity. Mucosal Immunol. 2015, 8, 558–568. [Google Scholar]
- Vernal, A. Levels of IL17 in GCF and tissue supernatants in chronic periodontitis. J. Clin. Periodontol. 2005, 32, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Dutzan, N.; Gamonal, J.; Silva, A.; Sanz, M.; Vernal, R. Over-expression of forkhead box P3 and its association with receptor activator of nuclear factor-kappa B ligand, interleukin (IL)-17, IL-10 and transforming growth factor-beta during the progression of chronic periodontitis. J. Clin. Periodontol. 2009, 36, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Dutzan, M. Interleukin21 expression and its association with proinflammatory cytokines in chronic periodontitis. J. Periodontol. 2012, 83, 1093–1103. [Google Scholar] [CrossRef]
- Díaz-Zúñiga, J.; Melgar-Rodríguez, S.; Rojas, C.; Alvarez, C.; Monasterio, G. Increased interleukin22 and aryl hydrocarbon receptor in periodontitis associate with osteoclast resorptive activity and disease severity. J. Periodont. Res. 2017, 52, 893–902. [Google Scholar] [CrossRef]
- Monasterio, G.; Rojas, C.; Alvarez, C.; Díaz-Zúñiga, J. IL22–expressing CD4+AhR+ T lymphocytes are associated with RANKLmediated alveolar bone resorption during experimental periodontitis. J. Periodont. Res. 2019, 54, 513–524. [Google Scholar] [CrossRef]
- Huang, N.; Dong, H.; Luo, Y.; Shao, B. Th17 cells in periodontitis and its regulation by A20. Front. Immunol. 2021, 12, 742925. [Google Scholar] [CrossRef] [PubMed]
- Neurath, N.; Kesting, M. Cytokines in gingivitis and periodontitis: From pathogenesis to therapy. Front. Immunol. 2024, 15, 1435054. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, A.R.; Hadge, P.; Chowdhry, S.; Patel, S.; Happy, D. Exploring the role of Th1 cytokines: Interleukin-17 and interleukin-18 in periodontal health and disease. J. Oral Sci. 2009, 51, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Buduneli, N.; Buduneli, E.; Kütükçüler, N. Interleukin-17, RANKL, and osteoprotegerin levels in gingival crevicular fluid from smoking and non-smoking patients with chronic periodontitis during initial periodontal treatment. J. Periodontol. 2009, 80, 1274–1280. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89, S159–S172. [Google Scholar] [CrossRef]
- Sanz, M.; Herrera, D.; Kebschull, M. Treatment of stage I–III periodontitis: EFP S3level clinical practice guideline. J. Clin. Periodontol. 2020, 47, 4–60. [Google Scholar] [CrossRef]
- Graziani, F.; Karapetsa, D.; Alonso, B.; Herrera, D. Nonsurgical periodontal therapy outcomes: The longevity of periodontal stability. Periodontol. 2000 2017, 75, 79–99. [Google Scholar]
- Takahashi, K. The potential role of interleukin17 in the immunopathology of periodontal disease. J. Clin. Periodontol. 2005, 32, 369–374. [Google Scholar] [CrossRef]
- Takayanagi, H. Osteoimmunology: Shared mechanisms and crosstalk. Nat. Rev. Immunol. 2007, 7, 292–304. [Google Scholar] [CrossRef]
- Udagawa, N.; Koide, M.; Nakamura, M.; Nakamichi, Y.; Yamashita, T.; Uehara, S.; Kobayashi, Y.; Furuya, Y.; Yasuda, H.; Fukuda, C.; et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J. Bone Miner. Metab. 2021, 39, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhou, Y.; Xu, Y.; Sun, Y.; Li, L.; Chen, W. Effect of non-surgical periodontal therapy on the levels of Th17/Th1/Th2 cytokines and their transcription factors in Chinese chronic periodontitis patients. J. Clin. Periodontol. 2011, 38, 509–516. [Google Scholar] [CrossRef]
- Teles, F.; Martin, L.; Patel, M.; Hu, W.; Bittinger, K.; Kallan, M.J.; Chandrasekaran, G.; Cucchiara, A.J.; Giannobile, W.V.; Stephens, D.; et al. Gingival Crevicular Fluid Biomarkers During Periodontitis Progression and After Periodontal Treatment. J. Clin. Periodontol. 2025, 52, 40–55. [Google Scholar] [CrossRef]
- Stadler, A.F. GCF levels of cytokines/chemokines in chronic periodontitis: Meta-analysis. J. Clin. Periodontol. 2016, 43, 727–745. [Google Scholar] [CrossRef] [PubMed]
- Koidou, V.P.; Chatzopoulos, G.S.; Tomas, I.; Nibali, L.; Donos, N. Expression of gingival crevicular fluid markers during early and late healing of intrabony defects after surgical treatment: A systematic review. Clin. Oral Investig. 2020, 24, 487–502. [Google Scholar] [CrossRef] [PubMed]
- Lamster, I.B. Evaluation of gingival crevicular fluid as a diagnostic test. Periodontol. 2000 1997, 24, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, G.S. Formation, collection and significance of gingival crevicular fluid. Periodontol. 2000 2003, 31, 32–42. [Google Scholar] [CrossRef]
- Goodson, J.M. Gingival crevicular fluid flow and measurement: Implications for inflammatory mediator quantitation. Periodontol. 2000 2003, 31, 43–54. [Google Scholar] [CrossRef]
- Szabo, Y.Z.; Slavish, D.C. Measuring salivary markers of inflammation in health and disease. Neurosci. Biobehav. Rev. 2021, 121, 134–146. [Google Scholar]
- Riis, J.L.; Ahmadi, H.; Hamilton, K.R.; Hand, T.; Granger, D.A. Best practice recommendations for the measurement and interpretation of salivary pro-inflammatory cytokines in biobehavioral research. Brain Behav. Immun. 2021, 91, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C. ROBINSI: Tool for assessing risk of bias in nonrandomised studies. BMJ 2016, 355, i4919. [Google Scholar]
- Cifcibasi, E.; Koyuncuoglu, C.; Ciblak, M.; Badur, S.; Kasali, K.; Firatli, E.; Cintan, S. Evaluation of Local and Systemic Levels of Interleukin-17, Interleukin-23, and Myeloperoxidase in Response to Periodontal Therapy in Patients with Generalized Aggressive Periodontitis. Inflammation 2015, 38, 1959–1968. [Google Scholar] [CrossRef]
- Jayakumar Sunandhakumari, V.; Sadasivan, A.; Koshi, E.; Krishna, A.; Alim, A.; Sebastian, A. Effect of NonSurgical Periodontal Therapy on Plasma Levels of IL-17 in Chronic Periodontitis Patients with Well Controlled Type-II Diabetes Mellitus—A Clinical Study. Dent. J. 2018, 6, 19. [Google Scholar] [CrossRef]
- Nile, C.J.; Apatzidou, D.A.; Awang, R.A.; Riggio, M.P.; Kinane, D.F.; Lappin, D.F. The effect of periodontal scaling and root polishing on serum IL-17E concentrations and the IL-17A:IL-17E ratio. Clin. Oral Investig. 2016, 20, 2529–2537. [Google Scholar] [CrossRef]
- Duarte, P.M.; Da Rocha, M.; Sampaio, E.; Mestnik, M.J.; Feres, M.; Figueiredo, L.C.; Bastos, M.F.; Faveri, M. Serum levels of cytokines in subjects with generalized chronic and aggressive periodontitis before and after non-surgical periodontal therapy: A pilot study. J. Periodontol. 2010, 81, 1056–1063. [Google Scholar] [CrossRef]
- Medara, N.; Lenzo, J.C.; Walsh, K.A.; Darby, I.B.; O’Brien-Simpson, N.M.; Reynolds, E.C. T helper 17 cell-related cytokines in serum and saliva during management of periodontitis. Cytokine 2020, 134, 155186. [Google Scholar] [CrossRef]
- Gur, A.T.; Guncu, G.N.; Akman, A.C.; Pinar, A.; Karabulut, E.; Nohutcu, R.M. Evaluation of GCF IL-17, IL-10, TWEAK, and sclerostin levels after scaling and root planing and adjunctive use of diode laser application in patients with periodontitis. J. Periodontol. 2022, 93, 1161–1172. [Google Scholar] [CrossRef]
- Haghi, M.; Sattari, M.; Shanei, F.; Taleghani, F. Effects of non-surgical periodontal therapy on gingival crevicular fluid levels of interleukin-17 and interleukin-23 in patients with periodontitis: A clinical trial. J. Islam. Dent. Assoc. Iran 2020, 32, 75–82. [Google Scholar] [CrossRef]
- Nejadi, R.; Sattari, M.; Taleghani, F. Assessment of non-surgical periodontal therapy on IL-22 and S100A12 concentration in gingival crevicular fluid. J. Clin. Diagn. Res. 2018, 12, 19–23. [Google Scholar] [CrossRef]
- Preianò, M.; Papale, M.; Serrao, S. Preanalytical and analytical variables affecting peptidome profiling of GCF by MALDITOF MS. Clin. Chim. Acta 2014, 437, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Suematsu, A.; Okamoto, K. Th17 functions in osteoclastogenesis in arthritis via RANKL induction. J. Exp. Med. 2006, 203, 2673–2682. [Google Scholar] [CrossRef]
- Teles, R.P.; Patel, M.; Fox, C.H. A longitudinal study of GCF biomarkers at progressing vs nonprogressing sites. J. Clin. Periodontol. 2014, 41, 113–120. [Google Scholar]
- McGeachy, H.F.; Cua, D.J.; Gaffen, S.L. The IL17 family in health and disease. Immunity 2019, 50, 892–906. [Google Scholar] [CrossRef]
- Stockinger, B.; Omenetti, S. The dichotomous nature of T helper 17 cells. Nat. Rev. Immunol. 2017, 17, 535–544. [Google Scholar] [CrossRef]
- Gaffen, S.L.; Moutsopoulos, N.M. Regulation of host-microbe interactions at oral mucosal barriers by type 17 immunity. Sci. Immunol. 2020, 5, eaau4594. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodríguez-Montaño, R.; Ruiz-Gutiérrez, A.d.C.; Martínez-Rodríguez, V.M.d.C.; Gómez-Sandoval, J.R.; Guzmán-Flores, J.M.; Becerra-Ruiz, J.S.; Zamora-Perez, A.L.; Guerrero-Velázquez, C. Levels of IL-23/IL-17 Axis in Plasma and Gingival Tissue of Periodontitis Patients According to the New Classification. Appl. Sci. 2022, 12, 8051. [Google Scholar] [CrossRef]
- Alarcón Sánchez, A.; Guerrero Velázquez, C.; Heboyan, A. Systematic review of the IL23/IL17 axis in gingival crevicular fluid. BMC Oral Health 2024, 24, 302. [Google Scholar] [CrossRef]
- Gaffen, S.L. Structure and signalling in the IL17 family. Nat. Rev. Immunol. 2009, 9, 556–567. [Google Scholar] [CrossRef]
- Korn, T.; Bettelli, E.; Oukka, M.; Kuchroo, V.K. IL17 and Th17 cells. Annu. Rev. Immunol. 2009, 27, 485–517. [Google Scholar] [CrossRef] [PubMed]
- Giannobile, W.V. Salivary diagnostics for periodontal diseases. J. Am. Dent. Assoc. 2012, 143, 6S–11S. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Choi, Y. Point-of-care diagnosis of periodontitis using saliva. Front. Cell. Infect. Microbiol. 2015, 5, 65. [Google Scholar] [CrossRef]
- Breen, E.C.; Reynolds, S.M.; Cox, C.; Jacobson, L.P.; Magpantay, L.; Mulder, C.B.; Dibben, O.; Margolick, J.B.; Bream, J.H.; Sambrano, E.; et al. Multisite comparison of high-sensitivity multiplex cytokine assays. Clin. Vaccine Immunol. 2011, 18, 1229–1242. [Google Scholar] [CrossRef]
- Lähteenmäki, H.; Tervahartiala, T.; Räisänen, I.T.; Pärnänen, P.; Mauramo, M.; Gupta, S.; Sampson, V.; Rathnayake, N.; Heikkinen, A.; Alassiri, S.; et al. Active MMP-8 point-of-care/chairside enzyme-test as an adjunctive tool for early, real-time diagnosis of peri-implantitis. Clin. Exp. Dent. Res. 2022, 8, 485–496. [Google Scholar] [CrossRef]
- Räisänen, I.T.; Lähteenmäki, H.; Gupta, S.; Grigoriadis, A.; Sahni, V.; Suojanen, J.; Seppänen, H.; Tervahartiala, T.; Sakellari, D.; Sorsa, T. An aMMP-8 point-of-care and questionnaire-based real-world study in periodontitis. Diagnostics 2021, 11, 711. [Google Scholar] [CrossRef]
- Sorsa, T.; Gursoy, U.K.; Nwhator, S.; Hernández, M.; Tervahartiala, T.; Leppilahti, J.; Gürsoy, M.; Könönen, E.; Emingil, G.; Pussinen, P.J.; et al. Analysis of matrix metalloproteinases, especially MMP-8, in gingival crevicular fluid, mouthrinse and saliva for monitoring periodontal diseases. Periodontol. 2000 2016, 70, 142–163. [Google Scholar] [CrossRef]
- Rautava, J.; Gürsoy, U.K.; Kullström, A.; Könönen, E.; Sorsa, T.; Tervahartiala, T.; Gürsoy, M. An oral rinse active matrix metalloproteinase-8 point-of-care immunotest may be less accurate in patients with Crohn’s disease. Biomolecules 2020, 10, 395. [Google Scholar] [CrossRef]
- Dong, A.; Proctor, G.; Zaric, S. Diagnostic accuracy of microbiome-derived biomarkers for periodontitis: Systematic review and meta-analysis. J. Periodontal Res. 2024, 60, 748–761. [Google Scholar] [CrossRef]
- Kebschull, M.; Papapanou, P.N. Mini but mighty: MicroRNAs in the pathobiology of periodontal disease. Periodontol. 2000 2015, 69, 201–220. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kinney, J.S.; Ramseier, C.A.; Giannobile, W.V. Oral fluidbased biomarkers of periodontitis. Ann. N. Y Acad. Sci. 2007, 1098, 230–251. [Google Scholar] [CrossRef] [PubMed]
- Bossuyt, P.M.; Reitsma, J.B.; Bruns, D.E.; Gatsonis, C.A.; Glasziou, P.P.; Irwig, L. STARD 2015: An updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015, 351, h5527. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; the QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529. [Google Scholar] [CrossRef] [PubMed]
- Arias-Bujanda, N.; Regueira-Iglesias, A.; Alonso-Sampedro, M.; González-Peteiro, M.M.; Mira, A.; Balsa-Castro, C.; Tomás, I. Cytokine thresholds in gingival crevicular fluid for diagnosis of chronic periodontitis, differentiating by smoking status. Sci. Rep. 2018, 8, 18003. [Google Scholar] [CrossRef]
- Romano, F.; Del Buono, W.; Bianco, L.; Arena, M.; Mariani, G.M.; Di Scipio, F.; Berta, G.N.; Aimetti, M. Gingival crevicular fluid cytokines in stage III periodontitis (Grade B vs Grade C) before and 6 months after non-surgical periodontal therapy. Biomedicines 2020, 8, 515. [Google Scholar]
- Himani, G.S.; Prabhuji, M.L.V.; Karthikeyan, B.V. Gingival crevicular fluid interleukin-23 in periodontal health and disease. J. Periodontal Res. 2014, 49, 237–245. [Google Scholar] [CrossRef]
- Ay, Z.Y.; Yılmaz, G.; Özdem, M.; Koçak, H.; Sütçü, R.; Uskun, E.; Tonguç, M.Ö.; Kırzıoğlu, F.Y. The gingival crevicular fluid levels of interleukin-11 and interleukin-17 in patients with aggressive periodontitis. J. Periodontol. 2012, 83, 1425–1431. [Google Scholar] [CrossRef]
| Study | Design & Sample | Compartment | Analytes | Follow-Up | Clinical Outcome | Main Th17-Relevant Finding |
|---|---|---|---|---|---|---|
| Zhao et al. (2011) [23] | Prospective pre–post; ~30 adults with chronic periodontitis | GCF; peripheral CD4+ T cells | IL-17A, IL-21, IL-4, IFN-γ (ELISA); RORC, T-bet, GATA-3 (qPCR); IL-17+ CD4+ (flow cytometry) | ≈6 weeks | ↓ PD, ↓ BOP, ↑ CAL | GCF IL-17A ↓, IL-21 ↓, IL-4 ↑; IFN-γ ↔; RORC ↓, GATA-3 ↑; circulating IL-17+(incl. IL-17+IFN-γ+) CD4+ cells ↓ |
| Buduneli et al. (2009) [16] | Prospective pre–post; smokers vs. nonsmokers | GCF | IL-17A, sRANKL, OPG (ELISA) | 4 weeks | Clinical improvement reported (PD/BOP) | IL-17 concentration ↑ but total amount ↔; sRANKL ↔; OPG ↓; trends not modified by smoking |
| Cifcibaşı et al. (2015) [34] | Prospective pre–post; generalized aggressive periodontitis | GCF; serum | IL-17, IL-23, MPO (ELISA) | 3 months | ↓ PD, ↑ CAL | Local and systemic IL-17 ↓ and IL-23 ↓; MPO ↓ |
| Jayakumar et al., (2018) [35] | Prospective pre–post; CP ± T2D | Plasma | IL-17A (ELISA) | 1 month | ↓ PD, ↑ CAL | Plasma IL-17A ↓ post-NSPT; magnitude may differ by diabetes status |
| Nile et al. (2016) [36] | Prospective pre–post; chronic periodontitis | Serum | IL-17A, IL-17E/IL-25 (ELISA); IL-17A:IL-17E ratio | 6 & 25 weeks | ↓ PD, ↑ CAL | Trajectory consistent with reduced Th17 bias over time (ratio shift) |
| Duarte et al. (2010) [37] | Prospective pre–post; generalized CP/AgP | Serum | IL-17, IL-23, TNF-α, IFN-γ, IL-4 (ELISA) | 6 months | ↓ PD, ↑ CAL | Serum IL-17 ↓ in AgP; IL-23 ↔; TNF-α ↓ in AgP; IFN-γ, IL-4 ↔. |
| Medara et al. (2020) [38] | Prospective cohort; 54 periodontitis vs. 40 healthy; baseline, 3, 6, 12 mo during supportive therapy | Serum & saliva (participant-level) | 15 Th17-related cytokines (Luminex Th17 panel) | 3, 6, 12 months | ↓ PD, ↓ BOP vs. baseline | Saliva: IL-17A/IL-17F/IL-23 ↓ with therapy; Serum detection low; Serum IL-31 ↑ |
| Teles et al. (2025) [24] | Longitudinal monitoring; H (n = 112), P (n = 302); site-level progressing vs. stable; bimonthly ×12 mo; post-NSPT 6 mo | GCF (site-level) | 64-plex inflammatory panel (Milliplex; Bio-Plex 200) | 12 mo natural history + 6 mo post-NSPT | ↓ PD, ↓ BOP, ↑ CAL (overall); ↑ sites with PD < 4 mm after NSPT | Monitoring: progressing sites showed higher IL-1β (±IL-17 profile); After NSPT: IL-17A ↓, IL-1β ↓, sCD40L ↓, RANTES ↓; IP-10 ↑ |
| Gür et al. (2022) [39] * | Prospective (RCT context: SRP ± laser) | GCF | IL-17, IL-10, TWEAK, sclerostin (ELISA) | ~1 and 3 months (primary window 3 months | ↓ PD, ↓ BOP, ↑ CAL | IL-17 ↓; IL-10 ↑; adjunct laser did not obscure NSPT-linked IL-17 ↓ |
| Haghi et al. (2020) [40] | Prospective pre–post; CP and AgP pooled | GCF | IL-17, IL-23 (ELISA) | ≈6 weeks | Clinical improvement reported | IL-17 ↓; IL-23 ↓ |
| Pradeep et al. (2009) [15] | Prospective pre–post; health/gingivitis/CP; CP re-sampled post-SRP | GCF | IL-17, IL-18 (ELISA) | 6–8 weeks | ↓ PD, ↓ BOP | Baseline: IL-17 variably detectable; post-NSPT: IL-18 ↓; IL-17 often undetectable |
| Nejadi et al. (2018) [41] | Prospective pre–post; chronic periodontitis | GCF | IL-22, S100A12 (ELISA) | ≈6 weeks | ↓ PD, ↓ BOP | No significant change in GCF IL-22 or S100A12 pre–post NSPT |
| Study (Ref) | Disease/Cohort | Specimen | Analyte | Unit | Follow-Up | n | Pre (Mean ± SD or Median [IQR]) | Post (Mean ± SD or Median [IQR]) | Reported p | Direction |
|---|---|---|---|---|---|---|---|---|---|---|
| Zhao 2011 [23] | CP (pre–post) | GCF | IL-17A | ng/mL | ≈6 weeks | 30 | 63.57 ± 19.60 | 39.91 ± 10.97 | <0.05 | decrease |
| Zhao 2011 [23] | CP (pre–post) | GCF | IL-21 | ng/mL | ≈6 weeks | 30 | 80.34 ± 39.42 | 31.77 ± 13.26 | <0.05 | decrease |
| Buduneli 2009—non-smokers [16] | CP (NS) | GCF | IL-17A (concentration) | pg/mL | 4 weeks | 10 | 2.18 ± 1.46 | 5.87 ± 8.43 | NR | increase |
| Buduneli 2009—non-smokers [16] | CP (NS) | GCF | IL-17A (total amount) | pg/two samples | 4 weeks | 10 | 1.41 ± 0.49 | 1.34 ± 0.37 | NR | no significant change |
| Buduneli 2009—smokers [16] | CP (S) | GCF | IL-17A (concentration) | pg/mL | 4 weeks | 10 | 1.57 ± 1.23 | 3.50 ± 4.10 | NR | increase |
| Buduneli 2009—smokers [16] | CP (S) | GCF | IL-17A (total amount) | pg/two samples | 4 weeks | 10 | 1.36 ± 0.42 | 1.17 ± 0.25 | NR | no significant change |
| Cifcibaşı 2015 [34] | GAgP | Serum | IL-17A | pg/mL | 3 months | 19 | 37.56 ± 17.41 | 27.69 ± 13.86 | 0.014 | decrease |
| Cifcibaşı 2015 [34] | GAgP | Serum | IL-23 | pg/mL | 3 months | 19 | 258.63 ± 241.75 | 168.39 ± 177.75 | <0.001 | decrease |
| Cifcibaşı 2015 [34] | GAgP | GCF | IL-17A | pg/mL | 3 months | 19 | 2246.92 ± 294.67 | 1858.58 ± 1425.85 | 0.013 | decrease |
| Cifcibaşı 2015 [34] | GAgP | GCF | IL-23 | pg/mL | 3 months | 19 | 675.49 ± 444.02 | 400.12 ± 241.63 | <0.001 | decrease |
| Jayakumar 2018 [35] | CP (non-diabetic) | Plasma | IL-17A | pg/mL | 1 month | 20 | 0.18 ± 0.03 | 0.16 ± 0.02 | 0.08 (not significant) | no significant change |
| Jayakumar 2018 [35] | CP + T2DM | Plasma | IL-17A | pg/mL | 1 month | 20 | 0.21 ± 0.04 | 0.16 ± 0.03 | 0.09 (not significant) | no significant change |
| Nile 2016 [36] | CP (FM-SRP & Q-SRP) | Serum | IL-17A:IL-17E ratio | unitless | 25 weeks | 40 | NR | NR | <0.05 (ratio lower than baseline) | decrease (ratio) |
| Duarte 2010 [37] | GAgP | Serum | IL-17A | pg/mL | 6 months | 14 | NR | NR | 0.04 | decrease |
| Duarte 2010 [37] | GCP | Serum | IL-23 | pg/mL | 6 months | 14 | NR | NR | not significant | no significant change |
| Medara 2020 [38] | Periodontitis | Serum | IL-17A | pg/mL | 3–12 months | 54 | often below detection | often below detection | NR | not detectable |
| Medara 2020 [38] | Periodontitis | Saliva | IL-17A | pg/mL | up to 12 months | 54 | NR (median reported in figure) | NR (median reported in figure) | <0.05 (overall) | decrease |
| Gür 2022—SRP arm [39] | CP (control) | GCF | IL-17A | ng/L | 3 months | 20 | 117.67 ± 10.77 | 115.19 ± 11.34 | 0.001 (within-arm) | decrease (small) |
| Gür 2022—SRP + laser [39] | CP (laser) | GCF | IL-17A | ng/L | 3 months | 20 | 117.66 ± 10.25 | 106.88 ± 7.56 | 0.001 (within-arm); 0.025 (between-arm at 3 months) | decrease |
| Haghi 2020 [40] | CP | GCF | IL-17A | pg/mL | ~6 weeks | 25 | 1.40 ± 1.36 | 0.96 ± 0.44 | 0.048 | decrease |
| Haghi 2020 [40] | CP | GCF | IL-23 | pg/mL | ~6 weeks | 25 | 80.60 ± 132.10 | 1.41 ± 3.63 | 0.001 | decrease |
| Haghi 2020 [40] | AgP | GCF | IL-17A | pg/mL | ~6 weeks | 29 | 0.91 ± 0.74 | 0.51 ± 0.54 | 0.022 | decrease |
| Haghi 2020 [40] | AgP | GCF | IL-23 | pg/mL | ~6 weeks | 29 | 32.27 ± 63.59 | 2.34 ± 4.70 | <0.001 | decrease |
| Nejadi 2018 [41] | CP | GCF | IL-22 | ng/mL | ~4 weeks | 22 | mean low; exact number not reported | no significant change | not significant | no significant change |
| Nejadi 2018 [41] | CP | GCF | S100A12 | ng/mL | ~4 weeks | 22 | mean low; exact number not reported | no significant change | not significant | no significant change |
| Pradeep 2009 [15] | CP (pre vs. post) | GCF | IL-18 | pg/µL | ~6–8 weeks | 20 | 450.54 ± 276.83 (pre-treatment CP) | 89.09 ± 66.69 (post-treatment CP) | <0.001 | decrease |
| Pradeep 2009 [15] | CP/healthy/gingivitis | GCF | IL-17A | pg/µL | — | — | approximately zero across groups | approximately zero across groups | — | not detectable |
| Teles 2025 [24] | Stage II–III periodontitis (site-level) | GCF | IL-17A | pg/mL | 6 months post-NSPT | 302 | model-based; raw means not reported | decrease after NSPT (mixed model) | reported as significant (q < 0.05) | decrease |
| Study | Site-Matching | Device + Dwell | Volume Capture | Elution Buffer | Storage (°C) | Freeze–Thaw Control | Unit Reported | LOD/LOQ Handling | Assay Brand/Model |
|---|---|---|---|---|---|---|---|---|---|
| Zhao 2011 [23] | Yes (same tooth pre/post) | Paper strip (2 × 20 mm), 30 s | Yes (strip weight/volume) | PBS 200 µL | −80 °C | NR | Concentration (ng/mL) | NR (brand LLDs available) | R&D Systems (Quantikine) |
| Buduneli 2009 [16] | Likely (same 4 sites); not explicit | PerioPaper, 30 s | Yes (Periotron 8000) | PBS 350 µL | −40 °C | NR | Amount & concentration | LLD given (IL-17 0.002 pg/mL); handling NR | ELISA kits (vendor NR) |
| Cifcibaşı 2015 [34] | Unclear (deepest 4 sites) | PerioPaper, 30 s | No | PBS 350 µL + 0.05% Tween-20; +4 °C overnight | −80 °C | NR | Concentration (GCF & serum) | LLDs reported; handling NR | Diaclone (IL-17/IL-23); eBioscience (MPO) |
| Jayakumar 2018 [35] | N/A (serum) | N/A | N/A | N/A | −80 °C | NR | Concentration (plasma) | NR | DIACLONE (ELISA development kit) |
| Nile 2016 [36] | N/A (serum) | N/A | N/A | N/A | −80 °C | NR | Concentration (serum) | LLD 1.9 pg/mL (IL-17A/E); handling NR | Peprotech (IL-17A/E); R&D (TNF/IL-6) |
| Duarte 2010 [37] | N/A (serum) | N/A | N/A | N/A | −80 °C | NR | Concentration (serum) | Below LOD scored 0 pg/mL | ELISA (vendor NR) |
| Medara 2020 [38] | N/A (serum & saliva) | N/A | N/A | N/A | −80 °C (serum & saliva; processed ≤ 2 h) | NR | Concentration (pg/mL) | Detection frequency noted (serum low); LOD/LOQ handling NR | Bio-Plex Pro Human Th17 Cytokine Panel (Bio-Rad) |
| Teles 2025 [24] | Yes (site-level; progressing vs. stable; no rescue sites in analyses) | PerioPaper; 30 s; 1–2 mm | No (concentration from elution) | NR (standard Milliplex protocols) | −80 °C (immediate freeze) | NR | Concentration (pg/mL) | Assay performance in Table S3; LOD/LOQ handling NR | Milliplex (MilliporeSigma); read on Bio-Plex 200 (Bio-Rad) |
| Gür 2022 [35] | Yes (deepest tooth sampled across time) | PerioPaper, 30 s | Yes (Periotron 8000) | PBS 800 µL (sterile) | −20 °C | NR | Concentration (GCF) | LLDs provided; handling NR | Bioassay (IL-17/IL-10/TWEAK); Elabscience (sclerostin) |
| Haghi 2020 [40] | Unclear (two deepest pockets at each time) | PerioPaper, 30 s | No | PBS 150 µL; 10,000 rpm × 30 min | −20 °C | NR | Concentration (GCF) | NR | Bender MedSystems (IL-17A, IL-23) |
| Pradeep 2009 [15] | Yes (same site pre/post) | Microcapillary pipettes; ≤10 min cap | Yes (fixed 1 µL/sample) | N/A (direct GCF) | −70 °C | NR | Concentration (pg/µL) | Kit sensitivity ≈15 pg/mL; ND frequently 0 | R&D Systems (IL-17); Bender MedSystems (IL-18) |
| Nejadi 2018 [41] | Yes (same two sites) | PerioPaper, 30 s | No | PBS 50 µL; centrifuge; −70 °C 24 h | −20 °C (then −70 °C 24 h) | NR (≥1 cycle implied) | Concentration (GCF) | NR | eBioscience (IL-22); Biovendor (S100A12) |
| Outcome | Specimen | Typical Timeframe | Direction After NSPT (Study-Level) | Studies | GRADE Certainty |
|---|---|---|---|---|---|
| IL-17A | GCF | 6–8 weeks (often persisting by ≈3 months) | Decrease in most studies | Decrease: Zhao 2011 [23]; Cifcibaşı 2015 (GCF) [34]; Haghi 2020 [40]; Gür 2022 [39]; Teles 2025 [24]. No significant change: Buduneli 2009 (amount prioritized) [16]. Indeterminate: Pradeep 2009 (undetectable IL-17A) [15]. | Low |
| IL-23 | GCF | ≈6–8 weeks | Decrease | Decrease: Haghi 2020 [40]; Cifcibaşı 2015 (GCF) [34]. Indeterminate: Teles 2025 [24] (model-based, no raw pre/post means). | Low |
| IL-21 | GCF | ≈6 weeks | Decrease (single study) | Zhao 2011 [23]. | Low |
| IL-22 | GCF | ≈4–6 weeks | No significant change (single study) | Nejadi 2018 [41]. | Very low |
| IL-17A | Serum/plasma | 1–6 months | Decrease or neutral; smaller effects | Decrease: Cifcibaşı 2015 (serum) [34]; Duarte 2010 (AgP subgroup) [37]. No significant change: Jayakumar 2018 (plasma) [35]. Indeterminate: Medara 2020 (often below detection) [38]. | Low |
| IL-23 | Serum | 3–6 months | Mixed | Decrease: Cifcibaşı 2015 (serum) [34]. No significant change: Duarte 2010 [37]. | Low |
| IL-17A | Saliva | 3–12 months | Decrease (single study) | Medara 2020 [38]. | Low |
| IL-17A:IL-17E ratio | Serum | 6–25 weeks | Decrease (ratio) | Nile 2016 [36]. | Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.J.H.; Baker, M.; Figueredo, C.M.S. Cytokine Changes in Gingival Crevicular Fluid and Serum After Non-Surgical Periodontal Therapy in Patients with Periodontitis: A Systematic Review and Narrative Synthesis. J. Clin. Med. 2025, 14, 8424. https://doi.org/10.3390/jcm14238424
Kim CJH, Baker M, Figueredo CMS. Cytokine Changes in Gingival Crevicular Fluid and Serum After Non-Surgical Periodontal Therapy in Patients with Periodontitis: A Systematic Review and Narrative Synthesis. Journal of Clinical Medicine. 2025; 14(23):8424. https://doi.org/10.3390/jcm14238424
Chicago/Turabian StyleKim, Christine J. H., Matthew Baker, and Carlos Marcelo S. Figueredo. 2025. "Cytokine Changes in Gingival Crevicular Fluid and Serum After Non-Surgical Periodontal Therapy in Patients with Periodontitis: A Systematic Review and Narrative Synthesis" Journal of Clinical Medicine 14, no. 23: 8424. https://doi.org/10.3390/jcm14238424
APA StyleKim, C. J. H., Baker, M., & Figueredo, C. M. S. (2025). Cytokine Changes in Gingival Crevicular Fluid and Serum After Non-Surgical Periodontal Therapy in Patients with Periodontitis: A Systematic Review and Narrative Synthesis. Journal of Clinical Medicine, 14(23), 8424. https://doi.org/10.3390/jcm14238424







