Comparative Long-Term Cardiovascular Outcomes of Empagliflozin and Dapagliflozin in Heart Failure Patients After Coronary Revascularization: A Retrospective Cohort Study
Abstract
1. Introduction
2. Statistical Analysis
3. Materials and Methods
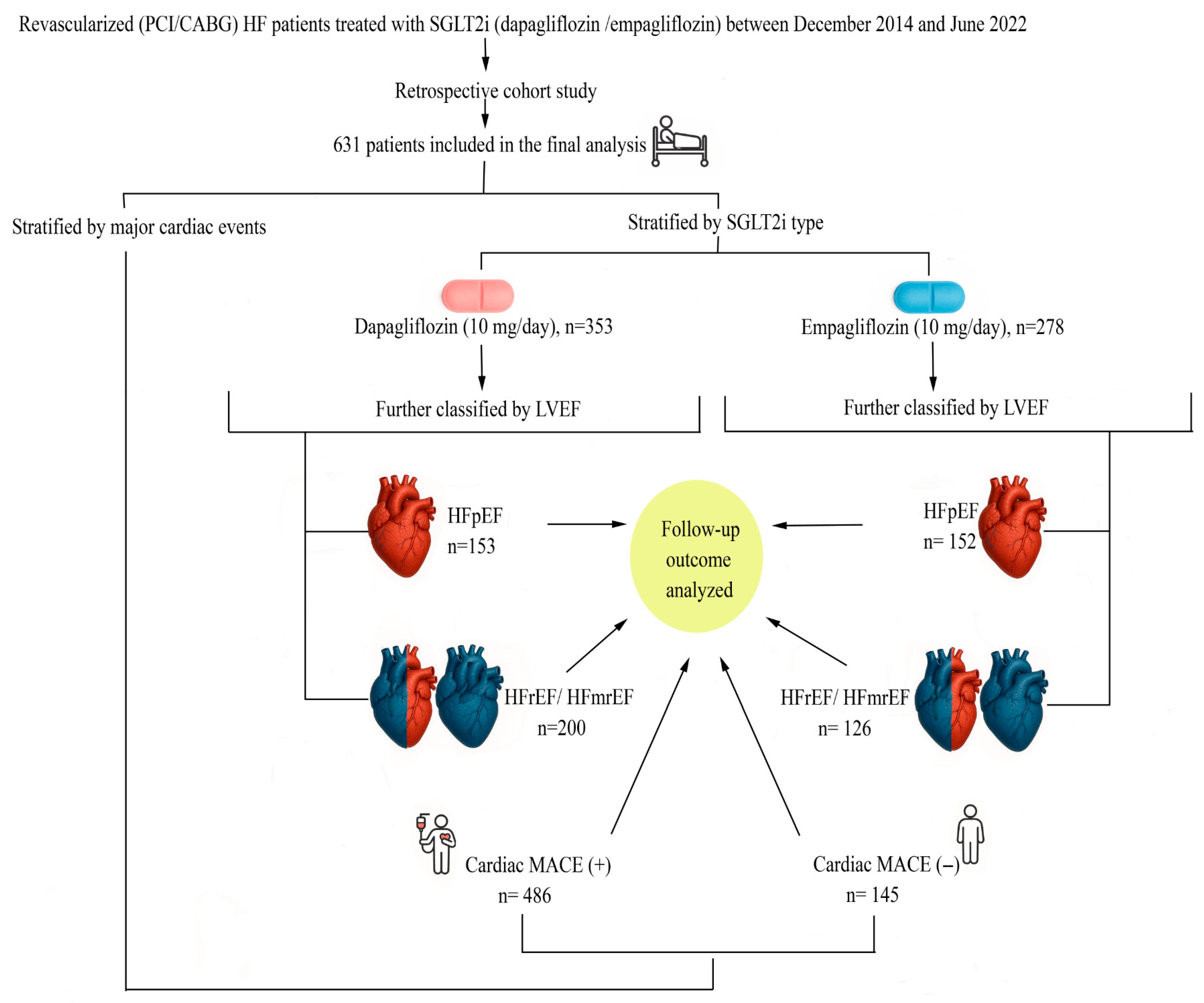
3.1. Study Design and Population
3.2. Data Collection and Echocardiographic Assessment
- HFpEF: LVEF ≥ 50%
- HFrEF/HFmrEF: LVEF < 50%
Laboratory Evaluations and Definitions
3.3. Outcome Definitions and Subgroup Classification
3.4. Ethical Approval
4. Results
5. Discussion
6. Limitations and Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hsia, D.S.; Grove, O.; Cefalu, W.T. An update on sodium-glucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Wilding, J.; Fernando, K.; Milne, N.; Evans, M.; Ali, A.; Bain, S.; Hicks, D.; James, J.; Newland-Jones, P.; Patel, D.; et al. SGLT2 inhibitors in type 2 diabetes management: Key evidence and implications for clinical practice. Diabetes Ther. 2018, 9, 1757–1773. [Google Scholar] [CrossRef] [PubMed]
- The Emerging Risk Factors Collaboration; Sarwar, N.; Gao, P.; Seshasai, S.R.; Gobin, R.; Kaptoge, S.; Di Angelantonio, E.; Ingelsson, E.; Lawlor, D.A.; Selvin, E.; et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Tang, H.; Fang, Z.; Wang, T.; Cui, W.; Zhai, S.; Song, Y. Meta-analysis of effects of sodium-glucose cotransporter 2 inhibitors on cardiovascular outcomes and all-cause mortality among patients with type 2 diabetes mellitus. Am. J. Cardiol. 2016, 118, 1774–1780. [Google Scholar] [CrossRef]
- Saad, M.; Mahmoud, A.N.; Elgendy, I.Y.; Abuzaid, A.; Barakat, A.F.; Elgendy, A.Y.; Al-Ani, M.; Mentias, A.; Nairooz, R.; Bavry, A.A.; et al. Cardiovascular outcomes with sodium-glucose cotransporter-2 inhibitors in patients with type II diabetes mellitus: A meta-analysis of placebo-controlled randomized trials. Int. J. Cardiol. 2017, 228, 352–358. [Google Scholar] [CrossRef]
- Wu, J.H.Y.; Foote, C.; Blomster, J.; Toyama, T.; Perkovic, V.; Sundström, J.; Neal, B. Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2016, 4, 411–419. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.; Martinez, F.; et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in heart failure with a preserved ejection fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Talha, K.M.; Anker, S.D.; Butler, J. SGLT-2 Inhibitors in Heart Failure: A Review of Current Evidence. Int. J. Heart Fail. 2023, 5, 82–90. [Google Scholar] [CrossRef]
- Hou, Y.C.; Zheng, C.M.; Yen, T.H.; Lu, K.C. Molecular Mechanisms of SGLT2 Inhibitor on Cardiorenal Protection. Int. J. Mol. Sci. 2020, 21, 7833. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Januzzi, J.L., Jr. Pleiotropic Effects of Sodium-Glucose Cotransporter-2 Inhibitors in Cardiovascular Disease and Chronic Kidney Disease. J. Clin. Med. 2023, 12, 2824. [Google Scholar] [CrossRef] [PubMed]
- Zelniker, T.A.; Wiviott, S.D.; Raz, I.; Im, K.; Goodrich, E.; Bonaca, M.P.; Mosenzon, O.; Kato, E.; Cahn, A.; Furtado, R.H.M.; et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019, 393, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Dhingra, N.K.; Hibino, M.; Gupta, V.; Verma, S. Sodium-glucose co-transporter 2 inhibitors in heart failure with reduced or preserved ejection fraction: A meta-analysis. ESC Heart Fail. 2022, 9, 942–946. [Google Scholar] [CrossRef]
- Banerjee, M.; Pal, R.; Nair, K.; Mukhopadhyay, S. SGLT2 inhibitors and cardiovascular outcomes in heart failure with mildly reduced and preserved ejection fraction: A systematic review and meta-analysis. Indian Heart J. 2023, 75, 122–127. [Google Scholar] [CrossRef]
- McGuire, D.K.; Shih, W.J.; Cosentino, F.; Charbonnel, B.; Cherney, D.Z.I.; Dagogo-Jack, S.; Pratley, R.; Greenberg, M.; Wang, S.; Huyck, S.; et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: A meta-analysis. JAMA Cardiol. 2021, 6, 148–158. [Google Scholar] [CrossRef]
- Lim, J.; Choi, Y.-J.; Kim, B.S.; Rhee, T.-M.; Lee, H.-J.; Han, K.-D.; Park, J.-B.; Na, J.O.; Kim, Y.-J.; Lee, H.; et al. Comparative cardiovascular outcomes in type 2 diabetes patients taking dapagliflozin versus empagliflozin: A nationwide population-based cohort study. Cardiovasc. Diabetol. 2023, 22, 188. [Google Scholar] [CrossRef]
- Shao, S.-C.; Chang, K.-C.; Hung, M.-J.; Yang, N.-I.; Chan, Y.-Y.; Chen, H.-Y.; Yang, Y.-H.K.; Lai, E.C.-C. Comparative risk evaluation for cardiovascular events associated with dapagliflozin vs. empagliflozin in real-world type 2 diabetes patients: A multi-institutional cohort study. Cardiovasc. Diabetol. 2019, 18, 120. [Google Scholar] [CrossRef] [PubMed]
- Modzelewski, K.L.; Pipilas, A.; Bosch, N.A. Comparative outcomes of empagliflozin to dapagliflozin in patients with heart failure. JAMA Netw. Open 2024, 7, e249305. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-Y.; Pan, H.-C.; Shiao, C.-C.; Chuang, M.-H.; See, C.Y.; Yeh, T.-H.; Yang, Y.; Chu, W.-K.; Wu, V.-C. Impact of SGLT2 inhibitors on patient outcomes: A network meta-analysis. Cardiovasc. Diabetol. 2023, 22, 290. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yang, P.; Fu, L.; Sun, L.; Shen, W.; Wu, Q. Comparative cardiovascular outcomes of SGLT2 inhibitors in type 2 diabetes mellitus: A network meta-analysis of randomized controlled trials. Front. Endocrinol. 2022, 13, 802992. [Google Scholar] [CrossRef]
- Shi, Z.; Gao, F.; Liu, W.; He, X. Comparative efficacy of dapagliflozin and empagliflozin of a fixed dose in heart failure: A network meta-analysis. Front. Cardiovasc. Med. 2022, 9, 869272. [Google Scholar] [CrossRef]
- Dhana, R.; Aqel, Y.; Rawat, A.; Mahato, A.; Abusal, A.M.; Munawar, N.; Wei, C.R.; Amin, A. Comparative cardiovascular outcomes of dapagliflozin versus empagliflozin in patients with type 2 diabetes: A meta-analysis. Cureus 2025, 17, e83449. [Google Scholar] [CrossRef]
- Usman, M.S.; Siddiqi, T.J.; Memon, M.M.; Khan, M.S.; Rawasia, W.F.; Ayub, M.T.; Sreenivasan, J.; Golzar, Y. Sodium-glucose co-transporter 2 inhibitors and cardiovascular outcomes: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2018, 25, 495–502. [Google Scholar] [CrossRef]
- Lim, J.; Hwang, I.C.; Choi, H.M.; Yoon, Y.E.; Cho, G.Y. Comparison of cardiovascular and renal outcomes between dapagliflozin and empagliflozin in patients with type 2 diabetes without prior cardiovascular or renal disease. PLoS ONE 2022, 17, e0269414. [Google Scholar] [CrossRef]
- Alhakak, A.; Kober, L.; Petrie, M.C.; Jhund, P.S.; Dridi, N.P.; Karacan, M.N.; Schou, M.; Elmegaard, M.; Rossing, P.; Fosbol, E.; et al. Dapagliflozin versus empagliflozin and major adverse cardiovascular events in type 2 diabetes: A nationwide cohort study. Eur. Heart J. 2024, 45 (Suppl. S1), ehae666.3317. [Google Scholar] [CrossRef]
- Kim, J.H.; Yoon, Y.C.; Kim, Y.H.; Park, J.I.; Choi, K.U.; Nam, J.H.; Lee, C.H.; Son, J.W.; Park, J.S.; Kim, U. Cardiovascular outcomes between dapagliflozin versus empagliflozin in patients with diabetes mellitus. Clin. Cardiol. 2024, 47, e24248. [Google Scholar] [CrossRef]
- Yang, I.N.; Chong, K.S.; Peng, Z.Y.; Ou, H.T.; Wang, M.C. Comparative effectiveness and prescribing patterns of dapagliflozin vs empagliflozin in type 2 diabetes patients: A target trial emulation. Diabetes Res. Clin. Pract. 2025, 228, 112427. [Google Scholar] [CrossRef]
| Variables | Dapagliflozin (n = 353) | Empagliflozin (n = 278) | p-Value |
|---|---|---|---|
| Age, years | 62.09 ± 11.31 | 61.29 ± 9.65 | 0.388 |
| Sex, n (%) | |||
| Male | 216 (61.2) | 151 (54.3) | 0.082 |
| Female | 137 (38.8) | 127 (45.7) | 0.082 |
| BMI, kg/m2 | 27.89 ± 4.74 | 28.13 ± 4.71 | 0.687 |
| Comorbidities, n (%) | |||
| Hypertension (HT) | 239 (67.7) | 194 (69.8) | 0.576 |
| Diabetes mellitus (DM) | 227 (64.3) | 176 (63.3) | 0.796 |
| Atrial fibrillation (AF) | 74 (24.3) | 49 (21.5) | 0.453 |
| Smoking | 61 (17.3) | 47 (16.9) | 0.901 |
| Coronary artery disease (CAD), n (%) | |||
| PCI | 227 (64.3) | 169 (60.8) | 0.365 |
| CABG | 126 (35.7) | 109 (39.2) | 0.365 |
| Ejection fraction (EF), % | 46.0 [38.0–55.0] | 49.00 [38.00–60.00] | 0.052 |
| Laboratory data | |||
| Fasting blood glucose (FBG), mg/dL | 123.20 ± 49.80 | 121.94 ± 41.88 | 0.623 |
| HbA1c, % | 6.68 ± 1.56 | 6.66 ± 1.35 | 0.616 |
| Total cholesterol (TC), mg/dL | 187.00 [150.00–222.00] | 189.50 [162.00–227.00] | 0.092 |
| LDL-C, mg/dL | 126.50 ± 45.07 | 131.49 ± 46.26 | 0.121 |
| HDL-C, mg/dL | 44.00 [35.75–52.00] | 45.00 [38.00–57.00] | 0.057 |
| Triglycerides (TG), mg/dL | 132.00 [97.00–178.00] | 129.00 [102.00–181.00] | 0.726 |
| BUN (mg/dL) | 20.78 ± 13.59 | 19.35 ± 9.19 | 0.952 |
| Creatinine (mg/dL) | 1.09 ± 0.77 | 1.00 ± 0.43 | 0.390 |
| Uric acid (mg/dL) | 6.35 ± 1.86 | 6.15 ± 1.80 | 0.155 |
| NT-proBNP (pg/mL) | 803.50 [330.50–1652.25] | 864.00 [127.68–2502.25] | 0.640 |
| Medications, n (%) | |||
| ARNi | 44 (12.5) | 37 (13.3) | 0.753 |
| ACEi/ARB | 288 (81.6) | 221 (79.5) | 0.509 |
| Beta-blocker | 345 (97.7) | 274 (98.6) | 0.450 |
| MRA | 137 (38.8) | 121 (43.5) | 0.232 |
| Diuretic | 260 (73.7) | 205 (73.7) | 0.980 |
| Oral anticoagulant (OAC) | 82 (23.2) | 75 (27) | 0.280 |
| Calcium channel blocker (CCB) | 79 (22.4) | 66 (23.7) | 0.687 |
| Outcomes, n (%) | |||
| Hospitalization | 84 (23.8) | 66 (23.7) | 0.987 |
| Total mortality | 68 (19.3) | 55 (19.8) | 0.870 |
| Cardiac mortality | 39 (11) | 34 (12.2) | 0.645 |
| MACE (total mortality and Hospitalization) | 91 (25.8) | 73 (26.3) | 0.891 |
| Cardiac MACE | 84 (23.8) | 61 (21.9) | 0.720 |
| Implantable cardiac devices, n (%) | |||
| ICD-CRT | 29 (8.2) | 23 (8.3) | 0.979 |
| Pacemaker | 30 (185) | 24 (8.6) | 0.952 |
| Follow-up period, months | 19.67 ± 1.50 | 19.47 ± 1.48 | 0.080 |
| Variables | Dapagliflozin (n = 153) | Empagliflozin (n = 152) | p-Value |
|---|---|---|---|
| Age, years | 60.86 ± 10.59 | 61.89 ± 9.61 | 0.116 |
| Sex, n (%) | |||
| Male | 89 (58.2) | 75 (49.3) | 0.122 |
| Female | 64 (41.8) | 77 (50.7) | 0.122 |
| BMI, kg/m2 | 27.71 ± 4.16 | 28.48 ± 4.84 | 0.067 |
| Comorbidities, n (%) | |||
| Hypertension (HT) | 110 (71.9) | 111 (73) | 0.732 |
| Diabetes mellitus (DM) | 91 (59.5) | 102 (67.1) | 0.167 |
| Atrial fibrillation (AF) | 26 (17) | 32 (21.1) | 0.366 |
| Smoking | 26 (17) | 30 (19.7) | 0.536 |
| Coronary artery disease (CAD), n (%) | |||
| PCI | 94 (61.4) | 92 (60.5) | 0.870 |
| CABG | 59 (38.6) | 60 (39.5) | 0.870 |
| Ejection fraction (EF), % | 55.00 [52.00–60.00] | 57.00 [52.00–60.00] | 0.270 |
| Laboratory data | |||
| Fasting blood glucose (FBG), mg/dL | 106.00 [96.00–132.00] | 107.00 [96.25–132.00] | 0.620 |
| HbA1c, % | 6.74 ± 1.67 | 6.74 ± 1.43 | 0.380 |
| Total cholesterol (TC), mg/dL | 196.00 [153.00–227.00] | 196.00 [167.00–231] | 0.391 |
| LDL-C, mg/dL | 130.60 ± 46.94 | 134.76 ± 49.59 | 0.315 |
| HDL-C, mg/dL | 45.00 [36.00–52.00] | 46.00 [39.25–58.00] | 0.132 |
| Triglycerides (TG), mg/dL | 139.50 [110.00–195.50] | 132.00 [106.00–180.50] | 0.513 |
| BUN (mg/dL) | 20.45 ± 15.65 | 19.22 ± 9.23 | 0.292 |
| Creatinine (mg/dL) | 1.10 ± 1.04 | 0.96 ± 0.35 | 0.920 |
| Uric acid (mg/dL) | 6.23 ± 2.01 | 6.34 ± 1.87 | 0.930 |
| NT-proBNP (pg/mL) | 745.50 [144.95–1350.50] | 593.62 [86.30–2420.00] | 0.314 |
| Medications, n (%) | |||
| ARNi | 12 (7.8) | 19 (12.5) | 0.178 |
| ACEi/ARB | 131 (85.6) | 117 (77) | 0.053 |
| Beta-blocker | 151 (98.7) | 150 (98.7) | 0.995 |
| MRA | 63 (41.2) | 61 (40.1) | 0.853 |
| Diuretic | 122 (79.7) | 119 (78.3) | 0.756 |
| Oral anticoagulant (OAC) | 36 (23.5) | 38 (25) | 0.765 |
| Calcium channel blocker (CCB) | 29 (19) | 41 (27) | 0.096 |
| Outcomes, n (%) | |||
| Hospitalization | 43 (28.1) | 30 (19.7) | 0.087 |
| Total mortality | 36 (23.5) | 24 (15.8) | 0.069 |
| Cardiac mortality | 19 (12.4) | 12 (7.9) | 0.191 |
| MACE (total mortality and Hospitalization) | 43 (28.1) | 33 (21.7) | 0.197 |
| Cardiac MACE | 41 (26.8) | 27 (17.8) | 0.140 |
| Implantable cardiac devices, n (%) | |||
| ICD-CRT | 1 (0.7) | 1 (0.7) | 0.996 |
| Pacemaker | 8 (5.2) | 7 (4.6) | 0.801 |
| Follow-up period, months | 19.52 ± 1.43 | 19.40 ± 1.34 | 0.582 |
| Variables | Dapagliflozin (n = 200) | Empagliflozin (n = 126) | p-Value |
|---|---|---|---|
| Age, years | 63.03 ± 11.77 | 62.78 ± 9.72 | 0.997 |
| Sex, n (%) | |||
| Male | 127 (63.5) | 76 (60.3) | 0.564 |
| Female | 73 (36.5) | 50 (39.7) | 0.564 |
| BMI, kg/m2 | 28.02 ± 5.15 | 27.71 ± 4.53 | 0.227 |
| Comorbidities, n (%) | |||
| Hypertension (HT) | 129 (64.6) | 83 (65.9) | 0.800 |
| Diabetes mellitus (DM) | 136 (68) | 74 (58.7) | 0.089 |
| Atrial fibrillation (AF) | 54 (27) | 30 (23.8) | 0.521 |
| Smoking | 35 (17.5) | 17 (13.5) | 0.336 |
| Coronary artery disease (CAD), n (%) | |||
| PCI | 133 (66.5) | 77 (61.1) | 0.322 |
| CABG | 67 (33.5) | 49 (38.9) | 0.322 |
| Ejection fraction (EF), % | 38.00 [35.00–45.00] | 38.00 [31.75–43.00] | 0.062 |
| Laboratory data | |||
| Fasting blood glucose (FBG), mg/dL | 107.00 [95.00–129.00] | 109.00 [96.50–124.00] | 0.893 |
| HbA1c, % | 6.64 ± 1.48 | 6.55 ± 1.23 | 0.706 |
| Total cholesterol (TC), mg/dL | 179.50 [149.50–213.25] | 188.00 [160.00–218.00] | 0.279 |
| LDL-C, mg/dL | 129.54 ± 43.41 | 127.34 ± 41.51 | 0.407 |
| HDL-C, mg/dL | 44.00 [35.00–52.00] | 44.00 [37.20–54.00] | 0.396 |
| Triglycerides (TG), mg/dL | 120.00 [91.50–171.50] | 125.00 [99.00–181.00] | 0.458 |
| BUN (mg/dL) | 21.03 ± 11.75 | 19.51 ± 9.16 | 0.561 |
| Creatinine (mg/dL) | 1.08 ± 0.49 | 1.06 ± 0.51 | 0.740 |
| Uric acid (mg/dL) | 6.40 ± 1.80 | 6.04 ± 1.73 | 0.081 |
| NT-proBNP (pg/mL) | 947.20 [410.43–2013.75] | 1148.80 [280.05–3008.00] | 0.728 |
| Medications, n (%) | |||
| ARNi | 32 (16) | 18 (14.3) | 0.676 |
| ACEi/ARB | 157 (78.5) | 104 (82.5) | 0.374 |
| Beta-blocker | 194 (97) | 124 (98.4) | 0.422 |
| MRA | 74 (37) | 60 (47.6) | 0.058 |
| Diuretic | 138 (69) | 86 (68.3) | 0.888 |
| Oral anticoagulant (OAC) | 46 (23) | 37 (29.3) | 0.199 |
| Calcium channel blocker (CCB) | 50 (25) | 25 (19.9) | 0.281 |
| Outcomes, n (%) | |||
| Hospitalization | 41 (20.5) | 36 (28.6) | 0.095 |
| Total mortality | 32 (16) | 31 (24.6) | 0.055 |
| Cardiac mortality | 20 (10) | 22 (17.5) | 0.053 |
| MACE (total mortality and Hospitalization) | 48 (24) | 40 (31.7) | 0.125 |
| Cardiac MACE | 43 (21.5) | 34 (26.9) | 0.130 |
| Implantable cardiac devices, n (%) | |||
| ICD-CRT | 28 (14) | 22 (17.5) | 0.399 |
| Pacemaker | 22 (11) | 17 (13.5) | 0.500 |
| Follow-up period, months | 19.79 ± 1.55 | 19.56 ± 1.63 | 0.146 |
| Variables | Cardiac MACE (−) (n = 486) | Cardiac MACE (+) (n = 145) | p-Value |
|---|---|---|---|
| Age, years | 61.38 ± 10.37 | 64.86 ± 10.98 | 0.004 |
| Sex, n (%) | |||
| Male | 286 (58.8) | 81 (55.9) | 0.522 |
| Female | 200 (41.2) | 64 (44.1) | 0.522 |
| BMI, kg/m2 | 28.09 ± 4.66 | 27.67 ± 4.94 | 0.413 |
| Comorbidities, n (%) | |||
| Hypertension (HT) | 338 (69.5) | 95 (65.5) | 0.359 |
| Diabetes mellitus (DM) | 318 (65.4) | 85 (58.6) | 0.134 |
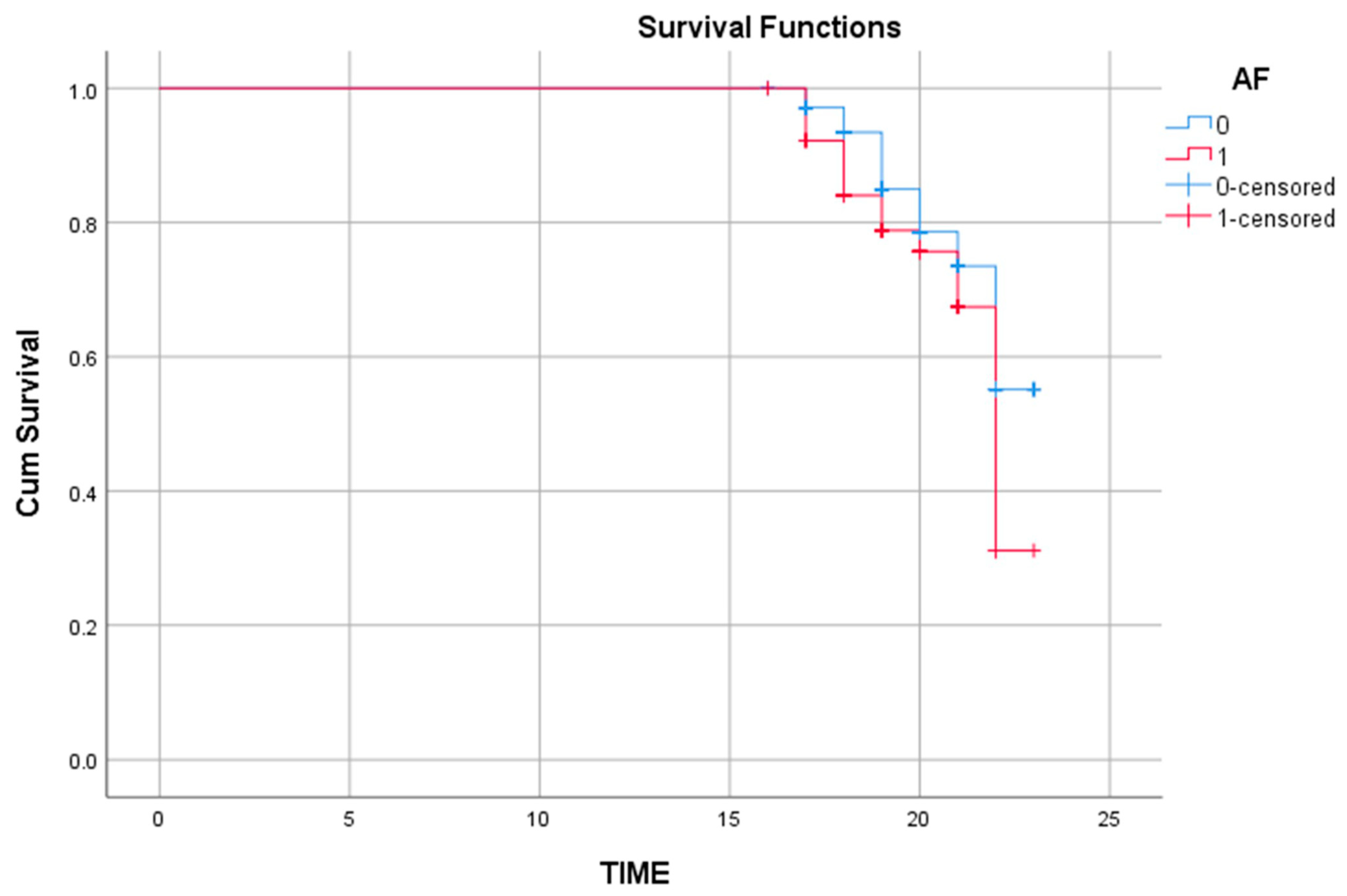
| Atrial fibrillation (AF) | 98 (20.2) | 44 (30.3) | 0.010 |
| Smoking | 81 (16.7) | 27 (18.6) | 0.584 |
| Coronary artery disease (CAD), n (%) | |||
| PCI | 148 (62.7) | 38 (55.1) | 0.252 |
| CABG | 88 (37.3) | 31 (44.9) | 0.252 |
| Ejection fraction (EF), % | 48.00 [38.00–60.00] | 45.00 [30.00–60.00] | 0.037 |
| Laboratory data | |||
| Fasting blood glucose (FBG), mg/dL | 107.00 [97.00–129.00] | 106.00 [93.00–132.00] | 0.921 |
| HbA1c, % | 6.66 ± 1.48 | 6.70 ± 1.42 | 0.768 |
| Total cholesterol (TC), mg/dL | 188.00 [153.00–224.50] | 189.00 [164.50–225.50] | 0.178 |
| LDL-C, mg/dL | 128.55 ± 46.47 | 129.17 ± 42.86 | 0.775 |
| HDL-C, mg/dL | 45.00 [36.00–54.00] | 44.00 [37.00–58.00] | 0.887 |
| Triglycerides (TG), mg/dL | 129.00 [99.00–178.00] | 132.00 [98.50–175.50] | 0.639 |
| BUN (mg/dL) | 19.36 ± 10.96 | 22.76 ± 14.01 | 0.001 |
| Creatinine (mg/dL) | 0.99 ± 0.41 | 1.24 ± 1.09 | 0.006 |
| Uric acid (mg/dL) | 6.31 ± 1.82 | 6.49 ± 1.93 | 0.051 |
| NT-proBNP (pg/mL) | 674.40 [150.80–1571.00] | 1289.00 [617.12–5443.00] | 0.001 |
| Medications, n (%) | |||
| ARNi | 57 (11.7) | 24 (16.6) | 0.128 |
| ACEi/ARB | 393 (80.9) | 116 (80) | 0.817 |
| Beta-blocker | 475 (97.7) | 144 (99.3) | 0.223 |
| MRA | 191 (39.3) | 67 (46.2) | 0.138 |
| Diuretic | 352 (72.4) | 113 (77.9) | 0.187 |
| Oral anticoagulant (OAC) | 105 (21.6) | 52 (35.9) | 0.001 |
| Calcium channel blocker (CCB) | 119 (24.5) | 26 (17.9) | 0.100 |
| Implantable cardiac devices, n (%) | |||
| ICD-CRT | 35 (7.5) | 17 (10.4) | 0.250 |
| Pacemaker | 38 (8.1) | 16 (9.8) | 0.534 |
| SGLT2i, n (%) | |||
| Dapagliflozin | 269 (55.3) | 84 (57.9) | 0.583 |
| Empagliflozin | 217 (44.7) | 61 (42.1) | 0.583 |
| Variables | p-Value | HR | 95% CI |
|---|---|---|---|
| Age | 0.001 | 1.026 | 1.011–1.042 |
| Atrial fibrillation (AF) | 0.040 | 1.452 | 1.018–2.207 |
| Oral anticoagulant use (OAC) | 0.001 | 2.029 | 1.357–3.034 |
| Ejection fraction (EF) | 0.068 | 0.987 | 0.973–1.001 |
| NT-proBNP | 0.007 | 1.001 | 1.001–1.002 |
| Serum creatinine | 0.041 | 1.141 | 1.005–1.294 |
| SGLT2i type (Dapagliflozin vs. Empagliflozin) | 0.968 | 1.007 | 0.725–1.398 |
| Variables | Coefficient (B) | Standard Error (SE) | Wald | p-Value | HR (Exp[B]) | 95% CI (Exp[B]) |
|---|---|---|---|---|---|---|
| Age | 0.737 | 0.098 | 56.127 | <0.001 | 2.089 | 1.723–2.533 |
| Serum creatinine | 0.157 | 0.139 | 1.289 | 0.256 | 1.170 | 0.892–1.536 |
| NT-proBNP | 0.000 | 0.000 | 0.585 | 0.444 | 1.000 | 1.000–1.000 |
| TIMEAGE (Age × Time interaction) | −0.038 | 0.005 | 54.121 | <0.001 | 0.963 | 0.953–0.973 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozgol, I.; Yildiz, C.; Yigit Gencer, E.; Karabulut, D.; Caglar, F.N.T.; Bicakhan, B.; Yilmaz, M.; Karabulut, U.; Gokkurt, Y.; Yigit, Z. Comparative Long-Term Cardiovascular Outcomes of Empagliflozin and Dapagliflozin in Heart Failure Patients After Coronary Revascularization: A Retrospective Cohort Study. J. Clin. Med. 2025, 14, 8383. https://doi.org/10.3390/jcm14238383
Ozgol I, Yildiz C, Yigit Gencer E, Karabulut D, Caglar FNT, Bicakhan B, Yilmaz M, Karabulut U, Gokkurt Y, Yigit Z. Comparative Long-Term Cardiovascular Outcomes of Empagliflozin and Dapagliflozin in Heart Failure Patients After Coronary Revascularization: A Retrospective Cohort Study. Journal of Clinical Medicine. 2025; 14(23):8383. https://doi.org/10.3390/jcm14238383
Chicago/Turabian StyleOzgol, Ilhan, Cennet Yildiz, Ece Yigit Gencer, Dilay Karabulut, Fatma Nihan Turhan Caglar, Burcu Bicakhan, Melek Yilmaz, Umut Karabulut, Yasar Gokkurt, and Zerrin Yigit. 2025. "Comparative Long-Term Cardiovascular Outcomes of Empagliflozin and Dapagliflozin in Heart Failure Patients After Coronary Revascularization: A Retrospective Cohort Study" Journal of Clinical Medicine 14, no. 23: 8383. https://doi.org/10.3390/jcm14238383
APA StyleOzgol, I., Yildiz, C., Yigit Gencer, E., Karabulut, D., Caglar, F. N. T., Bicakhan, B., Yilmaz, M., Karabulut, U., Gokkurt, Y., & Yigit, Z. (2025). Comparative Long-Term Cardiovascular Outcomes of Empagliflozin and Dapagliflozin in Heart Failure Patients After Coronary Revascularization: A Retrospective Cohort Study. Journal of Clinical Medicine, 14(23), 8383. https://doi.org/10.3390/jcm14238383






