Burden of Hematological Malignancies in East Asia from 1990 to 2021
Abstract
1. Introduction
2. Methods
2.1. Data Source
2.2. Statistical Analysis and Data Visualization
3. Results
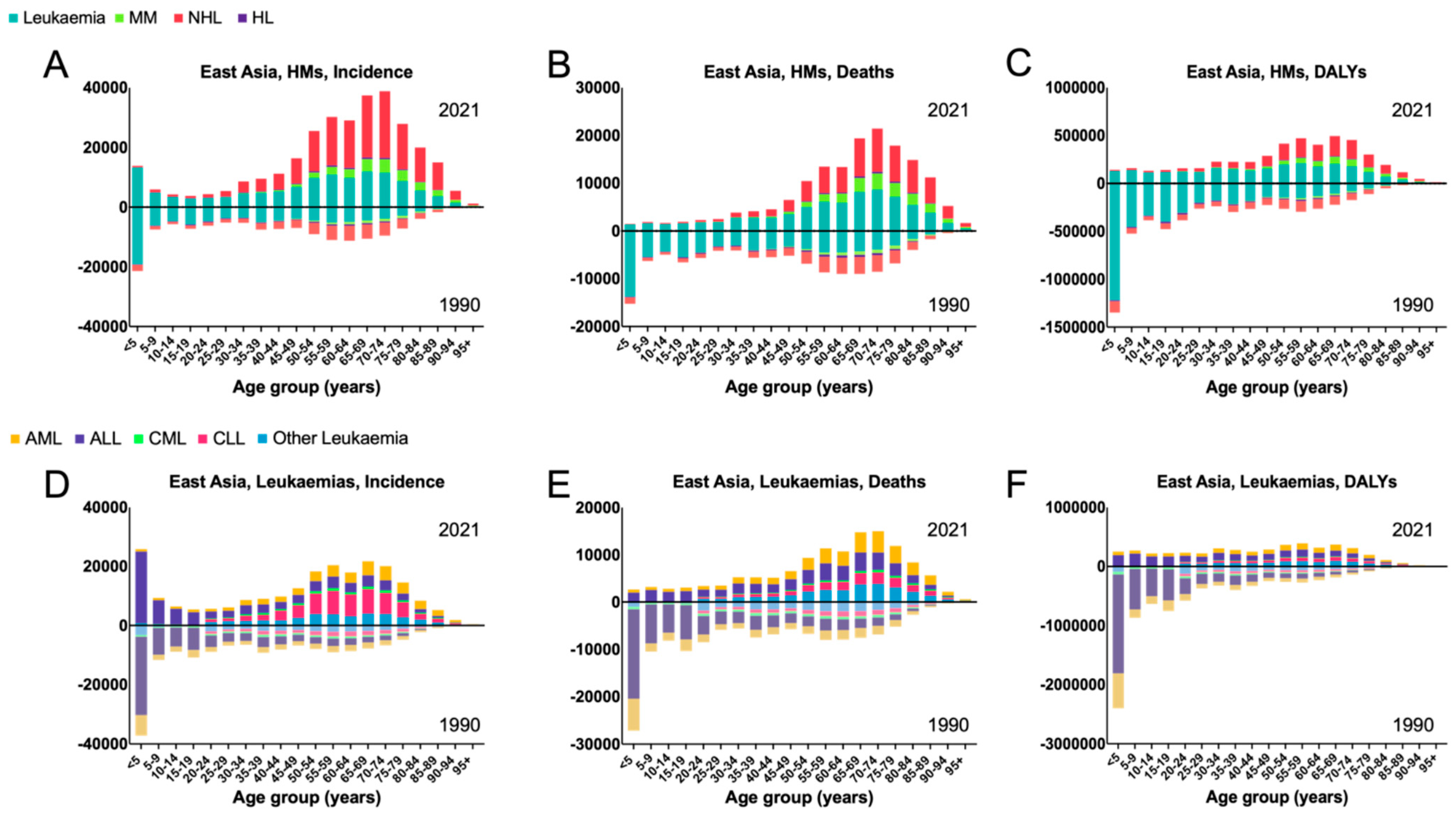
3.1. East Asia
3.2. China
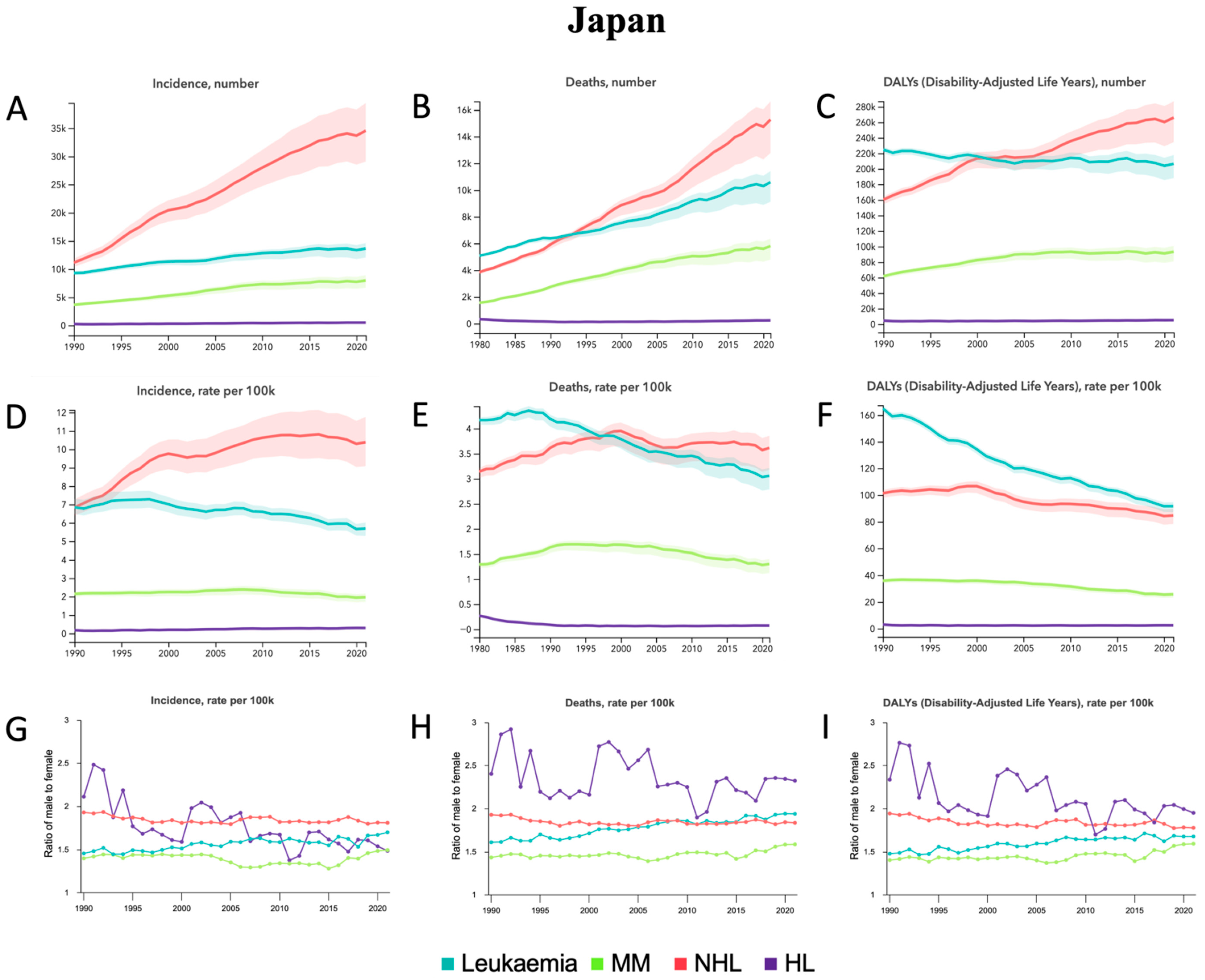
3.3. Japan
3.4. North Korea
3.5. Republic of Korea
3.6. Mongolia
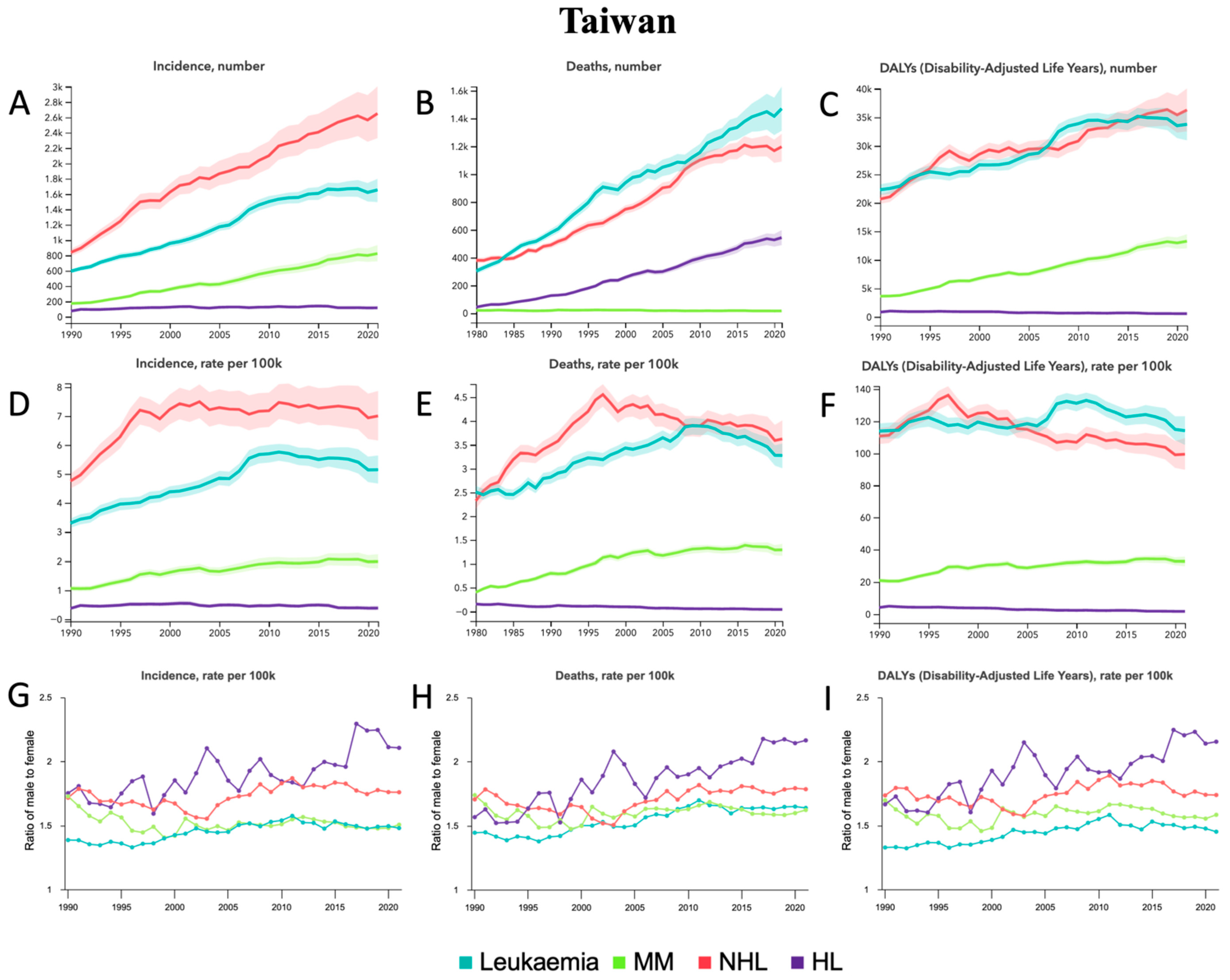
3.7. Taiwan
4. Discussion
4.1. East Asia
4.2. China
4.3. Japan
4.4. North Korea
4.5. Republic of Korea
4.6. Mongolia
4.7. Taiwan
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kocarnik, J. Cancer’s global epidemiological transition and growth. Lancet 2020, 395, 757–758. [Google Scholar] [CrossRef]
- Franceschi, S.; Wild, C.P. Meeting the global demands of epidemiologic transition—The indispensable role of cancer prevention. Mol. Oncol. 2013, 7, 1–13. [Google Scholar] [CrossRef]
- Kocarnik, J.M.; Compton, K.; Dean, F.E.; Fu, W.; Gaw, B.L.; Harvey, J.D.; Henrikson, H.J.; Lu, D.; Pennini, A.; Xu, R.; et al. Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life Years for 29 Cancer Groups from 2010 to 2019. JAMA Oncol. 2022, 8, 420. [Google Scholar] [CrossRef] [PubMed]
- Keykhaei, M.; Masinaei, M.; Mohammadi, E.; Azadnajafabad, S.; Rezaei, N.; Saeedi Moghaddam, S.; Rezaei, N.; Nasserinejad, M.; Abbasi-Kangevari, M.; Malekpour, M.-R.; et al. A global, regional, and national survey on burden and Quality of Care Index (QCI) of hematologic malignancies; global burden of disease systematic analysis 1990–2017. Exp. Hematol. Oncol. 2021, 10, 11. [Google Scholar] [CrossRef]
- Zhang, N.; Wu, J.; Wang, Q.; Liang, Y.; Li, X.; Chen, G.; Ma, L.; Liu, X.; Zhou, F. Global burden of hematologic malignancies and evolution patterns over the past 30 years. Blood Cancer J. 2023, 13, 82. [Google Scholar] [CrossRef] [PubMed]
- Sallman, D.; Chaudhury, A.; Nguyen, J.; Zhang, L. Handbook of Hematologic Malignancies, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Yi, M.; Li, A.; Zhou, L.; Chu, Q.; Song, Y.; Wu, K. The global burden and attributable risk factor analysis of acute myeloid leukemia in 195 countries and territories from 1990 to 2017: Estimates based on the global burden of disease study 2017. J. Hematol. Oncol. 2020, 13, 72. [Google Scholar] [CrossRef]
- Huang, J.; Chan, S.C.; Lok, V.; Zhang, L.; Lucero-Prisno, D.E., 3rd; Xu, W.; Zheng, Z.J.; Elcarte, E.; Withers, M.; Wong, M.C.S. The epidemiological landscape of multiple myeloma: A global cancer registry estimate of disease burden, risk factors, and temporal trends. Lancet Haematol. 2022, 9, e670–e677. [Google Scholar] [CrossRef] [PubMed]
- Standard Country or Area Codes for Statistical Use (M49): United Nations Statistics Division. Available online: https://unstats.un.org/unsd/methodology/m49/ (accessed on 8 November 2025).
- Rajappa, S.; Singh, M.; Uehara, R.; Schachterle, S.E.; Setia, S. Cancer incidence and mortality trends in Asia based on regions and human development index levels: An analyses from GLOBOCAN 2020. Curr. Med. Res. Opin. 2023, 39, 1127–1137. [Google Scholar] [CrossRef]
- Lin, K.; Jia, H.; Cao, M.; Xu, T.; Chen, Z.; Song, X.; Miao, Y.; Yao, T.; Dong, C.; Shao, J.; et al. Epidemiological characteristics of leukemia in China, 2005–2017: A log-linear regression and age-period-cohort analysis. BMC Public Health 2023, 23, 1647. [Google Scholar] [CrossRef]
- Huang, S.Y.; Yao, M.; Tang, J.L.; Lee, W.C.; Tsay, W.; Cheng, A.L.; Wang, C.H.; Chen, Y.C.; Shen, M.C.; Tien, H.F. Epidemiology of multiple myeloma in Taiwan. Cancer 2007, 110, 896–905. [Google Scholar] [CrossRef]
- Muto, R.; Miyoshi, H.; Sato, K.; Furuta, T.; Muta, H.; Kawamoto, K.; Yanagida, E.; Yamada, K.; Ohshima, K. Epidemiology and secular trends of malignant lymphoma in Japan: Analysis of 9426 cases according to the World Health Organization classification. Cancer Med. 2018, 7, 5843–5858. [Google Scholar] [CrossRef]
- Liu, W.; Liu, J.; Song, Y.; Zeng, X.; Wang, X.; Mi, L.; Cai, C.; Wang, L.; Ma, J.; Zhu, J. Burden of lymphoma in China, 2006–2016: An analysis of the Global Burden of Disease Study 2016. J. Hematol. Oncol. 2019, 12, 115. [Google Scholar] [CrossRef] [PubMed]
- Ko, B.S.; Chen, L.J.; Huang, H.H.; Wen, Y.C.; Liao, C.Y.; Chen, H.M.; Hsiao, F.Y. Subtype-specific epidemiology of lymphoid malignancies in Taiwan compared to Japan and the United States, 2002–2012. Cancer Med. 2018, 7, 5820–5831. [Google Scholar] [CrossRef]
- Huang, H.-H.; Chen, C.-M.; Wang, C.-Y.; Hsu, W.W.-Y.; Chen, H.-M.; Ko, B.-S.; Hsiao, F.-Y. The epidemiology, treatment patterns, healthcare utilizations and costs of Acute Myeloid Leukaemia (AML) in Taiwan. PLoS ONE 2022, 17, e0261871. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Park, E.-H.; Jung, K.-W.; Kong, H.-J.; Won, Y.-J.; Lee, J.Y.; Yoon, J.H.; Park, B.-K.; Lee, H.; Eom, H.-S.; et al. Statistics of hematologic malignancies in Korea: Incidence, prevalence and survival rates from 1999 to 2008. Korean J. Hematol. 2012, 47, 28. [Google Scholar] [CrossRef]
- Ferrari, A.J.; Santomauro, D.F.; Aali, A.; Abate, Y.H.; Abbafati, C.; Abbastabar, H.; Abd Elhafeez, S.; Abdelmasseh, M.; Abd-Elsalam, S.; Abdollahi, A.; et al. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: A systema. Lancet 2024, 403, 2133–2161. [Google Scholar] [CrossRef]
- Naghavi, M.; Ong, K.L.; Aali, A.; Ababneh, H.S.; Abate, Y.H.; Abbafati, C.; Abbasgholizadeh, R.; Abbasian, M.; Abbasi-Kangevari, M.; Abbastabar, H.; et al. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2100–2132. [Google Scholar] [CrossRef] [PubMed]
- Vollset, S.E.; Ababneh, H.S.; Abate, Y.H.; Abbafati, C.; Abbasgholizadeh, R.; Abbasian, M.; Abbastabar, H.; Abd Al Magied, A.H.A.; Abd Elhafeez, S.; Abdelkader, A.; et al. Burden of disease scenarios for 204 countries and territories, 2022–2050: A forecasting analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2204–2256. [Google Scholar] [CrossRef]
- Sharma, R.; Abbastabar, H.; Abdulah, D.M.; Abidi, H.; Abolhassani, H.; Abrehdari-Tafreshi, Z.; Absalan, A.; Ali, H.A.; Abu-Gharbieh, E.; Acuna, J.M.; et al. Temporal patterns of cancer burden in Asia, 1990–2019: A systematic examination for the Global Burden of Disease 2019 study. Lancet Reg. Health Southeast Asia 2024, 21, 100333. [Google Scholar] [CrossRef]
- Hammond, A.; Ranganathan, S.; Jain, U.; Jain, B.; Iyengar, P.; Jay, G.; Feliciano, E.; Swami, N.; Wu, J.F.; Gyawali, B.; et al. Hematologic Cancers in the SAARC Region: Current Burden in 2022 and Projections to 2050. Blood Glob. Hematol. 2025, 100036. [Google Scholar] [CrossRef]
- Wu, J.F.; Feliciano, E.J.G.; Singh, A.; Tremblay, D.; Abid, M.B.; Dee, E.C. National cancer system metrics and leukemia outcomes: An analysis of global data for pediatric and adult patients. Leukemia 2025, 39, 1783–1786. [Google Scholar] [CrossRef]
- Dee, E.C.; Wu, J.F.; Feliciano, E.J.G.; Ting, F.I.L.; Willmann, J.; Ho, F.D.V.; Jain, B.; Jain, U.; Chen, J.; Moraes, F.Y.; et al. National Cancer System Characteristics and Global Pan-Cancer Outcomes. JAMA Oncol. 2025, 11, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Bair, S.M.; Brandstadter, J.D.; Ayers, E.C.; Stadtmauer, E.A. Hematopoietic stem cell transplantation for blood cancers in the era of precision medicine and immunotherapy. Cancer 2020, 126, 1837–1855. [Google Scholar] [CrossRef] [PubMed]
- Stabellini, N.; Tomlinson, B.; Cullen, J.; Shanahan, J.; Waite, K.; Montero, A.J.; Barnholtz-Sloan, J.S.; Hamerschlak, N. Sex differences in adults with acute myeloid leukemia and the impact of sex on overall survival. Cancer Med. 2023, 12, 6711–6721. [Google Scholar] [CrossRef]
- Radkiewicz, C.; Bruchfeld, J.B.; Weibull, C.E.; Jeppesen, M.L.; Frederiksen, H.; Lambe, M.; Jakobsen, L.; El-Galaly, T.C.; Smedby, K.E.; Wästerlid, T. Sex differences in lymphoma incidence and mortality by subtype: A population-based study. Am. J. Hematol. 2023, 98, 23–30. [Google Scholar] [CrossRef]
- Ng, M.; Gakidou, E.; Lo, J.; Abate, Y.H.; Abbafati, C.; Abbas, N.; Abbasian, M.; Abd Elhafeez, S.; Abdel-Rahman, W.M.; Abd-Elsalam, S.; et al. Global, regional, and national prevalence of adult overweight and obesity, 1990–2021, with forecasts to 2050: A forecasting study for the Global Burden of Disease Study 2021. Lancet 2025, 405, 813–838. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.-V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef]
- Parikh, R.; Tariq, S.M.; Marinac, C.R.; Shah, U.A. A comprehensive review of the impact of obesity on plasma cell disorders. Leukemia 2022, 36, 301–314. [Google Scholar] [CrossRef]
- Teras, L.R.; Bertrand, K.A.; Deubler, E.L.; Chao, C.R.; Lacey, J.V.; Patel, A.V.; Rosner, B.A.; Shu, Y.H.; Wang, K.; Zhong, C.; et al. Body size and risk of non-Hodgkin lymphoma by subtype: A pooled analysis from six prospective cohorts in the United States. Br. J. Haematol. 2022, 197, 714–727. [Google Scholar] [CrossRef]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, Obesity, and Mortality from Cancer in a Prospectively Studied Cohort of U.S. Adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Su, B.; Zheng, X. Trends and Challenges for Population and Health During Population Aging-China, 2015–2050. China CDC Wkly. 2021, 3, 593–598. [Google Scholar] [CrossRef]
- Inaba, H.; Pui, C.-H. Advances in the Diagnosis and Treatment of Pediatric Acute Lymphoblastic Leukemia. J. Clin. Med. 2021, 10, 1926. [Google Scholar] [CrossRef]
- Chen, S.-L.; Zhang, H.; Gale, R.P.; Tang, J.-Y.; Pui, C.-H.; Chen, S.-J.; Liang, Y. Toward the Cure of Acute Lymphoblastic Leukemia in Children in China. JCO Glob. Oncol. 2021, 7, 1176–1186. [Google Scholar] [CrossRef]
- Tao, W.; Zeng, Z.; Dang, H.; Li, P.; Chuong, L.; Yue, D.; Wen, J.; Zhao, R.; Li, W.; Kominski, G. Towards universal health coverage: Achievements and challenges of 10 years of healthcare reform in China. BMJ Glob. Health 2020, 5, e002087. [Google Scholar] [CrossRef]
- Han, B.; Zheng, R.; Zeng, H.; Wang, S.; Sun, K.; Chen, R.; Li, L.; Wei, W.; He, J. Cancer incidence and mortality in China, 2022. J Natl. Cancer Cent. 2024, 4, 47–53. [Google Scholar] [CrossRef]
- Lancet, T. Cancer registries: The bedrock of global cancer care. Lancet 2025, 405, 353. [Google Scholar] [CrossRef]
- Narimatsu, H.; Sakaguchi, M.; Nakamura, S.; Katayama, K. Future Patient Incidence in Hemato-Oncology: A Study Using Data from Cancer Registries in Japan. Risk Manag. Healthc. Policy 2020, 13, 2407–2414. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.T.; Hori, M.; Matsuda, T.; Katanoda, K. Cancer Prevalence Projections in Japan and Decomposition Analysis of Changes in Cancer Burden, 2020–2050: A Statistical Modeling Study. Cancer Epidemiol. Biomark. Prev. 2023, 32, 1756–1770. [Google Scholar] [CrossRef] [PubMed]
- Itani, Y.; Obama, K.; Fujimori, M.; Saito, J.; Uchitomi, Y. Cancer treatment-related financial toxicity in Japan: A scoping review. Front. Psychol. 2023, 14, 1205016. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, K.; Hashimoto, H.; Ikegami, N.; Nishi, A.; Tanimoto, T.; Miyata, H.; Takemi, K.; Reich, M.R. Future of Japan’s system of good health at low cost with equity: Beyond universal coverage. Lancet 2011, 378, 1265–1273. [Google Scholar] [CrossRef]
- Lee, Y.H.; Yoon, S.-J.; Kim, Y.A.; Yeom, J.W.; Oh, I.-H. Overview of the Burden of Diseases in North Korea. J. Prev. Med. Public. Health 2013, 46, 111–117. [Google Scholar] [CrossRef]
- Park, S.M.; Lee, H.W. Current status of healthcare and effective health aid strategies in North Korea. J. Korean Med. Assoc. 2013, 56, 368. [Google Scholar] [CrossRef]
- Choi, S.; Kim, T.; Choi, S.; Shin, H.Y. Surgical Diseases in North Korea: An Overview of North Korean Medical Journals. Int. J. Environ. Res. Public Health 2020, 17, 9346. [Google Scholar] [CrossRef]
- Shim, T.-I. Ask a North Korean: What Is the Healthcare System in the DPRK Really Like? Available online: https://www.nknews.org/2020/02/ask-a-north-korean-what-is-the-healthcare-system-in-the-dprk-really-like/ (accessed on 9 October 2024).
- North Korea’s ‘Horrifying’ Health Care System. Available online: https://theweek.com/articles/492616/north-koreas-horrifying-health-care-system (accessed on 9 October 2024).
- World Health Organization 2025 Data.Who.Int, Democratic People’s Republic of Korea. Available online: https://data.who.int/countries/408 (accessed on 9 October 2024).
- Shin, B.K.; Jeon, W.T. National Health Priorities under the Kim Jong Un regime in Democratic People’s Republic of Korea (DPRK), 2012–2018. BMJ Glob. Health 2019, 4, e001518. [Google Scholar] [CrossRef]
- Beard, J.R.; Officer, A.; De Carvalho, I.A.; Sadana, R.; Pot, A.M.; Michel, J.-P.; Lloyd-Sherlock, P.; Epping-Jordan, J.E.; Peeters, G.M.E.E.; Mahanani, W.R.; et al. The World report on ageing and health: A policy framework for healthy ageing. Lancet 2016, 387, 2145–2154. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-Y.; Shin, K.; Kikkawa, A. Aging, automation, and productivity in Korea1. J. Jpn. Int. Econ. 2021, 59, 101109. [Google Scholar] [CrossRef]
- Park, B.; Yoon, J.; Lee, Y.; Park, Y.; Eom, H.-S. Trends in multiple myeloma incidence, prevalence, mortality, and survival rate in South Korea: A nationwide population-based study. Ann. Hematol. 2024, 103, 4111–4119. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Bang, S.-M. Epidemiological Change of Multiple Myeloma in Korea. Korean J. Hematol. 2006, 41, 225–234. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, K.; Kim, B.S.; Jo, D.Y.; Kang, H.J.; Kim, J.S.; Mun, Y.C.; Kim, C.S.; Sohn, S.K.; Eom, H.S.; et al. Clinical features and survival outcomes in patients with multiple myeloma: Analysis of web-based data from the Korean Myeloma Registry. Acta Haematol. 2009, 122, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Je-Jung, L.; Jae-Sook, A. Recently increasing hematologic diseases in Korea. Korean J. Med. 2010, 78, 557–563. [Google Scholar]
- Han, H.J.; Choi, K.; Suh, H.S. Impact of aging on acute myeloid leukemia epidemiology and survival outcomes: A real-world, population-based longitudinal cohort study. PLoS ONE 2024, 19, e0300637. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chan, S.C.; Ngai, C.H.; Lok, V.; Zhang, L.; Lucero-Prisno, D.E.; Xu, W.; Zheng, Z.-J.; Elcarte, E.; Withers, M.; et al. Disease Burden, Risk Factors, and Trends of Leukaemia: A Global Analysis. Front. Oncol. 2022, 12, 904292. [Google Scholar] [CrossRef]
- Klepin, H.D.; Estey, E.; Kadia, T. More Versus Less Therapy for Older Adults With Acute Myeloid Leukemia: New Perspectives on an Old Debate. Am. Soc. Clin. Oncol. Educ. Book. 2019, 39, 421–432. [Google Scholar] [CrossRef]
- Alsouqi, A.; Geramita, E.; Im, A. Treatment of Acute Myeloid Leukemia in Older Adults. Cancers 2023, 15, 5409. [Google Scholar] [CrossRef]
- World Health Organization 2025 Data.Who.Int, Mongolia. Available online: https://data.who.int/countries/496 (accessed on 8 October 2024).
- Chimed-Ochir, O.; Delgermaa, V.; Takahashi, K.; Purev, O.; Sarankhuu, A.; Fujino, Y.; Bayarmagnai, N.; Dugee, O.; Erkhembayar, R.; Lkhagvaa, B.; et al. Mongolia health situation: Based on the Global Burden of Disease Study 2019. BMC Public Health 2022, 22, 5. [Google Scholar] [CrossRef]
- Swabey-Van de Borne, E. Mongolia Takes Steps to Enhance Cancer Detection and Treatment Capacities. Available online: https://www.iaea.org/newscenter/news/mongolia-takes-steps-to-enhance-cancer-detection-and-treatment-capacities (accessed on 8 October 2024).
- Devi, S. Progress in NCD screening in Mongolia. Lancet Oncol. 2024, 25, e403. [Google Scholar] [CrossRef]
- Gonchigjav, B.; Bayaraa, A.; Enkhee, B.; Purevjal, M.; Pandaan, B. Organ Donation and Transplantation in Mongolia. Transplantation 2022, 106, 1–3. [Google Scholar] [CrossRef]
- Li, C.C.; Tsai, X.C.; Huang, W.H.; Wang, T.F. Recent advancements in hematopoietic stem cell transplantation in Taiwan. Tzu Chi Med. J. 2024, 36, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.-Y.; Wang, M.-Y. Progress, Challenges, and Development Directions of Long-Term Care Policies in Taiwan. In Proceedings of the 2024 4th International Conference on Public Management and Big Data Analysis (PMBDA 2024), Qingdao, China, 20–22 December 2024; Atlantis Press International BV: Dordrecht, The Netherlands, 2025; pp. 266–281. [Google Scholar]
- Kuo, C.N.; Liao, Y.M.; Kuo, L.N.; Tsai, H.J.; Chang, W.C.; Yen, Y. Cancers in Taiwan: Practical insight from epidemiology, treatments, biomarkers, and cost. J. Formos. Med. Assoc. 2020, 119, 1731–1741. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.Y.; Lin, C.-J. The Taiwan health-care system: Approaching a crisis point? Lancet 2024, 404, 745–746. [Google Scholar] [CrossRef] [PubMed]




| Location | Incidence | Deaths | DALYs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hematological Malignancy | Counts, 2021 | Percent change, 1990–2021 | ASR, 2021 | Percent change, 1990–2021 | Counts, 2021 | Percent change, 1990–2021 | ASR, 2021 | Percent change, 1990–2021 | Counts, 2021 | Percent change, 1990–2021 | ASR, 2021 | Percent change, 1990–2021 |
| East Asia | ||||||||||||
| Leukemia | 125,491.3 | 40.8% | ·· | ·· | 73,914.8 | -4.0% | ·· | ·· | 2,553,059.9 | −40.8% | ·· | ·· |
| Multiple Myeloma | 27,838.3 | 375.2% | ·· | ·· | 20,539.4 | 330.7% | ·· | ·· | 471,351.5 | 291.3% | ·· | ·· |
| Non-Hodgkin Lymphoma | 155,393.9 | 246.4% | ·· | ·· | 62,706.2 | 97.0% | ·· | ·· | 1,660,918.3 | 39.4% | ·· | ·· |
| Hodgkin Lymphoma | 5093.3 | −2.2% | ·· | ·· | 2797.9 | −39.8% | ·· | ·· | 83,423.8 | −56.4% | ·· | ·· |
| China | ||||||||||||
| Leukemia | 105,667.2 [75,275.7–132,236.9] | 38.7 [7.6–82.9] | 7.2 [4.9–9.1] | 0.9 [−23.3 to 35.1] | 58,903.5 [43,626.0–74,038.9] | −12.6 [−29.1 to 14.6] | 3.4 [2.5–4.3] | −47.0 [−56.7 to −31.4] | 2,205,220.6 [1,612,838.7–2,736,625.1] | −43.8 [−55.3 to −25.6] | 151.5 [108.7–185.1] | −55.9 [−65.3 to −41.6] |
| Multiple Myeloma | 17,249.5 [11,016.7–22,663.0] | 918.7 [310.4–1657.5] | 0.8 [0.5–1.1] | 312.5 [62.9–620.9] | 12,984.1 [8447.9–17,113.8] | 716.0 [229.5–1314.1] | 0.6 [0.4–0.8] | 221.5 [26.3–463.3] | 338,358.5 [213,668.7–447,634.9] | 622.1 [192.7–1140.6] | 16.1 [10.1–21.3] | 221.1 [28.5–459.2] |
| Non-Hodgkin Lymphoma | 110,923.5 [86,933.9–135,200.4] | 255.3 [172.7–367.9] | 5.5 [4.4–6.7] | 66.7 [28.7–117.0] | 42,856.9 [33,553.2–51,712.2] | 78.4 [37.2–134.7] | 2.1 [1.7–2.6] | −20.1 [−38.0 to 3.8] | 1,277,097.3 [997,263.0–1,551,799.7] | 33.1 [1.1–74.5] | 66.9 [52.5–80.2] | −27.6 [−4.5 to −5.7] |
| Hodgkin Lymphoma | 4211.1 [2542.0–5541.5] | −11.3 [−39.7 to 57.3] | 0.2 [0.1–0.3] | −52.5 [−67.8 to −13.6] | 2443.2 [1506.8–3231.6] | −44.4 [−62.6 to 0.4] | 0.13 [0.08–0.17] | −73.2 [−81.8 to −50.3] | 74,190.6 [46,299.5–100,603.2] | −59.2 [−72.6 to −26.4] | 4.1 [2.6–5.6] | −75.4 [−83.5 to −54.6] |
| Japan | ||||||||||||
| Leukemia | 13,647.1 [12,049.6–14,607.2] | 46.7 [33.6–56.8] | 5.7 [5.3–6.0] | −16.9 [−23.5 to −10.9] | 10,592.2 [9126.3–11,420.5] | 66.1 [48.6–76.4] | 3.1 [2.8–3.2] | −27.1 [−31.1 to −24.5] | 206,496.8 [188,223.6–217,895.6] | −8.1 [−15.1 to −3.8] | 91.6 [87.1–94.7] | −44.4 [−46.3 to −42.8] |
| Multiple Myeloma | 7969.1 [6706.2–8881.7] | 118.1 [92.5–141.8] | 2.0 [1.7–2.2] | −8.3 [−16.6 to 0.3] | 5806.5 [4799.9–6373.2] | 111.2 [87.1–125.7] | 1.3 [1.1–1.4] | −20.0 [−25.9. to −16.3] | 93,334.2 [80,942.2–101,130.6] | 51.0 [37.0–59.5] | 25.7 [23.2–27.3] | −28.4 [−32.5 to −25.7] |
| Non-Hodgkin Lymphoma | 34,577.7 [29,092.6–39,491.1] | 210.5 [165.7–261.9] | 10.4 [9.1–11.8] | 51.9 [33.7–74.1] | 15,272.4 [12,775.5–16,644.2] | 157.9 [127.1–174.4] | 3.6 [3.2–3.9] | 0.07 [−7.3 to 4.3] | 266,046.7 [234,467.9–287,122.6] | 66.0 [50.3–75.4] | 84.7 [78.3–89.6] | −16.5 [−20.9 to −13.3] |
| Hodgkin Lymphoma | 507.7 [466.1–542.0] | 97.5 [82.7–113.1] | 0.29 [0.27–0.32] | 69.4 [55.3–84.4] | 236.0 [207.9–253.1] | 65.0 [49.8–74.9] | 0.07 [0.07–0.08] | −17.6 [−21.8 to −14.0] | 5192.5 [4765.6–5515.2] | 13.9 [6.3–19.8] | 2.4 [2.3–2.6] | −20.0 [−23.5 to −16.6] |
| North Korea | ||||||||||||
| Leukemia | 1414.1 [1019.5–1974.1] | 40.1 [−0.6 to 95.3] | 4.9 [3.5–6.8] | −4.8 [−33.6 to 33.8] | 1201.2 [869.1–1689.8] | 30.7 [−7.2 to 84.9] | 4.1 [2.9–5.6] | −15.0 [−39.3 to 20.2] | 50,535.6 [36,097.7–72,235.7] | 1.0 [−29.2 to 45.0] | 187.6 [134.5–265.5] | −21.5 [−45.4 to 11.8] |
| Multiple Myeloma | 114.4 [64.9–191.9] | 124.5 [42.2–239.1] | 0.3 [0.2–0.6] | 11.1 [−29.0 to 66.0] | 97.0 [54.2–163.6] | 112.4 [33.3–218.7] | 0.3 [0.2–0.5] | 2.7 [−34.3 to 53.5] | 2705.5 [1534.7–4603.2] | 96.8 [24.7–196.0] | 8.0 [4.6–13.7] | 4.2 [−33.9 to 56.5] |
| Non-Hodgkin Lymphoma | 1011.9 [732.3–1476.9] | 119.6 [55.0–210.8] | 3.1 [2.3–4.5] | 21.6 [−13.1 to 71.5] | 789.8 [578.8–1145.4] | 93.5 [34.8–171.9] | 2.4 [1.8–3.5] | 3.1 [−27.3 to 44.5] | 26,082.4 [18,935.8–37,918.2] | 65.6 [14.5–140.0] | 81.6 [59.2–118.0] | 0.7 [−29.3 to 45.4] |
| Hodgkin Lymphoma | 106.3 [72.2–184.8] | 62.8 [−3.0 to 164.5] | 0.3 [0.2–0.6] | 0.4 [−38.3 to 64.2] | 49.5 [34.2–83.5] | 21.5 [−24.8 to 92.6] | 0.2 [0.1–0.3] | −32.1 [−57.2 to 6.5] | 1762.7 [1183.3–3066.8] | 2.1 [−40.7 to 66.8] | 5.7 [3.9–9.9] | −34.3 [−61.2 to 6.8] |
| Republic of Korea | ||||||||||||
| Leukemia | 3028.2 [1947.8–3706.7] | 52.4 [2.3–99.9] | 4.8 [2.9–6.2] | −7.4 [−43.3 to 23.5] | 1949.5 [1211.0–2389.6] | 11.2 [−28.9 to 40.2] | 2.4 [1.5–2.9] | −48.5 [−68.0 to −36.0] | 53,503.9 [34,349.8–64,911.7] | −40.0 [−57.6 to −19.7] | 82.0 [53.2–99.6] | −60.5 [−72.6 to −47.5] |
| Multiple Myeloma | 1672.6 [957.4–2207.4] | 487.7 [187.5–758.6] | 1.8 [1.0–2.3] | 73.8 [−18.3 to 159.4] | 1100.9 [624.8–1455.9] | 334.2 [111.9–539.5] | 1.2 [0.7–1.5] | 22.7 [−41.5 to 85.1] | 23,445.5 [13,793.7–30,965.4] | 247.4 [82.6–413.5] | 24.7 [14.6–32.6] | 13.6 [−41.2 to 64.2] |
| Non-Hodgkin Lymphoma | 6167.4 [4153.5–7432.6] | 426.0 [173.2–583.9] | 7.1 [4.9–8.5] | 111.7 [2.6–173.9] | 2279.8 [1493.8–2707.5] | 163.8 [27.1–233.0] | 2.5 [1.7–3.0] | −4.7 [−57.1 to 211.6] | 53,856.1 [37,017.1–62,843.0] | 59.6 [−8.6 to 96.9] | 64.2 [45.2–74.4] | −25.7 [−58.9 to −8.8] |
| Hodgkin Lymphoma | 132.5 [77.3–185.9] | 125.2 [30.0–242.5] | 0.2 [0.1–0.3] | 40.1 [−16.0 to 112.9] | 42.4 [23.8–58.9] | −4.5 [−44.2 to 43.6] | 0.05 [0.03–0.07] | −59.3 [−75.1 to −38.8] | 1247.8 [717.0–1745.4] | −34.2 [–62.1 to –1.3] | 1.8 [1.0–2.5] | −60.6 [−76.8 to −41.0] |
| Mongolia | ||||||||||||
| Leukemia | 79.5 [59.1–100.4] | 29.7 [−4.4 to 79.0] | 2.7 [2.0–3.3] | −19.9 [−39.2 to 7.2] | 71.0 [52.8–90.0] | 19.8 [−10.5 to 63.5] | 2.4 [1.8–3.1] | −24.8 [−43.1 to 0.5] | 3512.4 [2590.3–4496.3] | −4.4 [–31.4 to 31.2] | 108.1 [80.4–137.4] | −32.6 [−50.2 to −7.5] |
| Multiple Myeloma | 8.0 [5.6–10.8] | 312.9 [182.7–547.0] | 0.3 [0.2–0.4] | 77.1 [23.6–173.9] | 7.1 [5.0–9.7] | 279.0 [165.2–490.4] | 0.3 [0.2–0.4] | 66.5 [18.0–157.6] | 238.9 [168.1–328.8] | 295.7 [172.3–523.7] | 8.4 [5.9–11.5] | 68.9 [17.6–164.4] |
| Non-Hodgkin Lymphoma | 61.9 [46.9–81.6] | 56.7 [3.2–142.9] | 2.1 [1.6–2.8] | −14.9 [−43.1 to 31.8] | 36.4 [27.7–48.3] | 16.8 [−21.9 to 77.7} | 1.3 [1.0–1.8] | −35.5 [−56.8 to −1.9] | 1526.1 [1169.5–2036.5] | −6.5 [–38.0 to 46.0] | 49.1 [37.6–65.4] | −41.6 [−60.6 to −10.2] |
| Hodgkin Lymphoma | 18.7 [11.2–27.3] | 137.9 [34.8–379.4] | 0.6 [0.4–0.9] | 25.1 [−30.0 to 150.9] | 10.3 [6.2–14.9] | 63.6 [−9.5 to 229.6] | 0.3 [0.2–0.5] | −13.4 [−52.6 to 75.6] | 478.1 [291.3–696.8] | 45.5 [–17.4 to 193.5] | 14.8 [9.0–21.7] | −17.3 [−53.1 to 63.7] |
| Taiwan | ||||||||||||
| Leukemia | 1655.2 [1496.0–1798.8] | 178.3 [148.6–206.6] | 5.1 [4.7–5.6] | 55.7 [38.9–73.8] | 1197.2 [1090.9–1290.6] | 144.1 [120.7–165.8] | 3.3 [3.0–3.5] | 16.2 [5.5–26.2] | 33,790.7 [30,979.9–36,420.1] | 51.5 [37.5–65.3] | 114.2 [105.1–123.1] | 0.3 [−8.4 to 8.9] |
| Multiple Myeloma | 824.7 [724.5–935.7] | 374.5 [304.7–439.6] | 2.0 [1.8–2.2] | 86.8 [61.5–112.0] | 543.9 [490.1–598.1] | 329.1 [278.7–380.1] | 1.3 [1.2–1.4] | 60.6 [42.3–78.6] | 13,268.9 [12,022.6–14,507.0] | 266.9 [225.9–307.2] | 32.8 [29.8–35.7] | 56.5 [39.4–72.6] |
| Non-Hodgkin Lymphoma | 2651.4 [2330.0–3005.4] | 215.5 [175.1–262.1] | 7.0 [6.2–7.9] | 47.1 [29.1–67.3] | 1470.8 [1312.5–1629.8] | 154.3 [124.9–183.0] | 3.6 [3.3–4.0] | 3.8 [−7.6 to 15.4] | 36,309.7 [32,556.8–40,098.3] | 75.7 [56.6–94.2] | 99.4 [89.8–109.5] | −10.2 [−19.7 to −0.8] |
| Hodgkin Lymphoma | 117.0 [98.6–137.9] | 56.3 [25.0–91.7] | 0.4 [0.3–0.5] | 2.5 [−18.0 to 28.1] | 16.4 [14.1–19.0] | −14.7 [−27.7 to 0.7] | 0.04 [0.04–0.05] | −59.3 [−65.6 to −51.9] | 552.1 [473.9–644.5] | −33.3 [−44.1 to −20.8] | 1.7 [1.4–2.0] | −58.6 [−65.7 to −50.5] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.F.; Ho, F.D.V.; Columbres, R.C.; Tudisco, A.; Jain, U.; Selokar, A.; Swami, N.; Jain, B.; Hong, J.H.; Feliciano, E.J.G.; et al. Burden of Hematological Malignancies in East Asia from 1990 to 2021. J. Clin. Med. 2025, 14, 8381. https://doi.org/10.3390/jcm14238381
Wu JF, Ho FDV, Columbres RC, Tudisco A, Jain U, Selokar A, Swami N, Jain B, Hong JH, Feliciano EJG, et al. Burden of Hematological Malignancies in East Asia from 1990 to 2021. Journal of Clinical Medicine. 2025; 14(23):8381. https://doi.org/10.3390/jcm14238381
Chicago/Turabian StyleWu, James Fan, Frances Dominique V. Ho, Rod Carlo Columbres, Anthony Tudisco, Urvish Jain, Aryan Selokar, Nishwant Swami, Bhav Jain, Ji Hyun Hong, Erin Jay G. Feliciano, and et al. 2025. "Burden of Hematological Malignancies in East Asia from 1990 to 2021" Journal of Clinical Medicine 14, no. 23: 8381. https://doi.org/10.3390/jcm14238381
APA StyleWu, J. F., Ho, F. D. V., Columbres, R. C., Tudisco, A., Jain, U., Selokar, A., Swami, N., Jain, B., Hong, J. H., Feliciano, E. J. G., Ting, F. I. L., & Dee, E. C. (2025). Burden of Hematological Malignancies in East Asia from 1990 to 2021. Journal of Clinical Medicine, 14(23), 8381. https://doi.org/10.3390/jcm14238381







