Personalized Medicine in Pulmonary Arterial Hypertension: Utilizing Artificial Intelligence for Death Prevention
Abstract
1. Introduction
2. Materials and Methods
- TP (True Positive): Correctly predicted positive cases.
- TN (True Negative): Correctly predicted negative cases.
- FP (False Positive): Incorrectly predicted positive cases.
- FN (False Negative): Incorrectly predicted negative cases.
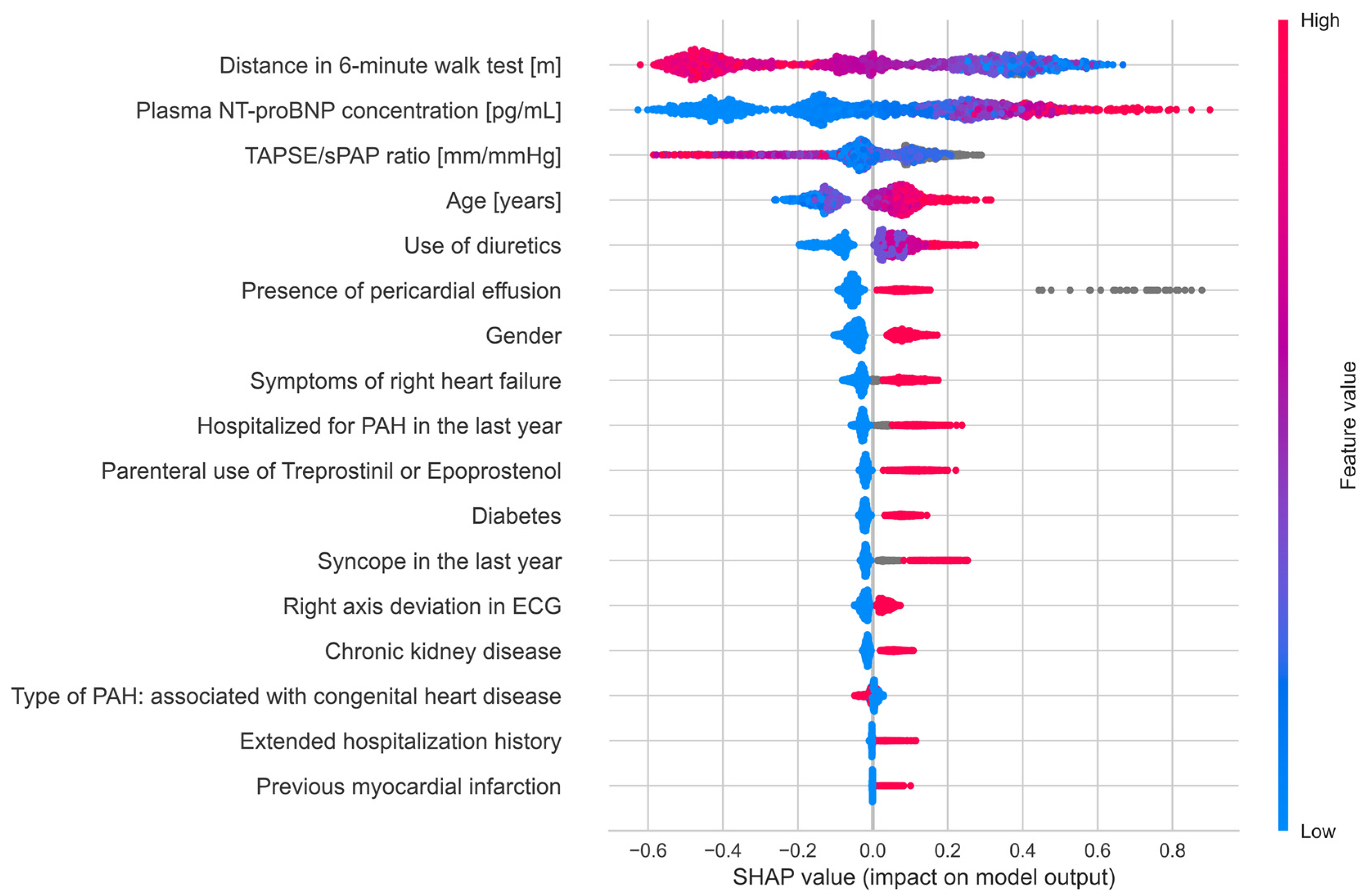
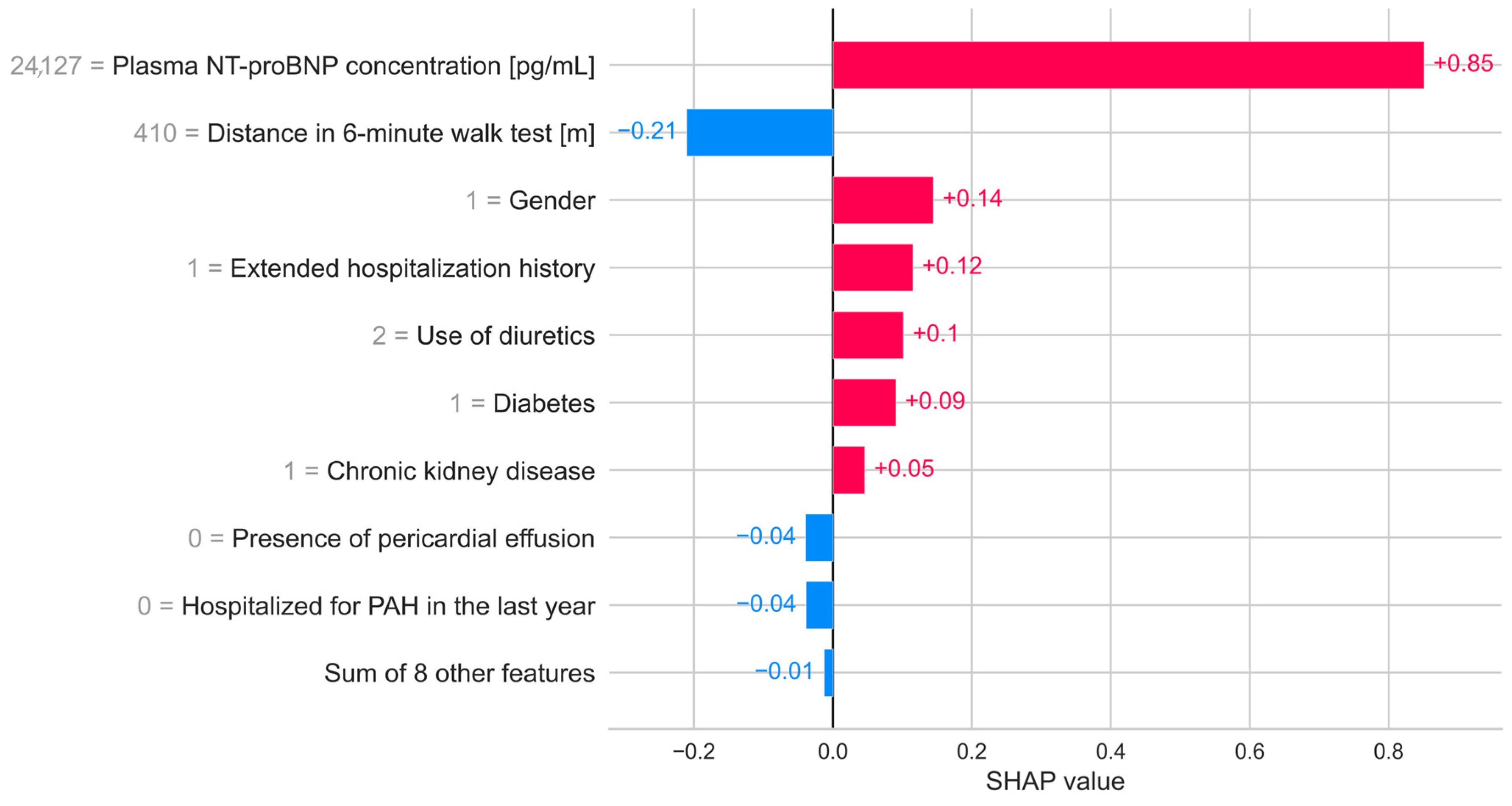
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| BNP-PL | Database of Pulmonary Hypertension in the Polish population |
| CTEPH | Chronic Thromboembolic Pulmonary Hypertension |
| LASSO | Least Absolute Shrinkage and Selection Operator algorithm |
| MCC | Matthews Correlation Coefficient |
| ML | Machine learning |
| PAH | Pulmonary Arterial Hypertension |
| ROC-AUC | The Receiver Operating Characteristic—Area Under the Curve |
| SFE | Sequential Feature Elimination |
| SHAP | Shapley Additive Explanations |
| sPAP | Systolic Pulmonary Arterial Pressure |
| TAPSE | Tricuspid Annular Plane Systolic Excursion |
| XAI | eXplainable Artificial Intelligence |
| XGBoost | eXtreme Gradient Boosting |
References
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef]
- Johnson, K.W.; Torres Soto, J.; Glicksberg, B.S.; Shameer, K.; Miotto, R.; Ali, M.; Ashley, E.; Dudley, J.T. Artificial Intelligence in Cardiology. J. Am. Coll. Cardiol. 2018, 71, 2668–2679. [Google Scholar] [CrossRef]
- Watson, X.; D’Souza, J.; Cooper, D.; Markham, R. Artificial Intelligence in Cardiology: Fundamentals and Applications. Intern. Med. J. 2022, 52, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Westcott, R.J.; Tcheng, J.E. Artificial Intelligence and Machine Learning in Cardiology. JACC Cardiovasc. Interv. 2019, 12, 1312–1314. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Shelly, M.; Attia, Z.I.; Friedman, P.A.; Ito, S.; Essayagh, B.A.; Ko, W.Y.; Murphree, D.H.; Michelena, H.I.; Enriquez-Sarano, M.; Carter, R.E.; et al. Electrocardiogram Screening for Aortic Valve Stenosis Using Artificial Intelligence. Eur. Heart J. 2021, 42, 2885–2896. [Google Scholar] [CrossRef]
- Pieszko, K.; Hiczkiewicz, J.; Budzianowski, J.; Musielak, B.; Hiczkiewicz, D.; Faron, W.; Rzeźniczak, J.; Burchardt, P. Clinical Applications of Artificial Intelligence in Cardiology on the Verge of the Decade. Cardiol. J. 2021, 28, 460–472. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska, J.; Zuk, M.; Migdal, A.; Kusa, J.; Skiba, E.; Zygielo, K.; Przetocka, K.; Werynski, P.; Banaszak, P.; Rzeznik-Bieniaszewska, A.; et al. Children and Adolescents with Pulmonary Arterial Hypertension: Baseline and Follow-Up Data from the Polish Registry of Pulmonary Hypertension (BNP-PL). J. Clin. Med. 2020, 9, 1717. [Google Scholar] [CrossRef]
- Braksator, M.; Kurzyna, M.; Kopeć, G.; Pruszczyk, P.; Mroczek, E.; Mularek-Kubzdela, T.; Skoczylas, I.; Błaszczak, P.; Chrzanowski, Ł.; Jaguszewski, M.; et al. Hemodynamic, Echocardiographic, and Demographic Profiles of Patients with Chronic Thromboembolic Pulmonary Hypertension and Atrial Fibrillation: A Multicenter Cohort Study. J. Heart Lung Transplant. 2025, 44, 1239–1248. [Google Scholar] [CrossRef]
- Kopeć, G.; Kurzyna, M.; Mroczek, E.; Chrzanowski, Ł.; Mularek-Kubzdela, T.; Skoczylas, I.; Kuśmierczyk, B.; Pruszczyk, P.; Błaszczak, P.; Lewicka, E.; et al. Characterization of Patients with Pulmonary Arterial Hypertension: Data from the Polish Registry of Pulmonary Hypertension (BNP-PL). J. Clin. Med. 2020, 9, 173. [Google Scholar] [CrossRef]
- Kopeć, G.; Kurzyna, M.; Mroczek, E.; Chrzanowski, Ł.; Mularek-Kubzdela, T.; Skoczylas, I.; Kuśmierczyk, B.; Pruszczyk, P.; Błaszczak, P.; Lewicka, E.; et al. Database of Pulmonary Hypertension in the Polish Population (BNP-PL): Design of the Registry. Kardiol. Pol. 2019, 77, 972–974. [Google Scholar] [CrossRef]
- Raschka, S. Model Evaluation, Model Selection, and Algorithm Selection in Machine Learning. arXiv 2020, arXiv:1811.12808. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. XGBoost. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13 August 2016; ACM: New York, NY, USA, 2016; pp. 785–794. [Google Scholar]
- Lundberg, S.; Lee, S.-I. A Unified Approach to Interpreting Model Predictions. In Proceedings of the Advances in Neural Information Processing Systems, Long Beach, CA, USA, 4–9 December 2017; pp. 3146–3154. [Google Scholar]
- Lundberg, S.M.; Erion, G.; Chen, H.; DeGrave, A.; Prutkin, J.M.; Nair, B.; Katz, R.; Himmelfarb, J.; Bansal, N.; Lee, S.-I. From Local Explanations to Global Understanding with Explainable AI for Trees. Nat. Mach. Intell. 2020, 2, 56–67. [Google Scholar] [CrossRef]
- Bergstra, J.; Yamins, D.; Cox, D. Making a Science of Model Search: Hyperparameter Optimization in Hundreds of Dimensions for Vision Architectures. In Proceedings of the 30th International Conference on Machine Learning, Atlanta, GA, USA, 16–21 June 2013; Dasgupta, S., McAllester, D., Eds.; PMLR: Atlanta, GA, USA, 2013; Volume 28, pp. 115–123. [Google Scholar]
- Agyemang, E.F.; Mensah, J.A.; Nyarko, E.; Arku, D.; Mbeah-Baiden, B.; Opoku, E.; Noye Nortey, E.N. Addressing Class Imbalance Problem in Health Data Classification: Practical Application From an Oversampling Viewpoint. Appl. Comput. Intell. Soft. Comput. 2025, 2025, 1013769. [Google Scholar] [CrossRef]
- Colalillo, A.; Hoffmann-Vold, A.-M.; Pellicano, C.; Romaniello, A.; Gabrielli, A.; Hachulla, E.; Smith, V.; Simeón-Aznar, C.-P.; Castellví, I.; Airò, P.; et al. The Role of TAPSE/SPAP Ratio in Predicting Pulmonary Hypertension and Mortality in the Systemic Sclerosis EUSTAR Cohort. Autoimmun. Rev. 2023, 22, 103290. [Google Scholar] [CrossRef]
- Palacios-Moguel, P.; Cueto-Robledo, G.; González-Pacheco, H.; Ortega-Hernández, J.; Torres-Rojas, M.B.; Navarro-Vergara, D.I.; García-Cesar, M.; González-Nájera, C.A.; Narváez-Oríani, C.A.; Sandoval, J. The Role of the TAPSE/SPAP Ratio as a Predictor of Mortality in Pulmonary Arterial Hypertension: Its Value for Patient Risk Stratification. JHLT Open 2025, 7, 100168. [Google Scholar] [CrossRef]
- Jain, S.; Kaur, A.; Qadeer, A.; Ghosh, V.; Thota, S.; Banala, M.; Lee, J.; Yerrapragada, G.; Elangovan, P.; Shariff, M.N.; et al. Leveraging Artificial Intelligence for the Diagnosis of Systemic Sclerosis Associated Pulmonary Arterial Hypertension: Opportunities, Challenges, and Future Perspectives. Adv. Respir. Med. 2025, 93, 47. [Google Scholar] [CrossRef] [PubMed]
- Benesch Vidal, M.L.; Arvanitaki, A.; Diller, G.-P. The Role of Artificial Intelligence and Mobile Health in Diagnosis and Management of Pulmonary Arterial Hypertension. Int. J. Cardiol. Congenit. Heart Dis. 2025, 22, 100622. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Shu, T.; Zhao, B.; Xiang, T.; Wang, J.; Huang, H.; Zhang, Y.; Xiao, P.; Zhou, B.; Xie, Z.; et al. Explainable Machine Learning Models for Predicting 30-Day Readmission in Pediatric Pulmonary Hypertension: A Multicenter, Retrospective Study. Front. Cardiovasc. Med. 2022, 9, 919224. [Google Scholar] [CrossRef]
- Alabed, S.; Uthoff, J.; Zhou, S.; Garg, P.; Dwivedi, K.; Alandejani, F.; Gosling, R.; Schobs, L.; Brook, M.; Shahin, Y.; et al. Machine Learning Cardiac-MRI Features Predict Mortality in Newly Diagnosed Pulmonary Arterial Hypertension. Eur. Heart J.-Digit. Health 2022, 3, 265–275. [Google Scholar] [CrossRef]
- Attaripour Esfahani, S.; Baba Ali, N.; Farina, J.M.; Scalia, I.G.; Pereyra, M.; Abbas, M.T.; Javadi, N.; Bismee, N.N.; Abdelfattah, F.E.; Awad, K.; et al. A Comprehensive Review of Artificial Intelligence (AI) Applications in Pulmonary Hypertension (PH). Medicina 2025, 61, 85. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.; Alabed, S.; Sharkey, M.; Maiter, A.; Dwivedi, K.; Yardibi, T.; Selej, M.; Hameed, A.; Charalampopoulos, A.; Kiely, D.G.; et al. Artificial Intelligence-Based Echocardiography Assessment to Detect Pulmonary Hypertension. ERJ Open Res. 2025, 11, 00592-2024. [Google Scholar] [CrossRef]
- Kwon, J.; Kim, K.-H.; Medina-Inojosa, J.; Jeon, K.-H.; Park, J.; Oh, B.-H. Artificial Intelligence for Early Prediction of Pulmonary Hypertension Using Electrocardiography. J. Heart Lung Transplant. 2020, 39, 805–814. [Google Scholar] [CrossRef]
- Celestin, B.; Bagherzadeh, S.P.; Santana, E.; Frost, M.; Iversen, M.; Hermansson, F.N.; Sweatt, A.; Zamanian, R.T.; Hummel, Y.M.; Rendon, G.G.; et al. Artificial Intelligence-Based Echocardiography in Pulmonary Arterial Hypertension. Chest 2025, in press. [Google Scholar] [CrossRef]
- Cersosimo, A.; Zito, E.; Pierucci, N.; Matteucci, A.; La Fazia, V.M. A Talk with ChatGPT: The Role of Artificial Intelligence in Shaping the Future of Cardiology and Electrophysiology. J. Pers. Med. 2025, 15, 205. [Google Scholar] [CrossRef] [PubMed]
- Saleh, K.M.; Mallat, J.; Mohammed, S.; Bodi, G.; Alazazzi, H.; Salim, S.; Elhennawi, M.; Iqbal, T.; Sabbour, H. Comparative Efficacy and Safety of Prostacyclin Therapies for Pulmonary Arterial Hypertension: A Systematic Review and Network Meta-Analysis. Front. Med. 2025, 12, 1643220. [Google Scholar] [CrossRef]
- Kaemmerer, H.; Diller, G.P.; Dähnert, I.; Achenbach, S.; Eichstaedt, C.A.; Eicken, A.; Freiberger, A.; Freilinger, S.; Geiger, R.; Gorenflo, M.; et al. Pulmonary Hypertension in Adults with Congenital Heart Defects (ACHDs) in Light of the 2022 ESC PAH Guidelines—Part II: Supportive Therapy, Special Situations (Pregnancy, Contraception, Non-Cardiac Surgery), Targeted Pharmacotherapy, Organ Transplantation, Special Management (Shunt Lesion, Left Ventricular Disease, Univentricular Hearts), Interventions, Intensive Care, ACHD Follow-up, Future Perspective. Cardiovasc. Diagn. Ther. 2024, 14, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Jonas, K.; Kurzyna, M.; Mroczek, E.; Chrzanowski, Ł.; Mularek-Kubzdela, T.; Skoczylas, I.; Błaszczak, P.; Grześk, G.; Mizia-Stec, K.; Kuśmierczyk, B.; et al. Impact of Diabetes Mellitus on Disease Severity and Patient Survival in Idiopathic Pulmonary Arterial Hypertension: Data from the Polish Multicentre Registry (BNP-PL). Cardiovasc. Diabetol. 2023, 22, 177. [Google Scholar] [CrossRef]
- Zheng, H.; Waqar, M.M.; Arif, S.; Sherazi, S.W.A.; Son, S.H.; Lee, J.Y. An Explainable Machine Learning-Based Prediction Model for In-Hospital Mortality in Acute Myocardial Infarction Patients with Typical Chest Pain. In Proceedings of the 2023 The 6th International Conference on Software Engineering and Information Management, Palmerston North, New Zealand, 31 January 2023; ACM: New York, NY, USA, 2023; pp. 44–49. [Google Scholar]
- Liao, Z.; Liu, K.; Ding, S.; Zhao, Q.; Jiang, Y.; Wang, L.; Huang, T.; Yang, L.; Luo, D.; Zhang, E.; et al. Automatic Echocardiographic Evaluation of the Probability of Pulmonary Hypertension Using Machine Learning. Pulm. Circ. 2023, 13, e12272. [Google Scholar] [CrossRef] [PubMed]
- Kogan, E.; Didden, E.-M.; Lee, E.; Nnewihe, A.; Stamatiadis, D.; Mataraso, S.; Quinn, D.; Rosenberg, D.; Chehoud, C.; Bridges, C. A Machine Learning Approach to Identifying Patients with Pulmonary Hypertension Using Real-World Electronic Health Records. Int. J. Cardiol. 2023, 374, 95–99. [Google Scholar] [CrossRef]
- Anand, V.; Weston, A.D.; Scott, C.G.; Kane, G.C.; Pellikka, P.A.; Carter, R.E. Machine Learning for Diagnosis of Pulmonary Hypertension by Echocardiography. Mayo Clin. Proc. 2024, 99, 260–270. [Google Scholar] [CrossRef]
- Durán, J.M.; Jongsma, K.R. Who Is Afraid of Black Box Algorithms? On the Epistemological and Ethical Basis of Trust in Medical AI. J. Med. Ethics 2021, 47, 329–335. [Google Scholar] [CrossRef]
- Patrascanu, O.S.; Tutunaru, D.; Musat, C.L.; Dragostin, O.M.; Fulga, A.; Nechita, L.; Ciubara, A.B.; Piraianu, A.I.; Stamate, E.; Poalelungi, D.G.; et al. Future Horizons: The Potential Role of Artificial Intelligence in Cardiology. J. Pers. Med. 2024, 14, 656. [Google Scholar] [CrossRef] [PubMed]
- Rosemann, A.; Zhang, X. Exploring the Social, Ethical, Legal, and Responsibility Dimensions of Artificial Intelligence for Health—A New Column in Intelligent Medicine. Intell. Med. 2022, 2, 103–109. [Google Scholar] [CrossRef]

| Variable | Survivors (n = 1629) | Non-Survivors (n = 126) | p-Value |
|---|---|---|---|
| Age [years], median (Q1,Q3) | 59 (40, 70) | 68 (57.75, 74) | 0.0000 |
| Gender, male, n (%) | 509 (31.25%) | 51 (40.48%) | 0.0411 |
| Type of PAH: associated with congenital heart disease, n (%) | 485 (28.12%) | 25 (19.84%) | 0.0574 |
| Chronic kidney disease, n (%) | 274 (16.82%) | 45 (35.71%) | 0.0000 |
| Diabetes, n (%) | 322 (19.77%) | 45 (35.71%) | 0.0000 |
| Previous myocardial infarction, n (%) | 78 (4.79%) | 21 (16.67%) | 0.0000 |
| Hospitalized for PAH in the last year, n (%) | 240 (15.81%) | 37 (31.36%) | 0.0000 |
| Extended hospitalization history, n (%) | 140 (8.59%) | 21 (16.67%) | 0.0042 |
| Syncope in the last year, n (%) | 97 (6.39%) | 18 (15.25%) | 0.0006 |
| Symptoms of right heart failure, n (%) | 493 (32.48%) | 75 (63.56%) | 0.0000 |
| Right axis deviation in ECG, n (%) | 626 (38.43%) | 69 (54.76%) | 0.0004 |
| Presence of pericardial effusion, n (%) | 290 (18.07%) | 39 (33.33%) | 0.0001 |
| TAPSE/sPAP ratio [mm/mmHg], median (Q1,Q3) | 0.25 (0.18, 0.38) | 0.21 (0.15, 0.28) | 0.0006 |
| Distance in 6-min walk test [m], median (Q1,Q3) | 360 (240, 460) | 231 (120, 322.5) | 0.0000 |
| Plasma NT-proBNP concentration [pg/mL], median (Q1,Q3) | 813.9 (250.55, 2482.5) | 2983.5 (1445.75, 5529.75) | 0.0000 |
| Use of diuretics, [0/1/2/3/4] n (%) | 487 (29.9%) 563 (34.56%) 461 (28.3%) 109 (6.69%) 9 (0.55%) | 11 (8.73%) 46 (36.51%) 53 (42.06%) 12 (9.52%) 4 (3.17%) | 0.0000 |
| Parenteral use of Treprostinil or Epoprostenol, n (%) | 281 (17.25%) | 49 (38.89%) | 0.0000 |
| Metric | Result | 95% Confidence Interval |
|---|---|---|
| Accuracy | 0.738 | 0.695–0.783 |
| Sensitivity | 0.800 | 0.636–0.947 |
| Specificity | 0.733 | 0.688–0.779 |
| MCC | 0.298 | 0.195–0.399 |
| ROC-AUC | 0.767 | 0.682–0.843 |
| Predicted | |||
|---|---|---|---|
| True | Negative | Positive | |
| Negative | TN = 239 | FP = 87 | |
| Positive | FN = 5 | TP = 20 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ledziński, Ł.; Grześk, G.; Ziołkowski, M.; Waligóra, M.; Kurzyna, M.; Mularek-Kubzdela, T.; Smukowska-Gorynia, A.; Skoczylas, I.; Chrzanowski, Ł.; Błaszczak, P.; et al. Personalized Medicine in Pulmonary Arterial Hypertension: Utilizing Artificial Intelligence for Death Prevention. J. Clin. Med. 2025, 14, 8325. https://doi.org/10.3390/jcm14238325
Ledziński Ł, Grześk G, Ziołkowski M, Waligóra M, Kurzyna M, Mularek-Kubzdela T, Smukowska-Gorynia A, Skoczylas I, Chrzanowski Ł, Błaszczak P, et al. Personalized Medicine in Pulmonary Arterial Hypertension: Utilizing Artificial Intelligence for Death Prevention. Journal of Clinical Medicine. 2025; 14(23):8325. https://doi.org/10.3390/jcm14238325
Chicago/Turabian StyleLedziński, Łukasz, Grzegorz Grześk, Michał Ziołkowski, Marcin Waligóra, Marcin Kurzyna, Tatiana Mularek-Kubzdela, Anna Smukowska-Gorynia, Ilona Skoczylas, Łukasz Chrzanowski, Piotr Błaszczak, and et al. 2025. "Personalized Medicine in Pulmonary Arterial Hypertension: Utilizing Artificial Intelligence for Death Prevention" Journal of Clinical Medicine 14, no. 23: 8325. https://doi.org/10.3390/jcm14238325
APA StyleLedziński, Ł., Grześk, G., Ziołkowski, M., Waligóra, M., Kurzyna, M., Mularek-Kubzdela, T., Smukowska-Gorynia, A., Skoczylas, I., Chrzanowski, Ł., Błaszczak, P., Jaguszewski, M., Kuśmierczyk-Droszcz, B., Ptaszyńska, K., Mizia-Stec, K., Malinowska, E., Peregud-Pogorzelska, M., Lewicka, E., Tomaszewski, M., Jacheć, W., ... Kopeć, G. (2025). Personalized Medicine in Pulmonary Arterial Hypertension: Utilizing Artificial Intelligence for Death Prevention. Journal of Clinical Medicine, 14(23), 8325. https://doi.org/10.3390/jcm14238325









