Histopathological Verification of Abnormal Cytology Results Suggesting High-Grade Intraepithelial Lesions in Women over 50 Years of Age—Evaluation of the Clinical Utility of Conventional Gynecological Cytology
Abstract
1. Introduction
Aim
2. Materials and Methods
2.1. Study Design and Population
2.2. Inclusion Criteria
- Age ≥ 50 years.
- Abnormal cytology result: HSIL, ASC-H or AGC diagnosed between 2018 and 2024, as the reason for referral to the Cervical Pathology Clinic at the Clinical Outpatient Clinic.
- Completion of in-depth diagnostics, including histopathological verification.
2.3. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Didkowska, J.; Wojciechowska, U.; Barańska, K.; Miklewska, M.; Michałek, I.; Olasek, P. Nowotwory Złośliwe w Polsce w 2021 Roku [Malignant Neoplasms in Poland in 2021]; Maria Skłodowska-Curie National Research Institute of Oncology, Polish National Cancer Registry: Warsaw, Poland, 2023; Available online: https://onkologia.org.pl/sites/default/files/publications/2024-01/biuletyn_2021_1.pdf (accessed on 8 October 2025).
- Singh, D.; Vignat, J.; Lorenzoni, V.; Eslahi, M.; Ginsburg, O.; Lauby-Secretan, B.; Arbyn, M.; Basu, P.; Bray, F.; Vaccarella, S. Global Estimates of Incidence and Mortality of Cervical Cancer in 2020: A Baseline Analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob. Health 2023, 11, e197–e206. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, R.; Sekiyama, K.; Higuchi, T.; Ikeda, M.; Mikami, M.; Kobayashi, Y.; Nagase, S.; Yokoyama, M.; Enomoto, T.; Katabuchi, H. Value and Limitation of Conization as a Diagnostic Procedure for Cervical Neoplasm. J. Obstet. Gynaecol. Res. 2019, 45, 2419–2424. [Google Scholar] [CrossRef]
- Łucyszyn, A.; Nowakowski, A.; Bidziński, M.; Kędzia, W.; Knapp, P.; Marszałek, A.; Sawicki, W.; Stojko, R.; Wolski, H.; Wydra, D.; et al. Algorytmy badań przesiewowych oraz postępowania w przypadku wyników nieprawidłowych w ramach Programu Profilaktyki Raka Szyjki Macicy refundowanego przez Narodowy Fundusz Zdrowia—Edycja po wprowadzeniu diagnostyki wirusa brodawczaka ludzkiego wysokiego ryzyka. Ginekol. Perinatol. Prakt. 2024, 9, 124–132. [Google Scholar]
- Ministerstwo Zdrowia. Rozporządzenie Ministra Zdrowia z Dnia 7 Marca 2025 r. Zmieniające Rozporządzenie w Sprawie Świadczeń Gwarantowanych z Zakresu Programów Zdrowotnych. Dziennik Ustaw 2025, poz. 298. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20250000298 (accessed on 8 October 2025).
- Schiffman, M.; Castle, P.E. The Promise of Global Cervical-Cancer Prevention. N. Engl. J. Med. 2005, 353, 2101–2104. [Google Scholar] [CrossRef]
- Bosch, F.X.; Lorincz, L.; Muñoz, N.; Meijer, C.J.L.M.; Shah, K.V. The Causal Relation Between Human Papillomavirus and Cervical Cancer. J. Clin. Pathol. 2002, 55, 244–265. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2007; Volume 90. [Google Scholar]
- Moscicki, A.B. HPV Infections in Adolescents. Dis. Markers 2007, 23, 229–234. [Google Scholar] [CrossRef]
- Madaan, N.; Pandhi, D.; Sharma, V.; Bhattacharya, S.N.; Guleria, K.; Mishra, K.; Bharadwaj, M. Association of Abnormal Cervical Cytology with Coinfection of Human Papillomavirus and Chlamydia trachomatis. Indian J. Sex. Transm. Dis. AIDS 2019, 40, 57–63. [Google Scholar] [CrossRef]
- Fonseca-Moutinho, J.A. Smoking and Cervical Cancer. Int. Sch. Res. Not. 2011, 2011, 847684. [Google Scholar] [CrossRef]
- Castle, P.E.; Einstein, M.H.; Sahasrabuddhe, V.V. Cervical Cancer Prevention and Control in Women Living with Human Immunodeficiency Virus. CA Cancer J. Clin. 2021, 71, 505–526. [Google Scholar] [CrossRef]
- Moscicki, A.B.; Flowers, L.; Huchko, M.J.; Long, M.E.; MacLaughlin, K.L.; Murphy, J.; Spiryda, L.B.; Gold, M.A. Guidelines for Cervical Cancer Screening in Immunosuppressed Women without HIV Infection. J. Low. Genit. Tract Dis. 2019, 23, 87–101. [Google Scholar] [CrossRef]
- Kiff, J.M.; Cotter, M.; Munro, E.G.; Leonard, M.E.; Morgan, T.K.; Bruegl, A.S. Cervical Cancer Screening in Postmenopausal Women: Is It Time to Move Toward Primary High-Risk Human Papillomavirus Screening? J. Womens Health 2021, 30, 972–978. [Google Scholar] [CrossRef]
- Nanda, K.; McCrory, D.C.; Myers, E.R.; Bastian, L.A.; Hasselblad, V.; Hickey, J.D.; Matchar, D.B. Accuracy of the Papanicolaou Test in Screening for and Follow-Up of Cervical Cytologic Abnormalities: A Systematic Review. Ann. Intern. Med. 2000, 132, 810–819. [Google Scholar] [CrossRef]
- Aminisani, N.; Armstrong, B.K.; Egger, S.; Canfell, K. Impact of Organised Cervical Screening on Cervical Cancer Incidence and Mortality in Migrant Women in Australia. BMC Cancer 2012, 12, 491. [Google Scholar] [CrossRef] [PubMed]
- Loopik, D.L.; Koenjer, L.M.; Siebers, A.G.; Melchers, W.J.; Bekkers, R.L. Benefit and Burden in the Dutch Cytology-Based vs High-Risk Human Papillomavirus-Based Cervical Cancer Screening Program. Am. J. Obstet. Gynecol. 2021, 224, 500.e1–500.e9. [Google Scholar] [CrossRef] [PubMed]
- Lönnberg, S.; Anttila, A.; Luostarinen, T.; Nieminen, P. Age-Specific Effectiveness of the Finnish Cervical Cancer Screening Programme. Cancer Epidemiol. Biomark. Prev. 2012, 21, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Australian Institute of Health and Welfare. National Cervical Screening Program Monitoring Report 2022; AIHW: Canberra, Australia, 2022. Available online: https://www.aihw.gov.au/getmedia/5c42bc77-589b-42ef-9bbd-fd91890e4920/aihw-can-149-ncsp-2022.pdf (accessed on 8 October 2025).
- Cancer Council Australia. Cervical Cancer Screening: National Cervical Screening Program; Cancer Council Australia: Sydney, Australia, 2025; Available online: https://www.cancer.org.au/clinical-guidelines/cervical-cancer/cervical-cancer-screening (accessed on 8 October 2025).
- Perkins, R.B.; Guido, R.S.; Castle, P.E.; Chelmow, D.; Einstein, M.H.; Garcia, F.; Huh, W.K.; Kim, J.J.; Moscicki, A.B.; Nayar, R.; et al. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J. Low. Genit. Tract Dis. 2020, 24, 102–131. [Google Scholar] [CrossRef]
- Schiffman, M.; Vaughan, L.M.; Raine-Bennett, T.R.; Castle, P.E.; Katki, H.A.; Gage, J.C.; Fetterman, B.; Befano, B.; Wentzensen, N. A Study of HPV Typing for the Management of HPV-Positive ASC-US Cervical Cytologic Results. Gynecol. Oncol. 2015, 138, 573–578. [Google Scholar] [CrossRef]
- Nayar, R.; Wilbur, D.C. The Bethesda System for Reporting Cervical Cytology: Definitions, Criteria, and Explanatory Notes, 3rd ed.; Springer: New York, NY, USA, 2015; pp. 1–289. [Google Scholar]
- Gupta, S.; Sodhani, P. Cytomorphologic patterns in atrophic cervical smears: A diagnostic challenge. Diagn. Cytopathol. 2004, 31, 81–85. [Google Scholar]
- Robboy, S.J.; Mutter, G.L.; Prat, J.; Bentley, R.C.; Russell, P.; Anderson, M.C. Robboy’s Pathology of the Female Reproductive Tract, 2nd ed.; Elsevier: Philadelphia, PA, USA, 2009; pp. 1–1400. [Google Scholar]
- Nayar, R. Pitfalls in cervical cytology: Errors in screening and interpretation. Semin. Diagn. Pathol. 1999, 16, 164–172. [Google Scholar]
- Jentschke, M.; Hoffmeister, V.; Soergel, P.; Hillemanns, P. Clinical Presentation, Treatment and Outcome of Vaginal Intraepithelial Neoplasia. Arch. Gynecol. Obstet. 2016, 293, 415–419. [Google Scholar] [CrossRef]
- Pesola, F.; Sasieni, P. Impact of Screening on Cervical Cancer Incidence in England: A Time Trend Analysis. BMJ Open 2019, 9, e026292. [Google Scholar] [CrossRef]
- Rokita, W. Wartość diagnostyczna cytologii i kolposkopii u kobiet ze śródnabłonkową neoplazją szyjki macicy [The Diagnostic Value of Cytology and Colposcopy in Women with Cervical Intraepithelial Neoplasia]. Ginekol. Pol. 2011, 82, 607–611. [Google Scholar]
- Li, X.; Zhao, Y.; Xiang, F.; Zhang, X.; Chen, Z.; Zhang, M.; Kang, X.; Wu, R. Evaluation of the diagnostic performance of colposcopy in the detection of cervical high-grade squamous intraepithelial lesions among women with transformation zone type 3. BMC Cancer 2024, 24, 381. [Google Scholar] [CrossRef]
- Benini, V.; Ruffolo, A.F.; Casiraghi, A.; Degliuomini, R.S.; Frigerio, M.; Braga, A.; Serati, M.; Torella, M.; Candiani, M.; Salvatore, S. New innovations for the treatment of vulvovaginal atrophy: An up-to-date review. Medicina 2022, 58, 770. [Google Scholar] [CrossRef]
- Lindahl, S.H. Reviewing the options for local estrogen treatment of vaginal atrophy. Int. J. Womens Health 2014, 6, 307–312. [Google Scholar] [CrossRef]
- Van Gerwen, O.T.; Smith, S.E.; Muzny, C.A. Bacterial vaginosis in postmenopausal women. Curr. Infect. Dis. Rep. 2023, 25, 7–15. [Google Scholar] [CrossRef] [PubMed]

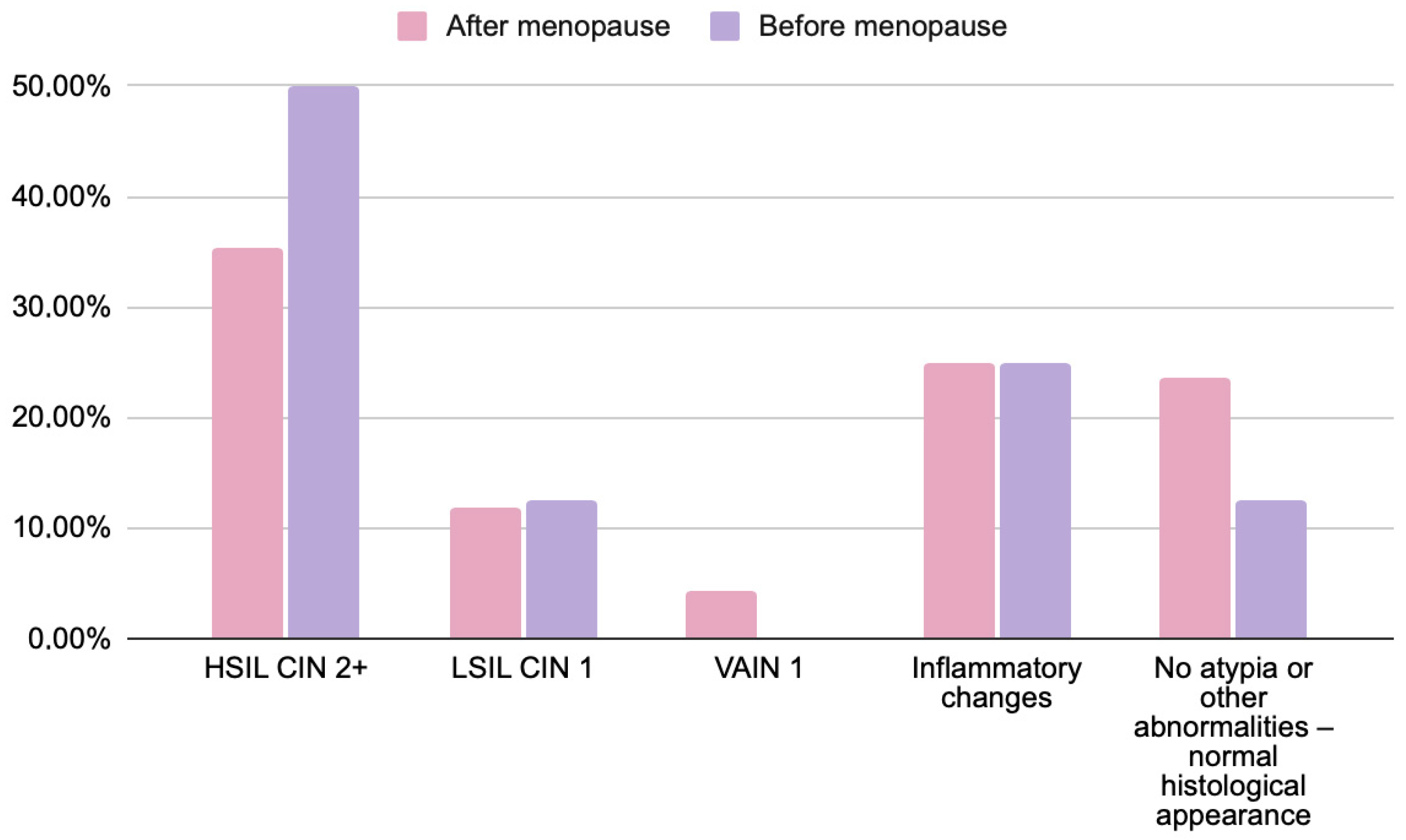
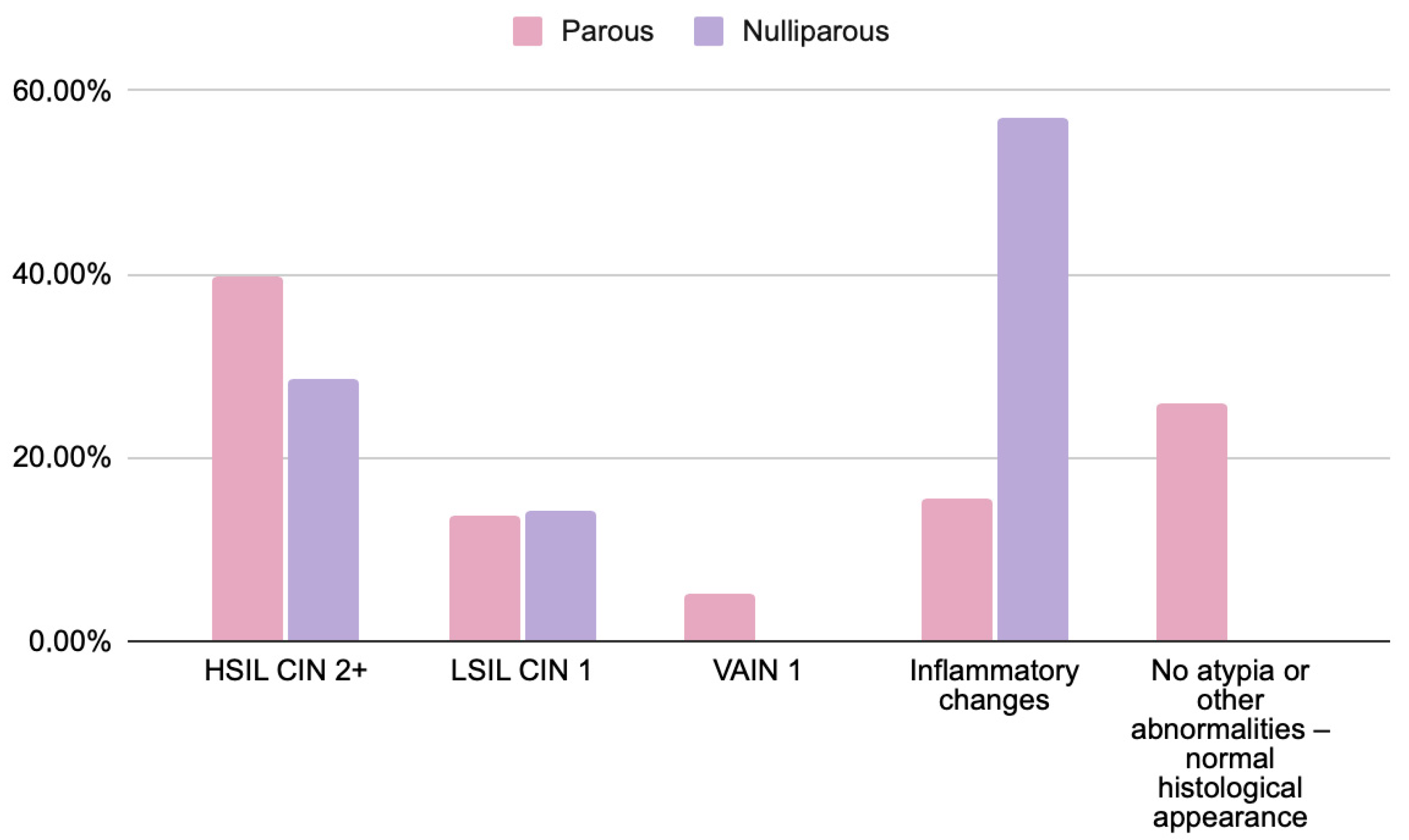
| Cytology Results | HSIL/CIN2+ | LSIL/CIN1 | VAIN1 | Inflammatory/No Atypia |
|---|---|---|---|---|
| HSIL (n = 59) | 28 (47.5%) | 5 (8.5%) | 1 (2%) | 25 (42%) |
| ASC-H (n = 18) | 2 (11%) | 3 (17%) | 2 (11%) | 11 (61%) |
| AGC (n = 2) | 0 (0%) | 1 (50%) | 0 (0%) | 1 (50%) |
| Total (n = 79) | 30 (38%) | 9 (11%) | 3 (4%) | 37 (47%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Utkowska, W.; Tucka, B.; Szyszkowski, J.; Krasuski, K.; Ludwin, A.; Suchońska, B. Histopathological Verification of Abnormal Cytology Results Suggesting High-Grade Intraepithelial Lesions in Women over 50 Years of Age—Evaluation of the Clinical Utility of Conventional Gynecological Cytology. J. Clin. Med. 2025, 14, 8305. https://doi.org/10.3390/jcm14238305
Utkowska W, Tucka B, Szyszkowski J, Krasuski K, Ludwin A, Suchońska B. Histopathological Verification of Abnormal Cytology Results Suggesting High-Grade Intraepithelial Lesions in Women over 50 Years of Age—Evaluation of the Clinical Utility of Conventional Gynecological Cytology. Journal of Clinical Medicine. 2025; 14(23):8305. https://doi.org/10.3390/jcm14238305
Chicago/Turabian StyleUtkowska, Wiktoria, Brygida Tucka, Jakub Szyszkowski, Krzysztof Krasuski, Artur Ludwin, and Barbara Suchońska. 2025. "Histopathological Verification of Abnormal Cytology Results Suggesting High-Grade Intraepithelial Lesions in Women over 50 Years of Age—Evaluation of the Clinical Utility of Conventional Gynecological Cytology" Journal of Clinical Medicine 14, no. 23: 8305. https://doi.org/10.3390/jcm14238305
APA StyleUtkowska, W., Tucka, B., Szyszkowski, J., Krasuski, K., Ludwin, A., & Suchońska, B. (2025). Histopathological Verification of Abnormal Cytology Results Suggesting High-Grade Intraepithelial Lesions in Women over 50 Years of Age—Evaluation of the Clinical Utility of Conventional Gynecological Cytology. Journal of Clinical Medicine, 14(23), 8305. https://doi.org/10.3390/jcm14238305






