Differential Risks of Dementia, Depression, and Injury Among Common α-Blockers, with Tamsulosin as the Reference Drug: A Real-World Cohort Study in Men with Lower Urinary Tract Symptoms
Abstract
1. Background
2. Methods
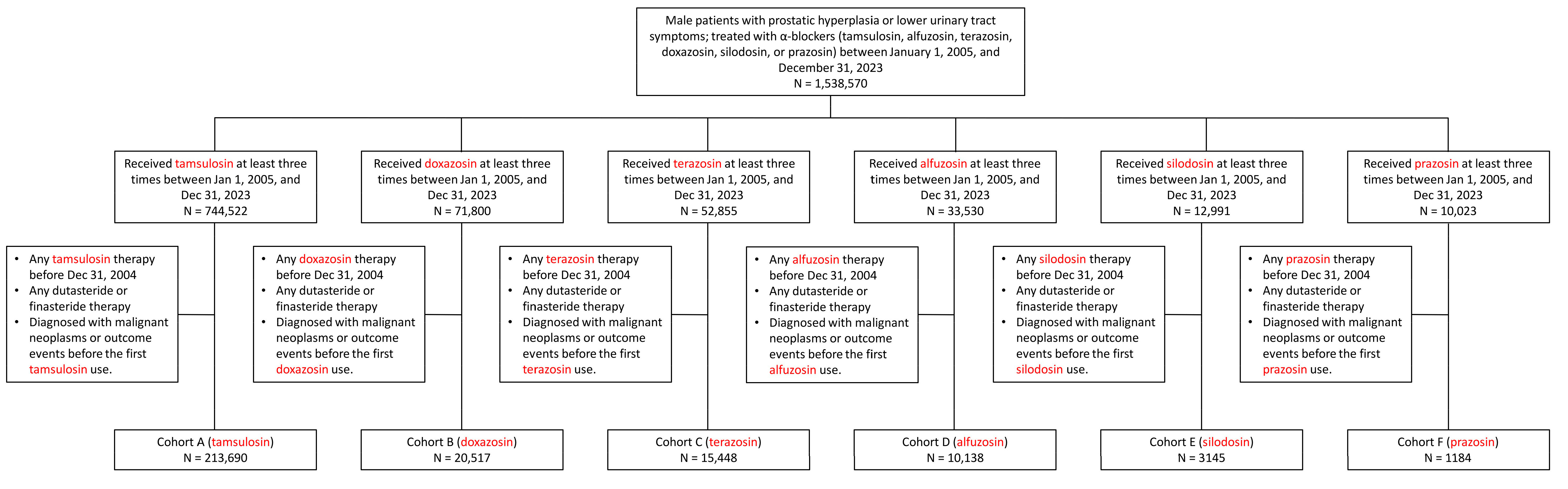
2.1. Data Source, Study Design, and Group Selection
2.2. Outcomes
2.3. Covariate Data
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of α-Blocker Users Before and After Matching
3.2. Comparative Risk Analysis of α-Blockers for Neuropsychiatric Outcomes and Injuries
3.3. Sensitivity Analyses of Cohort Selection Criteria
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stafford, R.S.; Furberg, C.D.; Finkelstein, S.N.; Cockburn, I.M.; Alehegn, T.; Ma, J. Impact of clinical trial results on national trends in alpha-blocker prescribing, 1996–2002. JAMA 2004, 291, 54–62. [Google Scholar] [CrossRef]
- McVary, K.T.; Roehrborn, C.G.; Avins, A.L.; Barry, M.J.; Bruskewitz, R.C.; Donnell, R.F.; Foster, H.E., Jr.; Gonzalez, C.M.; Kaplan, S.A.; Penson, D.F.; et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J. Urol. 2011, 185, 1793–1803. [Google Scholar] [CrossRef]
- Hollingsworth, J.M.; Wilt, T.J. Lower urinary tract symptoms in men. BMJ 2014, 349, g4474. [Google Scholar] [CrossRef] [PubMed]
- Filson, C.P.; Hollingsworth, J.M.; Clemens, J.Q.; Wei, J.T. The efficacy and safety of combined therapy with α-blockers and anticholinergics for men with benign prostatic hyperplasia: A meta-analysis. J. Urol. 2013, 190, 2153–2160. [Google Scholar] [CrossRef] [PubMed]
- Oelke, M.; Becher, K.; Castro-Diaz, D.; Chartier-Kastler, E.; Kirby, M.; Wagg, A.; Wehling, M. Appropriateness of oral drugs for long-term treatment of lower urinary tract symptoms in older persons: Results of a systematic literature review and international consensus validation process (LUTS-FORTA 2014). Age Ageing 2015, 44, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Aurora, R.N.; Zak, R.S.; Auerbach, S.H.; Casey, K.R.; Chowdhuri, S.; Karippot, A.; Maganti, R.K.; Ramar, K.; Kristo, D.A.; Bista, S.R.; et al. Best practice guide for the treatment of nightmare disorder in adults. J. Clin. Sleep. Med. 2010, 6, 389–401. [Google Scholar]
- Raskind, M.A.; Peskind, E.R.; Chow, B.; Harris, C.; Davis-Karim, A.; Holmes, H.A.; Hart, K.L.; McFall, M.; Mellman, T.A.; Reist, C.; et al. Trial of Prazosin for Post-Traumatic Stress Disorder in Military Veterans. N. Engl. J. Med. 2018, 378, 507–517. [Google Scholar] [CrossRef]
- Muderrisoglu, A.E.; Becher, K.F.; Madersbacher, S.; Michel, M.C. Cognitive and mood side effects of lower urinary tract medication. Expert. Opin. Drug Saf. 2019, 18, 915–923. [Google Scholar] [CrossRef]
- Duan, Y.; Grady, J.J.; Albertsen, P.C.; Helen Wu, Z. Tamsulosin and the risk of dementia in older men with benign prostatic hyperplasia. Pharmacoepidemiol. Drug Saf. 2018, 27, 340–348. [Google Scholar] [CrossRef]
- Yeon, B.; Suh, A.Y.; Choi, E.; Kim, B.; Noh, E.; Chung, S.Y.; Han, S.Y. Depression risk associated with the use of 5α-reductase inhibitors versus α-blockers: A retrospective cohort study in South Korea. PLoS ONE 2022, 17, e0265169. [Google Scholar] [CrossRef]
- Beers, M.H.; Passman, L.J. Antihypertensive medications and depression. Drugs 1990, 40, 792–799. [Google Scholar] [CrossRef]
- Welk, B.; McArthur, E.; Fraser, L.A.; Hayward, J.; Dixon, S.; Hwang, Y.J.; Ordon, M. The risk of fall and fracture with the initiation of a prostate-selective α antagonist: A population based cohort study. BMJ 2015, 351, h5398. [Google Scholar] [CrossRef]
- Souverein, P.C.; Van Staa, T.P.; Egberts, A.C.; De la Rosette, J.J.; Cooper, C.; Leufkens, H.G. Use of alpha-blockers and the risk of hip/femur fractures. J. Intern. Med. 2003, 254, 548–554. [Google Scholar] [CrossRef]
- Hart, A.; Aldridge, G.; Zhang, Q.; Narayanan, N.S.; Simmering, J.E. Association of Terazosin, Doxazosin, or Alfuzosin Use and Risk of Dementia With Lewy Bodies in Men. Neurology 2024, 103, e209570. [Google Scholar] [CrossRef] [PubMed]
- Sohn, J.H.; Lee, S.H.; Kwon, Y.S.; Kim, J.H.; Kim, Y.; Lee, J.J. The impact of tamsulosin on cognition in Alzheimer disease with benign prostate hyperplasia: A study using the Hallym Smart Clinical Data Warehouse. Medicine 2020, 99, e20240. [Google Scholar] [CrossRef] [PubMed]
- Fung, K.W.; Baye, F.; Baik, S.H.; McDonald, C.J. Tamsulosin use in benign prostatic hyperplasia and risks of Parkinson’s disease, Alzheimer’s disease and mortality: An observational cohort study of elderly Medicare enrollees. PLoS ONE 2024, 19, e0309222. [Google Scholar] [CrossRef] [PubMed]
- Tae, B.S.; Jeon, B.J.; Choi, H.; Cheon, J.; Park, J.Y.; Bae, J.H. α-Blocker and Risk of Dementia in Patients with Benign Prostatic Hyperplasia: A Nationwide Population Based Study Using the National Health Insurance Service Database. J. Urol. 2019, 202, 362–368. [Google Scholar] [CrossRef]
- Latvala, L.; Tiihonen, M.; Murtola, T.J.; Hartikainen, S.; Tolppanen, A.M. Use of α1-adrenoceptor antagonists tamsulosin and alfuzosin and the risk of Alzheimer’s disease. Pharmacoepidemiol. Drug Saf. 2022, 31, 1110–1120. [Google Scholar] [CrossRef]
- Garcia-Argibay, M.; Hiyoshi, A.; Fall, K.; Montgomery, S. Association of 5α-Reductase Inhibitors With Dementia, Depression, and Suicide. JAMA Netw. Open 2022, 5, e2248135. [Google Scholar] [CrossRef]
- Sommerlad, A.; Ruegger, J.; Singh-Manoux, A.; Lewis, G.; Livingston, G. Marriage and risk of dementia: Systematic review and meta-analysis of observational studies. J. Neurol. Neurosurg. Psychiatry 2018, 89, 231–238. [Google Scholar] [CrossRef]
- Hendriks, S.; Ranson, J.M.; Peetoom, K.; Lourida, I.; Tai, X.Y.; de Vugt, M.; Llewellyn, D.J.; Köhler, S. Risk Factors for Young-Onset Dementia in the UK Biobank. JAMA Neurol. 2024, 81, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Doze, V.A.; Papay, R.S.; Goldenstein, B.L.; Gupta, M.K.; Collette, K.M.; Nelson, B.W.; Lyons, M.J.; Davis, B.A.; Luger, E.J.; Wood, S.G.; et al. Long-term α1A-adrenergic receptor stimulation improves synaptic plasticity, cognitive function, mood, and longevity. Mol. Pharmacol. 2011, 80, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Doze, V.A.; Handel, E.M.; Jensen, K.A.; Darsie, B.; Luger, E.J.; Haselton, J.R.; Talbot, J.N.; Rorabaugh, B.R. alpha(1A)- and alpha(1B)-adrenergic receptors differentially modulate antidepressant-like behavior in the mouse. Brain Res. 2009, 1285, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.; Park, M.; Chun, Y.E.; Lee, J.; Shim, H.S.; Park, M.G.; Kim, S.; Sa, M.; Joo, J.; Kang, H.; et al. Astrocytes Render Memory Flexible by Releasing D-Serine and Regulating NMDA Receptor Tone in the Hippocampus. Biol. Psychiatry 2022, 91, 740–752. [Google Scholar] [CrossRef]
- Franco-Salinas, G.; de la Rosette, J.J.; Michel, M.C. Pharmacokinetics and pharmacodynamics of tamsulosin in its modified-release and oral controlled absorption system formulations. Clin. Pharmacokinet. 2010, 49, 177–188. [Google Scholar] [CrossRef]
- Nikolic, K.; Filipic, S.; Smoliński, A.; Kaliszan, R.; Agbaba, D. Partial least square and hierarchical clustering in ADMET modeling: Prediction of blood-brain barrier permeation of α-adrenergic and imidazoline receptor ligands. J. Pharm. Pharm. Sci. 2013, 16, 622–647. [Google Scholar] [CrossRef]
- Valletta, M.; Vetrano, D.L.; Xia, X.; Rizzuto, D.; Roso-Llorach, A.; Calderón-Larrañaga, A.; Marengoni, A.; Laukka, E.J.; Canevelli, M.; Bruno, G.; et al. Multimorbidity patterns and 18-year transitions from normal cognition to dementia and death: A population-based study. J. Intern. Med. 2023, 294, 326–335. [Google Scholar] [CrossRef]
- Mo, M.; Zacarias-Pons, L.; Hoang, M.T.; Mostafaei, S.; Jurado, P.G.; Stark, I.; Johnell, K.; Eriksdotter, M.; Xu, H.; Garcia-Ptacek, S. Psychiatric Disorders Before and After Dementia Diagnosis. JAMA Netw. Open 2023, 6, e2338080. [Google Scholar] [CrossRef]
- Perez, D.M. α(1)-Adrenergic Receptors in Neurotransmission, Synaptic Plasticity, and Cognition. Front. Pharmacol. 2020, 11, 581098. [Google Scholar] [CrossRef]
- Dewa, K.I.; Kaseda, K.; Kuwahara, A.; Kubotera, H.; Yamasaki, A.; Awata, N.; Komori, A.; Holtz, M.A.; Kasai, A.; Skibbe, H.; et al. The Astrocytic Ensemble Acts as a Multiday Trace to Stabilize Memory. Nature, 2025; ahead of print. [Google Scholar]
- Perez, D.M. Current Developments on the Role of α(1)-Adrenergic Receptors in Cognition, Cardioprotection, and Metabolism. Front. Cell Dev. Biol. 2021, 9, 652152. [Google Scholar] [CrossRef]
- Wahis, J.; Holt, M.G. Astrocytes, Noradrenaline, α1-Adrenoreceptors, and Neuromodulation: Evidence and Unanswered Questions. Front. Cell. Neurosci. 2021, 15, 645691. [Google Scholar] [CrossRef] [PubMed]
- Purkayastha, S.; Raven, P.B. The functional role of the alpha-1 adrenergic receptors in cerebral blood flow regulation. Indian J. Pharmacol. 2011, 43, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, Y.; Coresh, J.; Viswanathan, A.; Sullivan, K.J.; Walker, K.A.; Liu, C.; Lipsitz, L.A.; Selvin, E.; Sharrett, A.R.; et al. Orthostatic Blood Pressure Change, Dizziness, and Risk of Dementia in the ARIC Study. Hypertension 2024, 81, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Sara, S.J. The locus coeruleus and noradrenergic modulation of cognition. Nat. Rev. Neurosci. 2009, 10, 211–223. [Google Scholar] [CrossRef]
- Berridge, C.W.; Schmeichel, B.E.; España, R.A. Noradrenergic modulation of wakefulness/arousal. Sleep. Med. Rev. 2012, 16, 187–197. [Google Scholar] [CrossRef]
- Pudovkina, O.L.; Westerink, B.H. Functional role of alpha1-adrenoceptors in the locus coeruleus: A microdialysis study. Brain Res. 2005, 1061, 50–56. [Google Scholar] [CrossRef]
- Collins, L.; Francis, J.; Emanuel, B.; McCormick, D.A. Cholinergic and noradrenergic axonal activity contains a behavioral-state signal that is coordinated across the dorsal cortex. eLife 2023, 12, e81826. [Google Scholar] [CrossRef]
- Heneka, M.T.; Nadrigny, F.; Regen, T.; Martinez-Hernandez, A.; Dumitrescu-Ozimek, L.; Terwel, D.; Jardanhazi-Kurutz, D.; Walter, J.; Kirchhoff, F.; Hanisch, U.K.; et al. Locus ceruleus controls Alzheimer’s disease pathology by modulating microglial functions through norepinephrine. Proc. Natl. Acad. Sci. USA 2010, 107, 6058–6063. [Google Scholar] [CrossRef]
- Zou, H.L.; Li, J.; Zhou, J.L.; Yi, X.; Cao, S. Effects of norepinephrine on microglial neuroinflammation and neuropathic pain. Ibrain 2021, 7, 309–317. [Google Scholar] [CrossRef]
- Cai, R.; Zhang, Y.; Simmering, J.E.; Schultz, J.L.; Li, Y.; Fernandez-Carasa, I.; Consiglio, A.; Raya, A.; Polgreen, P.M.; Narayanan, N.S.; et al. Enhancing glycolysis attenuates Parkinson’s disease progression in models and clinical databases. J. Clin. Investig 2019, 129, 4539–4549. [Google Scholar] [CrossRef]
- Schultz, J.L.; Brinker, A.N.; Xu, J.; Ernst, S.E.; Tayyari, F.; Rauckhorst, A.J.; Liu, L.; Uc, E.Y.; Taylor, E.B.; Simmering, J.E.; et al. A pilot to assess target engagement of terazosin in Parkinson’s disease. Park. Relat. Disord. 2022, 94, 79–83. [Google Scholar] [CrossRef]
- Weber, M.A.; Sivakumar, K.; Tabakovic, E.E.; Oya, M.; Aldridge, G.M.; Zhang, Q.; Simmering, J.E.; Narayanan, N.S. Glycolysis-enhancing α(1)-adrenergic antagonists modify cognitive symptoms related to Parkinson’s disease. npj Park. Dis. 2023, 9, 32. [Google Scholar] [CrossRef]
- Richardson, C.D.; Donatucci, C.F.; Page, S.O.; Wilson, K.H.; Schwinn, D.A. Pharmacology of tamsulosin: Saturation-binding isotherms and competition analysis using cloned alpha 1-adrenergic receptor subtypes. Prostate 1997, 33, 55–59. [Google Scholar] [CrossRef]
- Kim, Y.J.; Tae, B.S.; Bae, J.H. Cognitive Function and Urologic Medications for Lower Urinary Tract Symptoms. Int. Neurourol. J. 2020, 24, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Hudson, S.M.; Whiteside, T.E.; Lorenz, R.A.; Wargo, K.A. Prazosin for the treatment of nightmares related to posttraumatic stress disorder: A review of the literature. Prim. Care Companion CNS Disord. 2012, 14, 11r01222. [Google Scholar] [CrossRef]
- Schultz, J.L.; Gander, P.E.; Workman, C.D.; Ponto, L.L.; Cross, S.; Nance, C.S.; Groth, C.L.; Taylor, E.B.; Ernst, S.E.; Xu, J.; et al. A pilot dose-finding study of Terazosin in humans. medRxiv 2024. [Google Scholar] [CrossRef] [PubMed]
- van Assema, D.M.E.; Lubberink, M.; Bauer, M.; van der Flier, W.M.; Schuit, R.C.; Windhorst, A.D.; Comans, E.F.I.; Hoetjes, N.J.; Tolboom, N.; Langer, O.; et al. Blood–brain barrier P-glycoprotein function in Alzheimer’s disease. Brain 2011, 135, 181–189. [Google Scholar] [CrossRef]
- van Assema, D.M.; Lubberink, M.; Boellaard, R.; Schuit, R.C.; Windhorst, A.D.; Scheltens, P.; Lammertsma, A.A.; van Berckel, B.N. P-glycoprotein function at the blood-brain barrier: Effects of age and gender. Mol. Imaging Biol. 2012, 14, 771–776. [Google Scholar] [CrossRef]
- Bennett, I.J.; Langley, J.; Sun, A.; Solis, K.; Seitz, A.R.; Hu, X.P. Locus coeruleus contrast and diffusivity metrics differentially relate to age and memory performance. Sci. Rep. 2024, 14, 15372. [Google Scholar] [CrossRef]
- Trujillo, P.; Aumann, M.A.; Claassen, D.O. Neuromelanin-sensitive MRI as a promising biomarker of catecholamine function. Brain 2024, 147, 337–351. [Google Scholar] [CrossRef]
- Ferreira, R.; Bastos-Leite, A.J. Arterial spin labelling magnetic resonance imaging and perfusion patterns in neurocognitive and other mental disorders: A systematic review. Neuroradiology 2024, 66, 1065–1081. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, R.F.; Egle, M.; Groechel, R.C.; Mughal, A. Blood pressure and the brain: The conundrum of hypertension and dementia. Cardiovasc. Res. 2025, 120, 2360–2372. [Google Scholar] [CrossRef]
- Alluri, S.R.; Kim, S.W.; Volkow, N.D.; Kil, K.E. PET Radiotracers for CNS-Adrenergic Receptors: Developments and Perspectives. Molecules 2020, 25, 4017. [Google Scholar] [CrossRef]
- Risgaard, R.; Ettrup, A.; Balle, T.; Dyssegaard, A.; Hansen, H.D.; Lehel, S.; Madsen, J.; Pedersen, H.; Püschl, A.; Badolo, L.; et al. Radiolabelling and PET brain imaging of the α1-adrenoceptor antagonist Lu AE43936. Nucl. Med. Biol. 2013, 40, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Taquet, M.; Luciano, S.; Geddes, J.R.; Harrison, P.J. Bidirectional associations between COVID-19 and psychiatric disorder: Retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry 2021, 8, 130–140. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.S.-Y.; Chang, Y.-C.; Yu, C.-Y.; Hsieh, T.-Y.; Sung, W.-W. Differential Risks of Dementia, Depression, and Injury Among Common α-Blockers, with Tamsulosin as the Reference Drug: A Real-World Cohort Study in Men with Lower Urinary Tract Symptoms. J. Clin. Med. 2025, 14, 8302. https://doi.org/10.3390/jcm14238302
Chen SS-Y, Chang Y-C, Yu C-Y, Hsieh T-Y, Sung W-W. Differential Risks of Dementia, Depression, and Injury Among Common α-Blockers, with Tamsulosin as the Reference Drug: A Real-World Cohort Study in Men with Lower Urinary Tract Symptoms. Journal of Clinical Medicine. 2025; 14(23):8302. https://doi.org/10.3390/jcm14238302
Chicago/Turabian StyleChen, Sunny Ssu-Yu, Ya-Chuan Chang, Chia-Ying Yu, Tzuo-Yi Hsieh, and Wen-Wei Sung. 2025. "Differential Risks of Dementia, Depression, and Injury Among Common α-Blockers, with Tamsulosin as the Reference Drug: A Real-World Cohort Study in Men with Lower Urinary Tract Symptoms" Journal of Clinical Medicine 14, no. 23: 8302. https://doi.org/10.3390/jcm14238302
APA StyleChen, S. S.-Y., Chang, Y.-C., Yu, C.-Y., Hsieh, T.-Y., & Sung, W.-W. (2025). Differential Risks of Dementia, Depression, and Injury Among Common α-Blockers, with Tamsulosin as the Reference Drug: A Real-World Cohort Study in Men with Lower Urinary Tract Symptoms. Journal of Clinical Medicine, 14(23), 8302. https://doi.org/10.3390/jcm14238302







