Subchondral Phosphate Injection During Hip Arthroscopy Safely Treats Acetabular Bone Marrow Lesions in Early Osteoarthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients Selection
2.2. Surgical Procedure
2.3. Patients Evaluation
2.4. Statistical Analysis
3. Results
3.1. Patients Characteristics
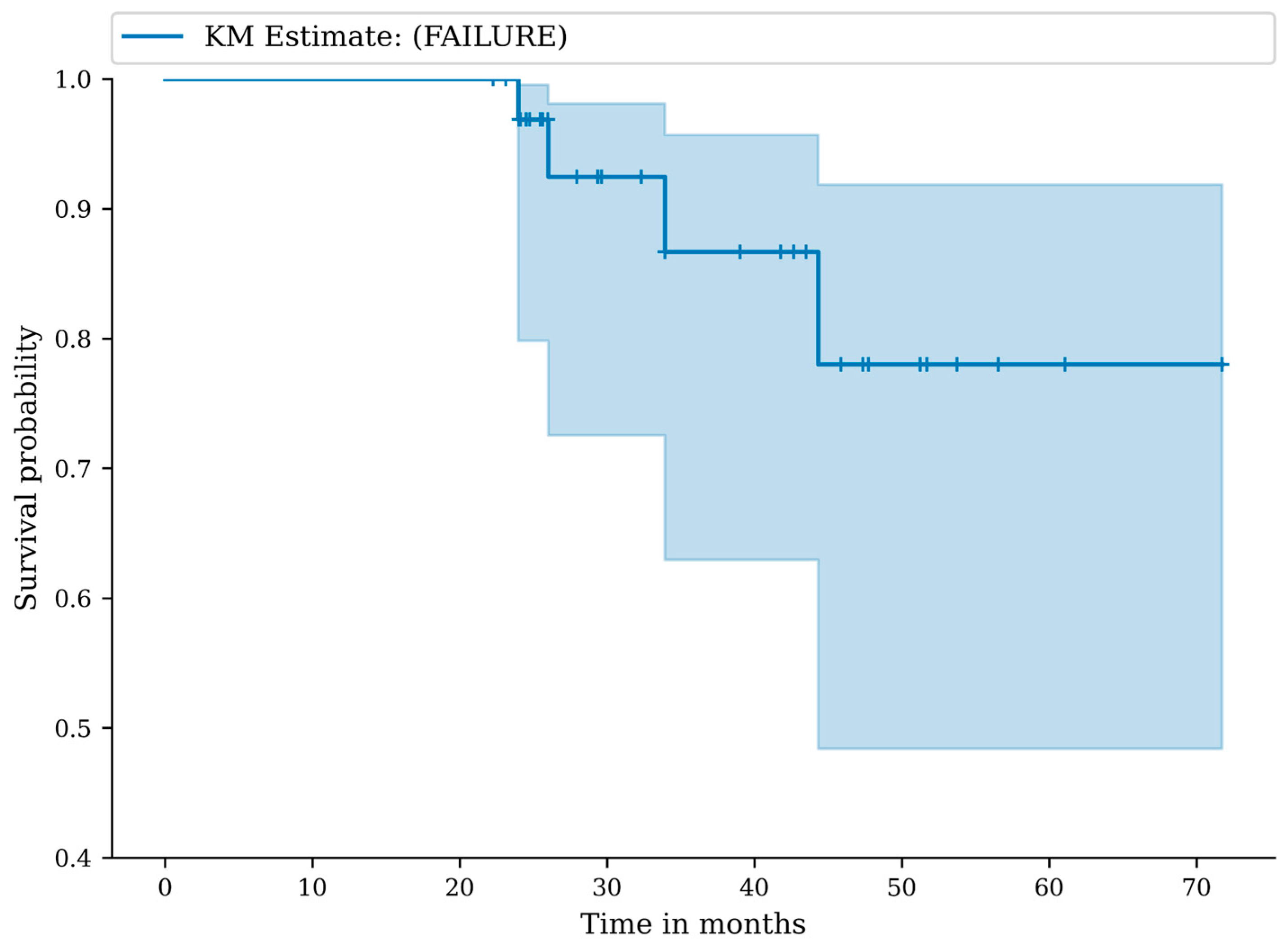
3.2. Complications, Adverse Events and Failures
3.3. Clinical Outcomes
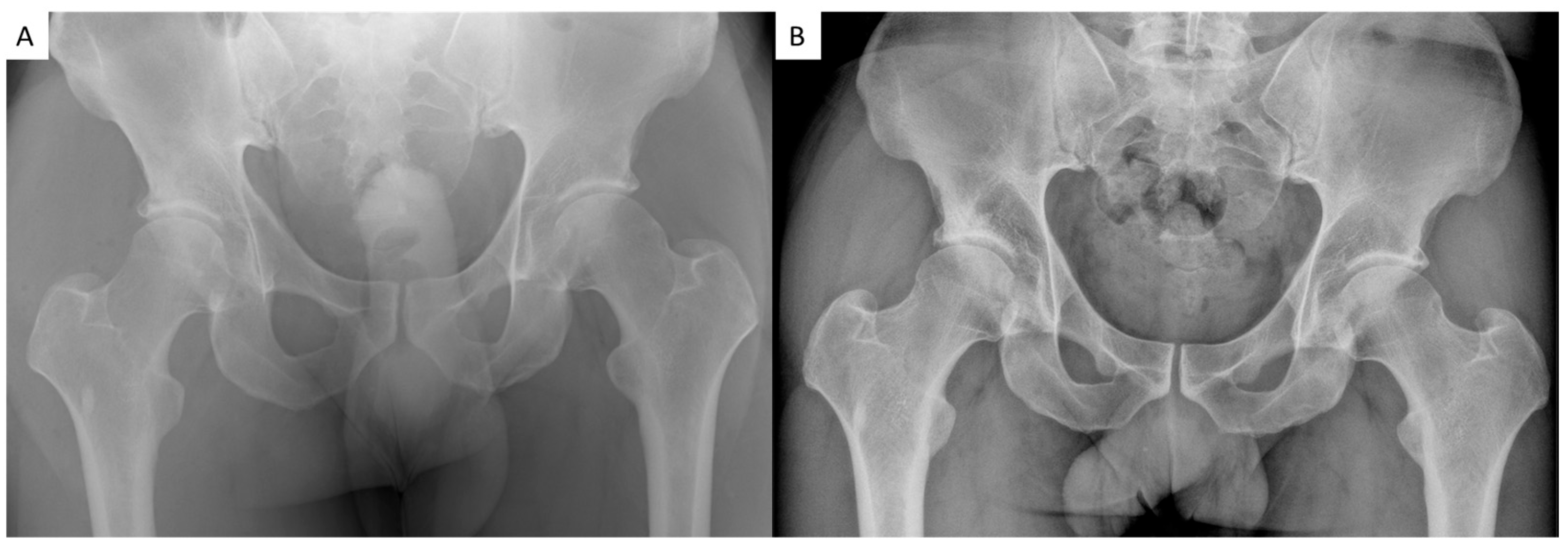
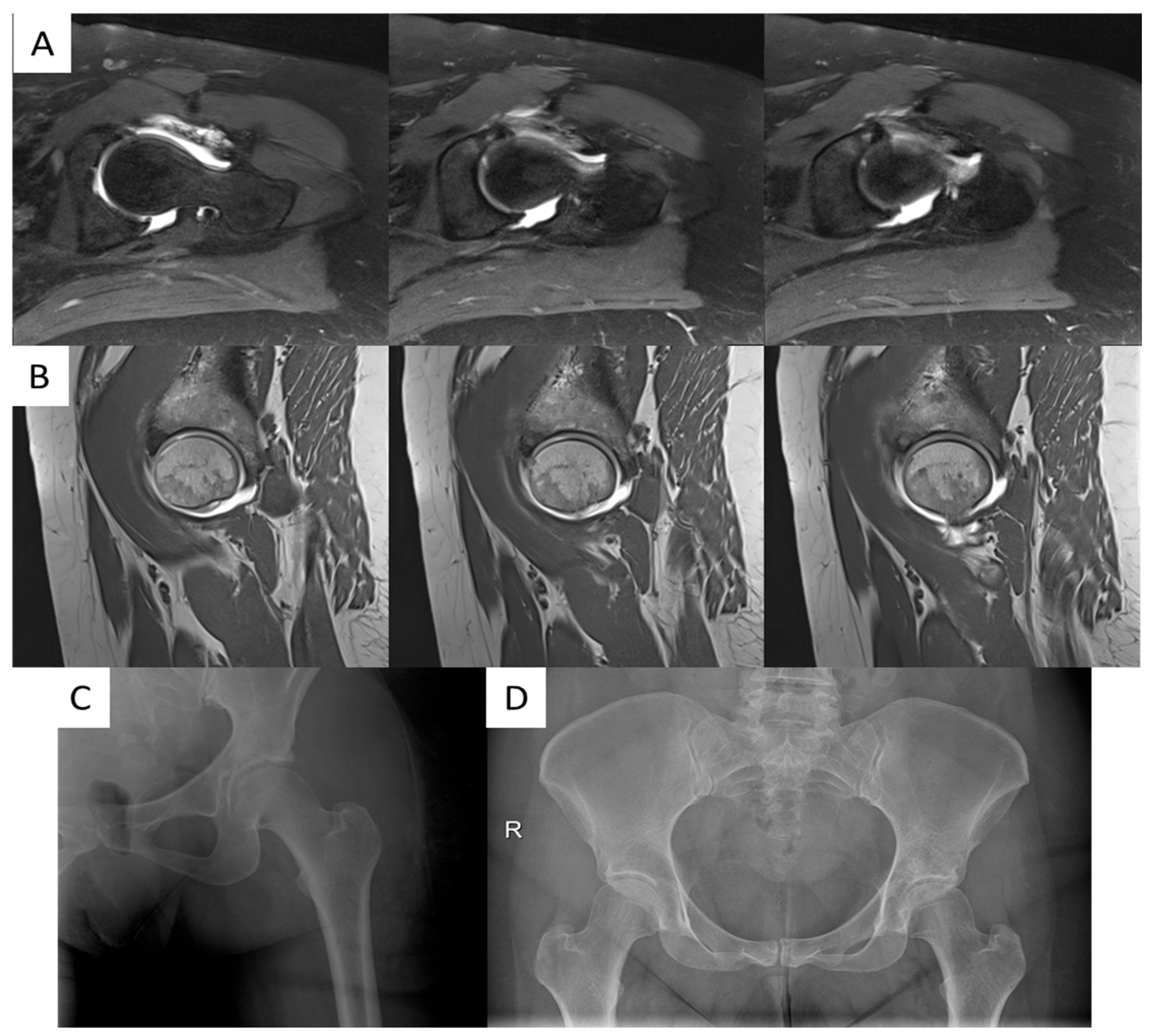
3.4. Radiographic Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Matteo, B.; Polignano, A.; Onorato, F.; La Porta, A.; Iacono, F.; Bonanzinga, T.; Raspugli, G.; Marcacci, M.; Kon, E. Knee Intraosseous Injections: A Systematic Review of Clinical Evidence of Different Treatment Alternatives. Cartilage 2021, 13 (Suppl. S1), 1165S–1177S. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roemer, F.W.; Frobell, R.; Hunter, D.J.; Crema, M.D.; Fischer, W.; Bohndorf, K.; Guermazi, A. MRI-detected subchondral bone marrow signal alterations of the knee joint: Terminology, imaging appearance, relevance and radiological differential diagnosis. Osteoarthr. Cartil. 2009, 17, 1115–1131. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Ronga, M.; Filardo, G.; Farr, J.; Madry, H.; Milano, G.; Andriolo, L.; Shabshin, N. Bone marrow lesions and subchondral bone pathology of the knee. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 1797–1814. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Wyatt, C.R.; Lee, S.; Nardo, L.; Link, T.M.; Majumdar, S.; Souza, R.B. Association of cartilage defects, and other MRI findings with pain and function in individuals with mild-moderate radiographic hip osteoarthritis and controls. Osteoarthr. Cartil. 2013, 21, 1685–1692. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krych, A.J.; King, A.H.; Berardelli, R.L.; Sousa, P.L.; Levy, B.A. Is Subchondral Acetabular Edema or Cystic Change on MRI a Contraindication for Hip Arthroscopy in Patients With Femoroacetabular Impingement? Am. J. Sports Med. 2016, 44, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Taljanovic, M.S.; Graham, A.R.; Benjamin, J.B.; Gmitro, A.F.; Krupinski, E.A.; Schwartz, S.A.; Hunter, T.B.; Resnick, D.L. Bone marrow edema pattern in advanced hip osteoarthritis: Quantitative assessment with magnetic resonance imaging and correlation with clinical examination, radiographic findings, and histopathology. Skelet. Radiol. 2008, 37, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Bessa, F.; Rasio, J.; Newhouse, A.; Nwachukwu, B.U.; Nho, S. Surgical Treatment of Subchondral Bone Cysts of the Acetabulum With Calcium Phosphate Bone Substitute Material in Patients Without Advanced Arthritic Hips. Arthrosc. Tech. 2020, 9, e1375–e1379. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Di Matteo, B.; Anzillotti, G.; Conte, P.; Angele, P.; Emans, P.; Minguell-Monyart, J.; Woodell-May, J.; Correa-Tapia, M.; Kon, E. Subchondroplasty® (SCP) Provides Resolution of Symptoms and Functional Improvements in Mild-to-Moderate Knee Osteoarthritis with Persistent Bone Marrow Lesions: 12-Month Follow-Up Results from a Multicentric Open-Label Prospective Clinical Trial. Cartilage 2024. ahead of print. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cohen, S.B.; Sharkey, P.F. Subchondroplasty for Treating Bone Marrow Lesions. J. Knee Surg. 2016, 29, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Kapil, N.; Samuel, L.T.; Kamath, A.F. Management of Bone Marrow Lesions of the Hip With Subchondral Calcium Phosphate Injection: Surgical Technique and Tips. Arthrosc. Tech. 2020, 9, e863–e875. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kohn, M.D.; Sassoon, A.A.; Fernando, N.D. Classifications in Brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin. Orthop. Relat. Res. 2016, 474, 1886–1893. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kovalenko, B.; Bremjit, P.; Fernando, N. Classifications in Brief: Tönnis Classification of Hip Osteoarthritis. Clin. Orthop. Relat. Res. 2018, 476, 1680–1684. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Monazzam, S.; Bomar, J.D.; Cidambi, K.; Kruk, P.; Hosalkar, H. Lateral center-edge angle on conventional radiography and computed tomography. Clin. Orthop. Relat. Res. 2013, 471, 2233–2337. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McQuivey, K.S.; Secretov, E.; Domb, B.G.; Levy, B.A.; Krych, A.J.; Neville, M.; Hartigan, D.E. A Multicenter Study of Radiographic Measures Predicting Failure of Arthroscopy in Borderline Hip Dysplasia: Beware of the Tönnis Angle. Am. J. Sports Med. 2020, 48, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- Clohisy, J.C.; Carlisle, J.C.; Beaulé, P.E.; Kim, Y.J.; Trousdale, R.T.; Sierra, R.J.; Leunig, M.; Schoenecker, P.L.; Millis, M.B. A systematic approach to the plain radiographic evaluation of the young adult hip. J. Bone Jt. Surg. Am. 2008, 90 (Suppl. S4), 47–66. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Slattery, C.; Kweon, C.Y. Classifications in Brief: Outerbridge Classification of Chondral Lesions. Clin. Orthop. Relat. Res. 2018, 476, 2101–2104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- El-Radi, M.A.; Marin-Peña, O.R.; Said, H.G.; Tey-Pons, M. Basics in hip chondrolabral lesions and state of the art. SICOT J. 2017, 3, 73. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zimmer-Biomet. The Subchondroplasty ® (SCP ®) Procedure. 2025. Available online: https://www.zimmerbiomet.com/en/products-and-solutions/products/restorative-therapies/subchondroplasty-procedure.html (accessed on 2 May 2025).
- Edwards, P.K.; Queen, R.M.; Butler, R.J.; Bolognesi, M.P.; Lowry Barnes, C. Are Range of Motion Measurements Needed When Calculating the Harris Hip Score? J. Arthroplast. 2016, 31, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Randelli, F.; Fioruzzi, A.; Clemente, A.; Radaelli, A.; Menon, A.; Di Via, D.; Caria, C.; Mazzoleni, M.G.; Buono, C.; Di Benedetto, P.; et al. The international Hip Outcome Tool 12 questionnaire (iHOT-12): An Italian language cross-cultural adaptation and validation. J. Orthop. Traumatol. 2024, 25, 59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maldonado, D.R.; Kyin, C.; Shapira, J.; Rosinsky, P.J.; Meghpara, M.B.; Ankem, H.K.; Lall, A.C.; Domb, B.G. Defining the Maximum Outcome Improvement of the Modified Harris Hip Score, the Nonarthritic Hip Score, the Visual Analog Scale For Pain, and the International Hip Outcome Tool-12 in the Arthroscopic Management for Femoroacetabular Impingement Syndrome and Labral Tear. Arthroscopy 2021, 37, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Kemp, J.L.; Collins, N.J.; Roos, E.M.; Crossley, K.M. Psychometric properties of patient-reported outcome measures for hip arthroscopic surgery. Am. J. Sports Med. 2013, 41, 2065–2073. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.L.; Kivlan, B.R.; Christoforetti, J.J.; Wolff, A.B.; Nho, S.J.; Salvo, J.P., Jr.; Ellis, T.J.; Van Thiel, G.; Matsuda, D.K.; Carreira, D.S. Minimal Clinically Important Difference and Substantial Clinical Benefit Values for the 12-Item International Hip Outcome Tool. Arthroscopy 2019, 35, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Nwachukwu, B.U.; Beck, E.C.; Kunze, K.N.; Chahla, J.; Rasio, J.; Nho, S.J. Defining the Clinically Meaningful Outcomes for Arthroscopic Treatment of Femoroacetabular Impingement Syndrome at Minimum 5-Year Follow-up. Am. J. Sports Med. 2020, 48, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Bonadio, M.B.; Giglio, P.N.; Helito, C.P.; Pécora, J.R.; Camanho, G.L.; Demange, M.K. Subchondroplasty for treating bone marrow lesions in the knee—Initial experience. Rev. Bras. Ortop. 2017, 52, 325–330. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Connor, M.; Minkara, A.A.; Westermann, R.W.; Rosneck, J.; Lynch, T.S. Return to Play After Hip Arthroscopy: A Systematic Review and Meta-analysis. Am. J. Sports Med. 2018, 46, 2780–2788. [Google Scholar] [CrossRef] [PubMed]
- Frank, R.M.; Lee, S.; Bush-Joseph, C.A.; Salata, M.J.; Mather RC3rd Nho, S.J. Outcomes for Hip Arthroscopy According to Sex and Age: A Comparative Matched-Group Analysis. J. Bone Jt. Surg. Am. 2016, 98, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.J.; Akpinar, B.; Bloom, D.A.; Youm, T. Age and Outcomes in Hip Arthroscopy for Femoroacetabular Impingement: A Comparison Across 3 Age Groups. Am. J. Sports Med. 2021, 49, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Kemp, J.L.; MacDonald, D.; Collins, N.J.; Hatton, A.L.; Crossley, K.M. Hip arthroscopy in the setting of hip osteoarthritis: Systematic review of outcomes and progression to hip arthroplasty. Clin. Orthop. Relat. Res. 2015, 473, 1055–1073. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Piuzzi, N.S.; Slullitel, P.A.; Bertona, A.; Oñativia, J.I.; Albergo, I.; Zanotti, G.; Buttaro, M.A.; Piccaluga, F.; Comba, F.M. Hip arthroscopy in osteoarthritis: A systematic review of the literature. Hip Int. 2016, 26, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Daivajna, S.; Bajwa, A.; Villar, R. Outcome of arthroscopy in patients with advanced osteoarthritis of the hip. PLoS ONE 2015, 10, e0113970. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sansone, M.; Ahldén, M.; Jonasson, P.; Thomeé, C.; Swärd, L.; Collin, D.; Baranto, A.; Karlsson, J.; Thomeé, R. Outcome of hip arthroscopy in patients with mild to moderate osteoarthritis-A prospective study. J. Hip Preserv. Surg. 2015, 3, 61–67. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, H.J.; Dang, H.H.; Mamtimin, M.; Yang, G.; Zhang, X.; Wang, J.Q. Hip Arthroscopy for Femoroacetabular Impingement Syndrome Shows Good Outcomes and Low Revision Rates, with Young Age and Low Postoperative Pain Score Predicting Excellent 5-Year Outcomes. Arthroscopy 2023, 39, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, T.; Guan, M.; Zhao, W.; Leung, F.K.; Pan, H.; Cao, X.; Guo, X.E.; Lu, W.W. Bone turnover and articular cartilage differences localized to subchondral cysts in knees with advanced osteoarthritis. Osteoarthr. Cartil. 2015, 23, 2174–2183. [Google Scholar] [CrossRef] [PubMed]
- Audrey, H.X.; Abd Razak, H.R.; Andrew, T.H. The truth behind subchondral cysts in osteoarthritis of the knee. Open Orthop. J. 2014, 8, 7–10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eriksen, E.F.; Ringe, J.D. Bone marrow lesions: A universal bone response to injury? Rheumatol. Int. 2012, 32, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Ankem, H.K.; Diulus, S.C.; Maldonado, D.R.; Ortiz-Declet, V.; Rosinsky, P.J.; Meghpara, M.B.; Shapira, J.; Lall, A.C.; Domb, B.G. Arthroscopic-Assisted Intraosseous Bioplasty of the Acetabulum. Arthrosc. Tech. 2020, 9, e1531–e1539. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roemer, F.W.; Neogi, T.; Nevitt, M.C.; Felson, D.T.; Zhu, Y.; Zhang, Y.; Lynch, J.A.; Javaid, M.K.; Crema, M.D.; Torner, J.; et al. Subchondral bone marrow lesions are highly associated with, and predict subchondral bone attrition longitudinally: The MOST study. Osteoarthr. Cartilage. 2010, 18, 47–53. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tanamas, S.K.; Wluka, A.E.; Pelletier, J.P.; Pelletier, J.M.; Abram, F.; Berry, P.A.; Wang, Y.; Jones, G.; Cicuttini, F.M. Bone marrow lesions in people with knee osteoarthritis predict progression of disease and joint replacement: A longitudinal study. Rheumatology 2010, 49, 2413–2419. [Google Scholar] [CrossRef] [PubMed]
- Moradi, K.; Mohammadi, S.; Roemer, F.W.; Momtazmanesh, S.; Hathaway, Q.; Ibad, H.A.; Hunter, D.J.; Guermazi, A.; Demehri, S. Progression of Bone Marrow Lesions and the Development of Knee Osteoarthritis: Osteoarthritis Initiative Data. Radiology 2024, 312, e240470. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sofat, N.; Howe, F.A. Bone marrow lesions in osteoarthritis: Characterising genetic and histological changes to understand disease pathophysiology. Osteoarthr. Cart. Open 2024, 6, 100531. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, Y.N.; Fan, J.J.; Li, Z.Q.; Liu, Y.W.; Wu, Y.P.; Liu, J. Effects of Pore Size on the Osteoconductivity and Mechanical Properties of Calcium Phosphate Cement in a Rabbit Model. Artif. Organs 2017, 41, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, O. Octacalcium phosphate: Osteoconductivity and crystal chemistry. Acta Biomater. 2010, 6, 3379–3387. [Google Scholar] [CrossRef] [PubMed]
- Chahla, J.; Beck, E.C.; Okoroha, K.; Cancienne, J.M.; Kunze, K.N.; Nho, S.J. Prevalence and Clinical Implications of Chondral Injuries After Hip Arthroscopic Surgery for Femoroacetabular Impingement Syndrome. Am. J. Sports Med. 2019, 47, 2626–2635. [Google Scholar] [CrossRef] [PubMed]
- Egerton, T.; Hinman, R.S.; Takla, A.; Bennell, K.L.; O’Donnell, J. Intraoperative cartilage degeneration predicts outcome 12 months after hip arthroscopy. Clin. Orthop. Relat. Res. 2013, 471, 593–599. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


| Patient | Gender | Age at Surgery | BMI | Preoperative Tonnis | Preoperative Kellgren–Lawrence |
|---|---|---|---|---|---|
| P01 | F | 29.0 | 19.13 | 1 | 1 |
| P02 | M | 35.0 | 22.72 | 1 | 2 |
| P03 | M | 40.0 | 25.26 | 1 | 2 |
| P04 | M | 41.0 | 26.59 | 1 | 3 |
| P05 | F | 25.0 | 24.09 | 1 | 1 |
| P06 | M | 37.0 | 26.3 | 1 | 2 |
| P07 | M | 38.0 | 29.54 | 1 | 2 |
| P08 | F | 28.0 | 24.22 | 1 | 2 |
| P09 | M | 23.0 | 22.86 | 2 | 2 |
| P10 | M | 38.0 | 24.38 | 1 | 2 |
| P11 | M | 28.0 | 24.58 | 1 | 2 |
| P12 | M | 33.0 | 27.14 | 1 | 2 |
| P13 | M | 35.0 | 24.58 | 1 | 2 |
| P14 | M | 46.0 | 25.9 | 2 | 3 |
| P15 | M | 53.0 | 22.22 | 2 | 3 |
| P16 | M | 43.0 | 23.55 | 2 | 2 |
| P17 | M | 46.0 | 25.93 | 1 | 2 |
| P18 | M | 36.0 | 24.65 | 2 | 3 |
| P19 | M | 42.0 | 26.96 | 1 | 1 |
| P20 | M | 47.0 | 26.22 | 2 | 3 |
| P21 | M | 50.0 | 22.2 | 1 | 2 |
| P22 | F | 50.0 | 22.2 | 1 | 2 |
| P23 | M | 39.0 | 27.47 | 1 | 1 |
| P24 | M | 33.0 | 26.3 | 1 | 1 |
| P25 | M | 26.0 | 25.98 | 2 | 3 |
| P26 | M | 50.0 | 24.34 | 2 | 2 |
| P27 | M | 32.0 | 27.13 | 1 | 2 |
| P28 | M | 23.0 | 23.29 | 1 | 2 |
| P29 | M | 26.0 | 26.78 | 2 | 2 |
| P30 | M | 29.0 | 21.47 | 2 | 3 |
| P31 | M | 29.0 | 21.47 | 2 | 3 |
| P32 | M | 49.0 | 24.58 | 1 | 2 |
| P33 | M | 46.0 | 20.94 | 1 | 2 |
| P34 | M | 46.0 | 20.94 | 1 | 2 |
| Patient | Surgical Time (min) | Acetabular Trimming | Anterior Superior Partial Labral Resection | Labral Reconstruction | Femur Neck Reshaping | Bone Substitute Injected (cc) | Acetabulum Intraoperative Grade (I–IV) |
|---|---|---|---|---|---|---|---|
| P01 | 188 | no | no | no | yes | 3 | 1 |
| P02 | 65 | no | no | no | yes | 3 | 4 |
| P03 | 77 | no | no | no | yes | 3 | 4 |
| P04 | 80 | no | yes | no | yes | 3 | 3 |
| P05 | 80 | no | no | no | yes | 3 | 2 |
| P06 | 112 | no | yes | yes | yes | 3 | 3 |
| P07 | 104 | no | no | no | yes | 3 | 3 |
| P08 | 95 | no | no | no | yes | 9 | 4 |
| P09 | 114 | no | no | no | yes | 15 | 4 |
| P10 | 108 | no | no | no | yes | 3 | 4 |
| P11 | 115 | no | no | no | yes | 15 | 4 |
| P12 | 85 | no | no | no | yes | 9 | 4 |
| P13 | 107 | yes | no | no | yes | 3 | 2 |
| P14 | 89 | no | no | no | yes | 3 | 4 |
| P15 | 98 | no | yes | no | yes | 3 | 3 |
| P16 | 79 | no | no | no | yes | 3 | 3 |
| P17 | 115 | no | no | no | yes | 3 | 2 |
| P18 | 83 | no | yes | no | yes | 2.5 | 4 |
| P19 | 97 | no | no | no | yes | 3 | 3 |
| P20 | 108 | yes | no | no | yes | 3.5 | 4 |
| P21 | 114 | no | yes | no | yes | 3 | 3 |
| P22 | 114 | no | yes | no | yes | 3 | 3 |
| P23 | 115 | no | no | no | yes | 3 | 2 |
| P24 | 81 | no | no | no | yes | 3 | 3 |
| P25 | 47 | no | yes | no | yes | 3 | 3 |
| P26 | 109 | no | no | no | yes | 3 | 3 |
| P27 | 109 | no | no | no | yes | 3 | 3 |
| P28 | 98 | no | no | no | yes | 3 | 4 |
| P29 | 110 | no | no | no | yes | 3 | 4 |
| P30 | 55 | yes | yes | no | yes | 3 | 4 |
| P31 | 55 | yes | yes | no | yes | 3 | 2 |
| P32 | 106 | no | no | no | yes | 3 | 4 |
| P33 | 110 | yes | no | no | yes | 3 | 4 |
| P34 | 111 | yes | no | no | yes | 3 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minelli, M.; Di Matteo, B.; Longobardi, V.; D’Addona, A.; Rosolani, M.; Pitronaci, S.; Kon, E.; Della Rocca, F. Subchondral Phosphate Injection During Hip Arthroscopy Safely Treats Acetabular Bone Marrow Lesions in Early Osteoarthritis. J. Clin. Med. 2025, 14, 8298. https://doi.org/10.3390/jcm14238298
Minelli M, Di Matteo B, Longobardi V, D’Addona A, Rosolani M, Pitronaci S, Kon E, Della Rocca F. Subchondral Phosphate Injection During Hip Arthroscopy Safely Treats Acetabular Bone Marrow Lesions in Early Osteoarthritis. Journal of Clinical Medicine. 2025; 14(23):8298. https://doi.org/10.3390/jcm14238298
Chicago/Turabian StyleMinelli, Marco, Berardo Di Matteo, Vincenzo Longobardi, Alessio D’Addona, Marco Rosolani, Sebiano Pitronaci, Elizaveta Kon, and Federico Della Rocca. 2025. "Subchondral Phosphate Injection During Hip Arthroscopy Safely Treats Acetabular Bone Marrow Lesions in Early Osteoarthritis" Journal of Clinical Medicine 14, no. 23: 8298. https://doi.org/10.3390/jcm14238298
APA StyleMinelli, M., Di Matteo, B., Longobardi, V., D’Addona, A., Rosolani, M., Pitronaci, S., Kon, E., & Della Rocca, F. (2025). Subchondral Phosphate Injection During Hip Arthroscopy Safely Treats Acetabular Bone Marrow Lesions in Early Osteoarthritis. Journal of Clinical Medicine, 14(23), 8298. https://doi.org/10.3390/jcm14238298







