Choice of Treatment Modality and Validity of Direct Surgery for Complex Posterior Inferior Cerebellar Artery-Related Aneurysms
Abstract
1. Introduction
2. Materials and Methods
2.1. Strategic Choice of Endovascular or Open Surgical Management
2.2. Operative Technique
2.3. Outcome Assessment
3. Results
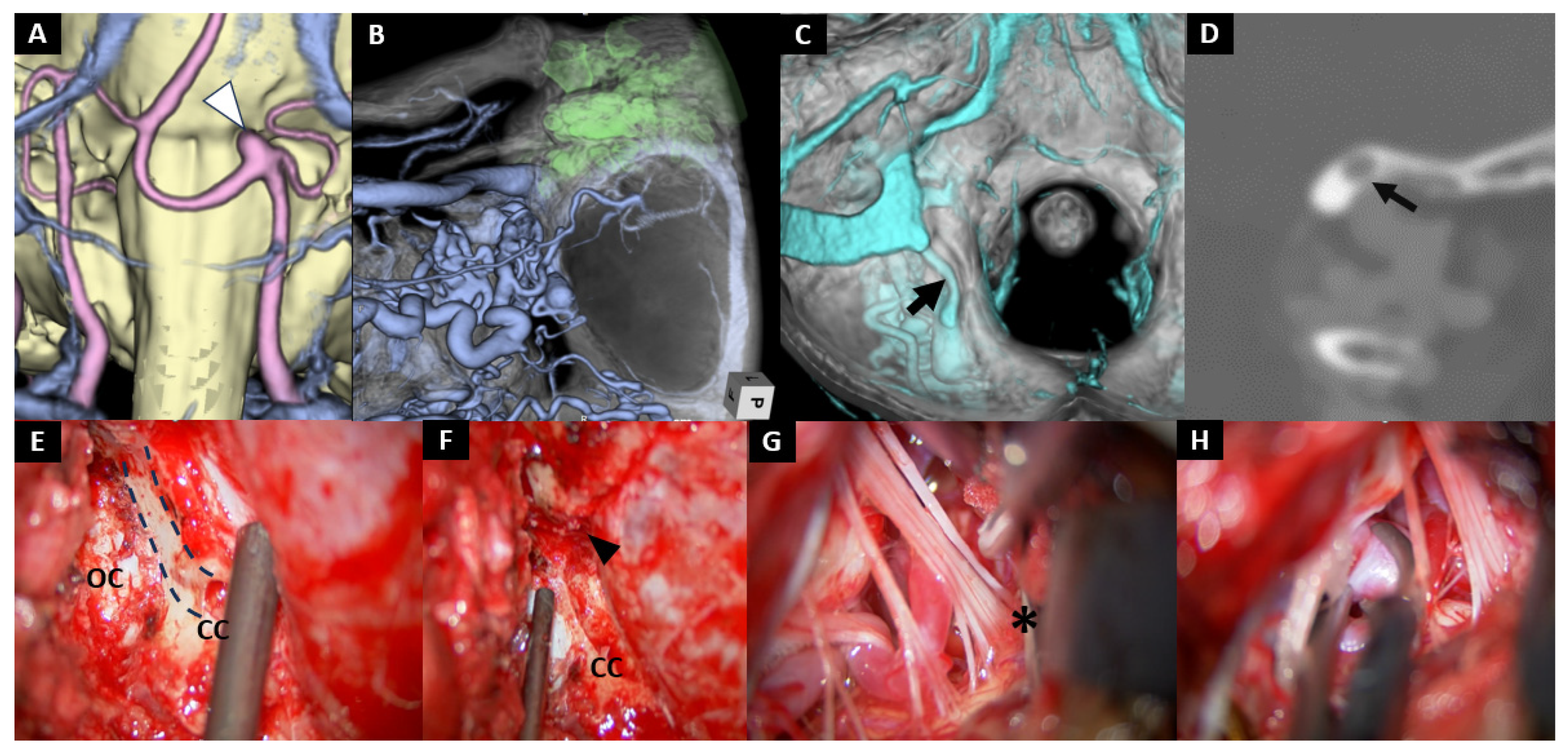
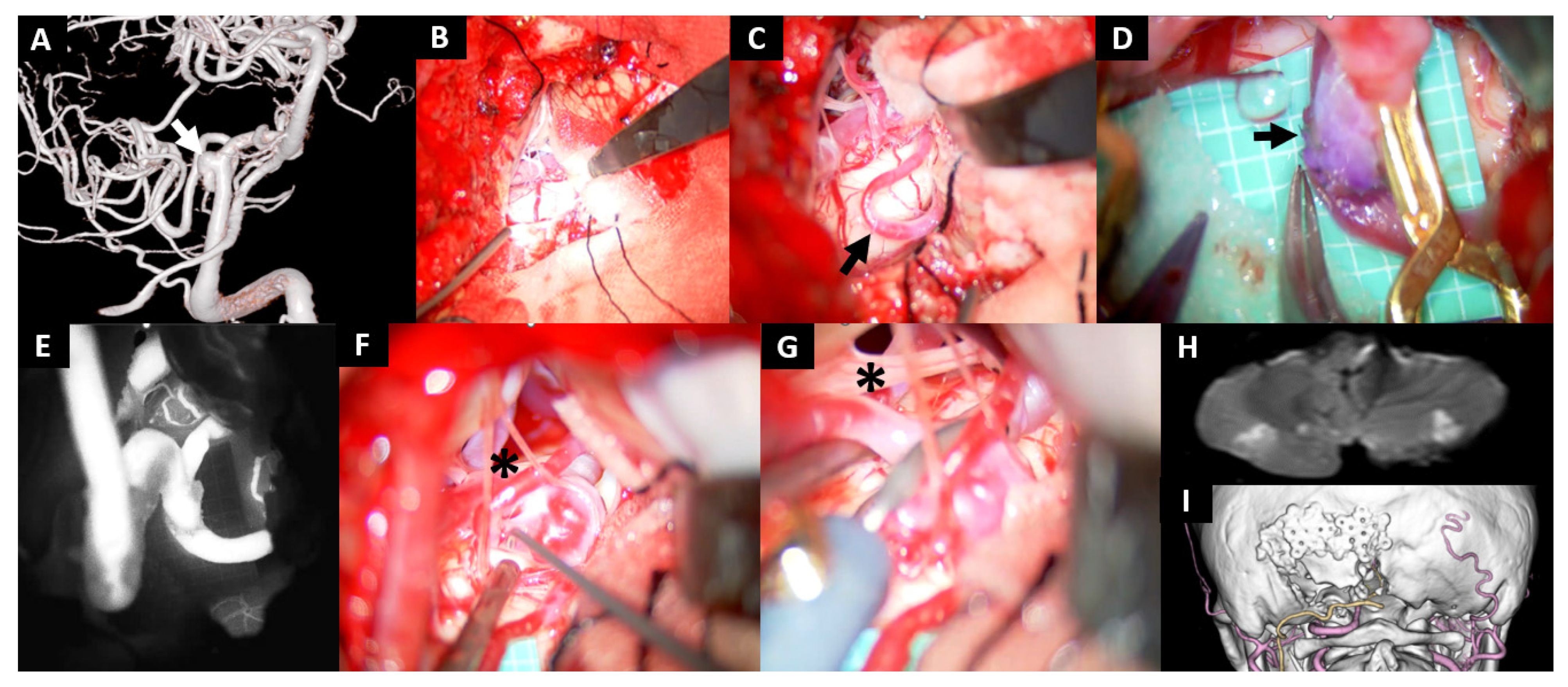
Illustrative Cases
- Case No. 9
- Case No. 10
- Case No. 8
4. Discussion
4.1. Clinical Outcomes
4.2. Technical Considerations
4.2.1. Perioperative Antiplatelet Therapy
4.2.2. Craniotomy
4.2.3. Intradural Maneuvers
4.2.4. Closure
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AICA | Anterior inferior cerebellar artery |
| BA | Basilar artery |
| CC | Condylar canal |
| CMC | Cerebellomedullary cistern |
| CMF | Cerebellomedullary fissure |
| CN | Cranial nerve |
| CSF | Cerebrospinal fluid |
| CT | Computed tomography |
| DA | Dissecting aneurysm |
| DAPT | Dual antiplatelet therapy |
| DWI | Diffusion-weighted imaging |
| FD | Flow diverter |
| EVT | Endovascular treatment |
| ICG | Indocyanine green |
| INL | Inferior nuchal line |
| JT | Jugular tubercle |
| mRS | Modified Rankin Scale |
| OA | Occipital artery |
| EPAO | Endovascular parent artery occlusion |
| PICA | Posterior inferior cerebellar artery |
| SAH | Subarachnoid hemorrhage |
| STA-MCA | Superficial temporal artery—middle cerebral artery |
| VA | Vertebral artery |
References
- Park, J.S.; Lee, T.H.; Seo, E.K.; Cho, Y.J. Aneurysms of distal posterior inferior cerebellar artery. J. Korean Neurosurg. Soc. 2008, 44, 205–210. [Google Scholar] [CrossRef]
- Xu, F.; Hong, Y.; Zheng, Y.; Xu, Q.; Leng, B. Endovascular treatment of posterior inferior cerebellar artery aneurysms: A 7-year single-center experience. J. Neurointerv. Surg. 2017, 9, 45–51. [Google Scholar] [CrossRef]
- Wallace, A.N.; Kamran, M.; Madaelil, T.P.; Kayan, Y.; Osbun, J.W.; Roy, A.K.; Almandoz, J.E.D.; Moran, C.J.; Howard, B.M.; Yasin, J.; et al. Endovascular Treatment of Posterior Inferior Cerebellar Artery Aneurysms with Flow Diversion. World Neurosurg. 2018, 114, e581–e587. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wei, L.; Li, L.; Niu, Y.; Chen, Q.; Feng, H.; Zhu, G.; Chen, Z. Endovascular treatment of distal posterior inferior cerebellar artery aneurysms. Neurosciences 2016, 21, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Sriamornrattanakul, K.; Akharathammachote, N.; Chonhenchob, A.; Mongkolratnan, A.; Niljianskul, N.; Phoominaonin, I.S.; Ariyaprakai, C. Far-lateral approach without C1 laminectomy for microsurgical treatment of vertebral artery and proximal posterior inferior cerebellar artery aneurysms: Experience from 48 patients. World Neurosurg. X 2023, 19, 100216. [Google Scholar] [CrossRef]
- Graffeo, C.S.; Srinivasan, V.M.; Scherschinski, L.; Winkler, E.A.; Baranoski, J.F.; Albuquerque, F.C.; Lawton, M.T. Far Lateral Approach and Occipital Artery to Posterior Inferior Cerebellar Artery Bypass with Staged Flow Diversion for Treatment of De Novo Vertebrobasilar Junction Aneurysm: 2-Dimensional Operative Video. Oper. Neurosurg. 2024, 27, 510–511. [Google Scholar] [CrossRef]
- Matsushima, T.; Kawashima, M.; Masuoka, J.; Mineta, T.; Inoue, T. Transcondylar fossa (supracondylar transjugular tubercle) approach: Anatomic basis for the approach, surgical procedures, and surgical experience. Skull Base 2010, 20, 83–91. [Google Scholar] [CrossRef]
- Kawashima, M.; Takase, Y.; Matsushima, T. Surgical treatment for vertebral artery-posterior inferior cerebellar artery aneurysms: Special reference to the importance of the cerebellomedullary fissure dissection. J. Neurosurg. 2013, 118, 460–464. [Google Scholar] [CrossRef]
- Matsushima, T.; Kawashima, M.; Inoue, K.; Matsushima, K.; Miki, K. Exposure of wide cerebellomedullary cisterns for vascular lesion surgeries in cerebellomedullary cisterns: Opening of unilateral cerebellomedullary fissures combined with lateral foramen magnum approach. World Neurosurg. 2014, 82, e615–e621. [Google Scholar] [CrossRef]
- Matsuno, H.; Rhoton, A.L., Jr.; Peace, D. Microsurgical anatomy of the posterior fossa cisterns. Neurosurgery 1988, 23, 58–80. [Google Scholar] [CrossRef]
- Hudgins, R.J.; Day, A.L.; Quisling, R.G.; Rhoton, A.L., Jr.; Sypert, G.W.; Garcia-Bengochea, F. Aneurysms of the posterior inferior cerebellar artery: A clinical and anatomical analysis. J. Neurosurg. 1983, 58, 381–387. [Google Scholar] [CrossRef]
- Matsushima, T.; Matsukado, K.; Natori, Y.; Inamura, T.; Hitotsumatsu, T.; Fukui, M. Surgery on a saccular vertebral artery-posterior inferior cerebellar artery aneurysm via the transcondylar fossa (supracondylar transjugular tubercle) approach or the transcondylar approach: Surgical results and indications for using two different lateral skull base approaches. J. Neurosurg. 2001, 95, 268–274. [Google Scholar] [CrossRef]
- Garner, M.; Fries, F.; Kettner, M.; Haußmann, A.; Bachhuber, A.; Reith, W.; Yilmaz, U. Endovascular Treatment Strategies for Aneurysms of the Origin of the Posterior Inferior Cerebellar Artery. World Neurosurg. 2023, 172, e412–e417. [Google Scholar] [CrossRef] [PubMed]
- Heros, R.C. Lateral suboccipital approach for vertebral and vertebrobasilar artery lesions. J. Neurosurg. 1986, 64, 559–562. [Google Scholar] [CrossRef]
- Origitano, T.C.; Anderson, D.E.; Tarassoli, Y.; Reichman, O.H.; al-Mefty, O. Skull base approaches to complex cerebral aneurysms. Surg. Neurol. 1993, 40, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.N.; Sekhar, L.N. An extreme lateral approach to intradural lesions of the cervical spine and foramen magnum. Neurosurgery 1990, 27, 197–204. [Google Scholar] [CrossRef]
- Bertalanffy, H.; Seeger, W. The dorsolateral, suboccipital, transcondylar approach to the lower clivus and anterior portion of the craniocervical junction. Neurosurgery 1991, 29, 815–821. [Google Scholar] [CrossRef]
- Matsushima, T.; Natori, Y.; Katsuta, T.; Ikezaki, K.; Fukui, M.; Rhoton, A.L. Microsurgical anatomy for lateral approaches to the foramen magnum with special reference to transcondylar fossa (supracondylar transjugular tubercle) approach. Skull Base Surg. 1998, 8, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Kochi, R.; Endo, H.; Fujimura, M.; Sato, K.; Sugiyama, S.; Osawa, S.; Tominaga, T. Outflow Occlusion with Occipital Artery-Posterior Inferior Cerebellar Artery Bypass for Growing Vertebral Artery Fusiform Aneurysm with Ischemic Onset: A Case Report. J. Stroke Cerebrovasc. Dis. 2015, 24, e223–e226. [Google Scholar] [CrossRef] [PubMed]
- Cavagnaro, M.J.; Orenday-Barraza, J.M.; Dowell, A.; Lee, M.; Jabre, R.; Nakaji, P. Occipital Artery-Posterior Inferior Cerebellar Artery (PICA) Bypass for the Treatment of a Ruptured Fusiform Aneurysm of the Left PICA: 2-Dimensional Operative Video. World Neurosurg. 2022, 161, 105. [Google Scholar] [CrossRef]
- Schubert, G.A.; Biermann, P.; Weiss, C.; Seiz, M.; Vajkoczy, P.; Schmiedek, P.; Thomé, C. Risk profile in extracranial/intracranial bypass surgery--the role of antiplatelet agents, disease pathology, and surgical technique in 168 direct revascularization procedures. World Neurosurg. 2014, 82, 672–677. [Google Scholar] [CrossRef]
- Nakamura, A.; Takasu, S.; Seki, Y.; Saito, R. Safety of antiplatelet therapy during the perioperative period of revascularization surgery for moyamoya disease patients with ischemic onset. Nagoya J. Med. Sci. 2024, 86, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, F.; Araki, Y.; Yokoyama, K.; Uda, K.; Mamiya, T.; Nishihori, M.; Izumi, T.; Okamoto, S.; Natsume, A. Effects of aspirin and heparin treatment on perioperative outcomes in patients with Moyamoya disease. Acta Neurochir. 2021, 163, 1485–1491. [Google Scholar] [CrossRef]
- Palanisamy, D.; Yasuhiro, Y.; Kyosuke, M.; Tsukasa, K.; Katsumi, T.; Kato, Y. Transcondylar Fossa Approach to Unruptured Vertebral Artery and Vertebral Artery-Posterior Inferior Cerebellar Artery Aneurysms: Surgical Outcome. World Neurosurg. 2018, 119, e783–e791. [Google Scholar] [CrossRef]
- Campero, A.; Baldoncini, M.; Villalonga, J.F.; Paíz, M.; Giotta Lucifero, A.; Luzzi, S. Transcondylar Fossa Approach for Resection of Anterolateral Foramen Magnum Meningioma: 2-Dimensional Operative Video. World Neurosurg. 2021, 154, 91–92. [Google Scholar] [CrossRef]
- Kawashima, M.; Rhoton, A.L., Jr.; Tanriover, N.; Ulm, A.J.; Yasuda, A.; Fujii, K. Microsurgical anatomy of cerebral revascularization. Part II: Posterior circulation. J. Neurosurg. 2005, 102, 132–147. [Google Scholar] [CrossRef] [PubMed]
- Khodadad, G.; Singh, R.S.; Olinger, C.P. Possible prevention of brain stem stroke by microvascular anastomosis in the vertebrobasilar system. Stroke 1977, 8, 316–321. [Google Scholar] [CrossRef]
- Sundt, T.M., Jr.; Piepgras, D.G. Occipital to posterior inferior cerebellar artery bypass surgery. J. Neurosurg. 1978, 48, 916–928. [Google Scholar] [CrossRef] [PubMed]
- Nisson, P.L.; Ding, X.; Tayebi Meybodi, A.; Palsma, R.; Benet, A.; Lawton, M.T. Revascularization of the Posterior Inferior Cerebellar Artery Using the Occipital Artery: A Cadaveric Study Comparing the p3 and p1 Recipient Sites. Oper. Neurosurg. 2020, 19, E122–E129. [Google Scholar] [CrossRef]
- Abe, H.; Miki, K.; Kobayashi, H.; Ogata, T.; Iwaasa, M.; Matsushima, T.; Inoue, T. Unilateral Trans-cerebellomedullary Fissure Approach for Occipital Artery to Posterior Inferior Cerebellar Artery Bypass during Aneurysmal Surgery. Neurol. Med. Chir. 2017, 57, 284–291. [Google Scholar] [CrossRef]
- Anichini, G.; Evins, A.I.; Boeris, D.; Stieg, P.E.; Bernardo, A. Three-dimensional endoscope-assisted surgical approach to the foramen magnum and craniovertebral junction: Minimizing bone resection with the aid of the endoscope. World Neurosurg. 2014, 82, e797–e805. [Google Scholar] [CrossRef] [PubMed]
- Nanda, A.; Vincent, D.A.; Vannemreddy, P.S.; Baskaya, M.K.; Chanda, A. Far-lateral approach to intradural lesions of the foramen magnum without resection of the occipital condyle. J. Neurosurg. 2002, 96, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Rhoton, A.L., Jr. The far-lateral approach and its transcondylar, supracondylar, and paracondylar extensions. Neurosurgery 2000, 47 (Suppl. 3), S195–S209. [Google Scholar] [CrossRef]
- Fukuda, H.; Evins, A.I.; Iwasaki, K.; Hattori, I.; Murao, K.; Kurosaki, Y.; Chin, M.; Stieg, P.E.; Yamagata, S.; Bernardo, A. The role of alternative anastomosis sites in occipital artery-posterior inferior cerebellar artery bypass in the absence of the caudal loop using the far-lateral approach. J. Neurosurg. 2017, 126, 634–644. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, X.; Han, L.; Tuo, Y.; Wu, B.; Ding, X. Occipital artery-posterior inferior cerebellar artery bypass: A cadaveric feasibility study. Surg. Radiol. Anat. 2023, 45, 839–848. [Google Scholar] [CrossRef]
- Wen, H.T.; Rhoton, A.L., Jr.; Katsuta, T.; de Oliveira, E. Microsurgical anatomy of the transcondylar, supracondylar, and paracondylar extensions of the far-lateral approach. J. Neurosurg. 1997, 87, 555–585. [Google Scholar] [CrossRef]
- Salas, E.; Sekhar, L.N.; Ziyal, I.M.; Caputy, A.J.; Wright, D.C. Variations of the extreme-lateral craniocervical approach: Anatomical study and clinical analysis of 69 patients. J. Neurosurg. 1999, 90 (Suppl. 2), 206–219. [Google Scholar] [CrossRef]
- Matsushima, T.; Inoue, T.; Inamura, T.; Natori, Y.; Ikezaki, K.; Fukui, M. Transcerebellomedullary fissure approach with special reference to methods of dissecting the fissure. J. Neurosurg. 2001, 94, 257–264. [Google Scholar] [CrossRef]
- Matsushima, T.; Fukui, M.; Inoue, T.; Natori, Y.; Baba, T.; Fujii, K. Microsurgical and magnetic resonance imaging anatomy of the cerebello-medullary fissure and its application during fourth ventricle surgery. Neurosurgery 1992, 30, 325–330. [Google Scholar] [CrossRef]
- Kawashima, M.; Matsushima, T.; Nakahara, Y.; Takase, Y.; Masuoka, J.; Ohata, K. Trans-cerebellomedullary fissure approach with special reference to lateral route. Neurosurg. Rev. 2009, 32, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, K.; Yagmurlu, K.; Kohno, M.; Rhoton, A.L., Jr. Anatomy and approaches along the cerebellar-brainstem fissures. J. Neurosurg. 2016, 124, 248–263. [Google Scholar] [CrossRef]
- Ozveren, M.F.; Türe, U.; Ozek, M.M.; Pamir, M.N. Anatomic landmarks of the glossopharyngeal nerve: A microsurgical anatomic study. Neurosurgery 2003, 52, 1400–1410; discussion 1410. [Google Scholar] [CrossRef] [PubMed]
- Ozveren, M.F.; Türe, U. The microsurgical anatomy of the glossopharyngeal nerve with respect to the jugular foramen lesions. Neurosurg. Focus 2004, 17, E3. [Google Scholar] [CrossRef] [PubMed]
- Narotam, P.K.; Qiao, F.; Nathoo, N. Collagen matrix duraplasty for posterior fossa surgery: Evaluation of surgical technique in 52 adult patients. Clinical article. J. Neurosurg. 2009, 111, 380–386. [Google Scholar] [CrossRef] [PubMed]



| Case | Age | Sex | Morphology | Location | Dome Size | Procedure | Antithrombotic | CSF Leak | New Deficits | Hemorrhage | DWI | mRS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 75 | F | saccular (SAH) | VA-PICA | 2 mm | OA-PICA & trapping | None | - | - | - | small | 0 |
| 2 | 84 | F | saccular (SAH) | VA-PICA | 8 mm | clipping | None | - | dysphagia | - | 4 | |
| 3 | 64 | F | saccular | VA-PICA | 14 mm | OA-PICA & trapping | None | - | Transient dysphagia | - | small | 3 Parkinson’s disease |
| 4 | 78 | F | DA | VA (PICA involved) | FD | DAPT | - | - | - | 0 | ||
| 5 | 45 | M | DA | VA (PICA involved) | EPAO | DAPT | - | - | - | 0 | ||
| 6 | 66 | M | DA | VA (PICA involved) | OA-PICA & trapping | Aspirin | - | - | - | - | 0 | |
| 7 | 33 | M | thrombosed DA | PICA proximal | 20 mm | OA-PICA & trapping | Aspirin | - | - | - | - | 0 |
| 8 | 67 | M | saccular | BA-AICA (AICA-PICA) | 7 mm | Hybrid | Aspirin+ Heparin | - | - | + | - | 1 |
| 9 | 70 | F | saccular | VA-PICA | 4.5 mm | clipping | None | - | - | - | - | 0 |
| 10 | 57 | F | saccular | VA-PICA | 3 mm | OA-PICA & trapping | None | - | - | - | moderate | 0 |
| 11 | 54 | F | saccular | VA-PICA | 11 mm | coil embolization | DAPT | - | - | - | 4 aneurysmal mass effect | |
| 12 | 44 | F | fusiform | PICA origin | EPAO | DAPT | - | - | - | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamada, F.; Fukuda, H.; Fukui, N.; Ueba, Y.; Nonaka, M.; Takemura, M.; Kida, N.; Ueba, T. Choice of Treatment Modality and Validity of Direct Surgery for Complex Posterior Inferior Cerebellar Artery-Related Aneurysms. J. Clin. Med. 2025, 14, 8270. https://doi.org/10.3390/jcm14238270
Hamada F, Fukuda H, Fukui N, Ueba Y, Nonaka M, Takemura M, Kida N, Ueba T. Choice of Treatment Modality and Validity of Direct Surgery for Complex Posterior Inferior Cerebellar Artery-Related Aneurysms. Journal of Clinical Medicine. 2025; 14(23):8270. https://doi.org/10.3390/jcm14238270
Chicago/Turabian StyleHamada, Fumihiro, Hitoshi Fukuda, Naoki Fukui, Yusuke Ueba, Motonobu Nonaka, Mitsuhiro Takemura, Namito Kida, and Tetsuya Ueba. 2025. "Choice of Treatment Modality and Validity of Direct Surgery for Complex Posterior Inferior Cerebellar Artery-Related Aneurysms" Journal of Clinical Medicine 14, no. 23: 8270. https://doi.org/10.3390/jcm14238270
APA StyleHamada, F., Fukuda, H., Fukui, N., Ueba, Y., Nonaka, M., Takemura, M., Kida, N., & Ueba, T. (2025). Choice of Treatment Modality and Validity of Direct Surgery for Complex Posterior Inferior Cerebellar Artery-Related Aneurysms. Journal of Clinical Medicine, 14(23), 8270. https://doi.org/10.3390/jcm14238270






