Beyond Iron: The Roles of CD71 in the Pathophysiology of Cancer—A Comprehensive Review
Abstract
1. Introduction
2. CD71 and Iron Metabolism in Cancer
2.1. CD71-Mediated Iron Uptake
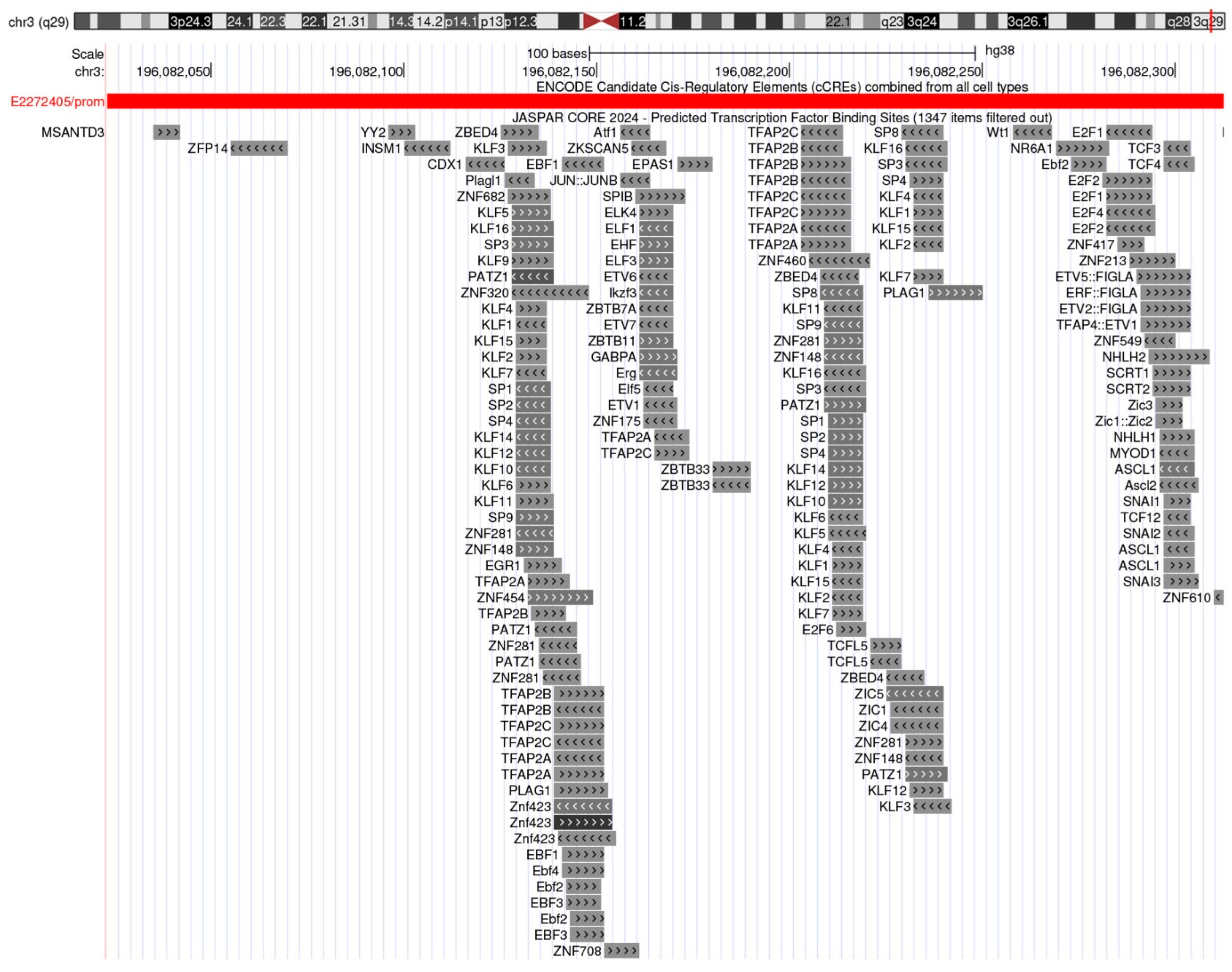
2.2. Transcriptional Regulation of TFRC Gene
2.3. CD71 Expression Across Tumors and Its Impact
3. Non-Canonical Functions of CD71
3.1. Signal Transduction Pathways
3.2. Regulation of Apoptosis and Autophagy
3.3. Cell Adhesion and Migration
4. CD71 in the Tumor Microenvironment
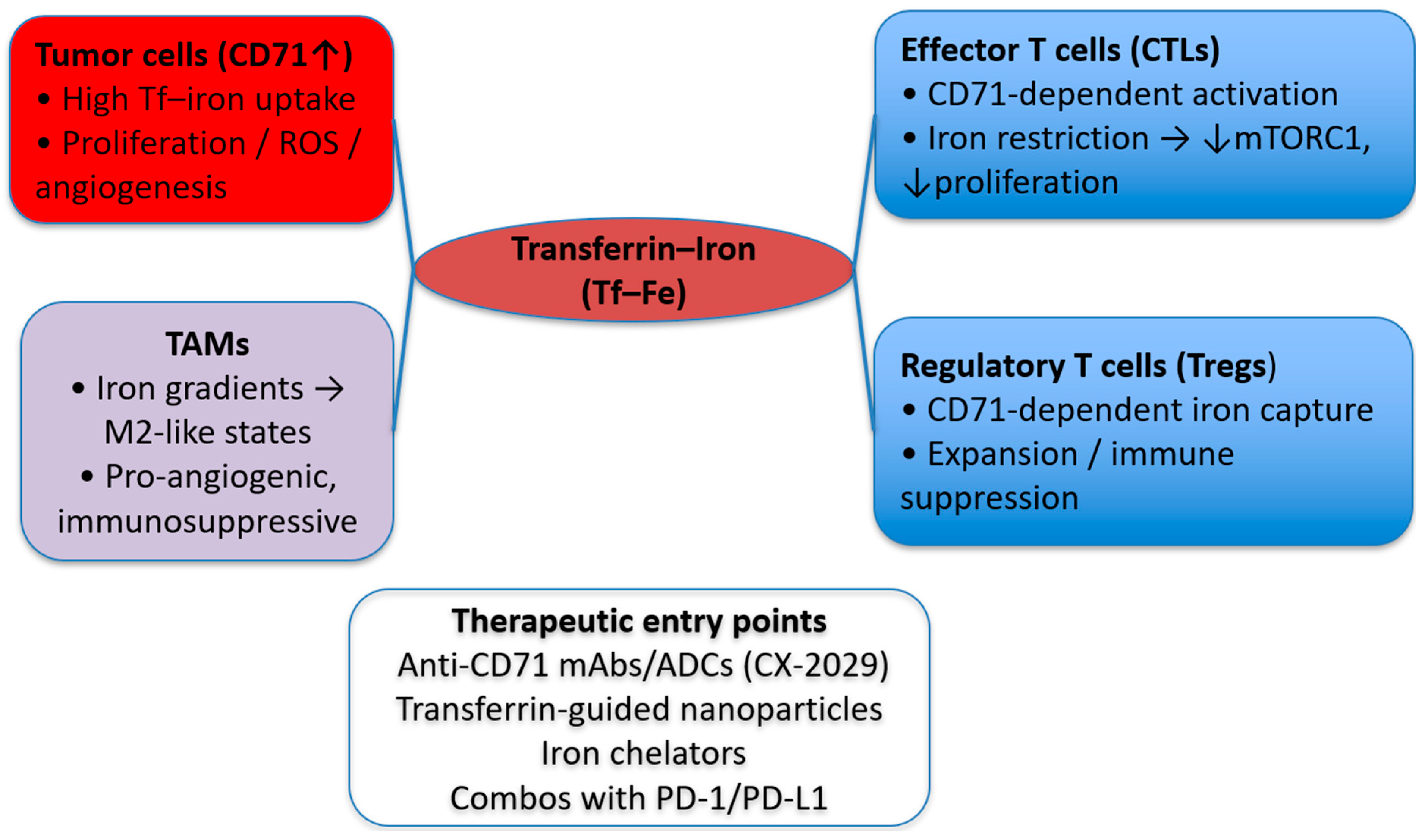
Interaction with Immune Cells
5. CD71 and the Crosstalk Between Cancer and Chronic Disease
5.1. CD71 in Cardiac Disease
5.2. CD71 in Neuronal Disease
5.3. CD71 in Gastrointestinal Disease
5.4. Summary on CD71 in Chronic Disease
6. Methods
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Force, L.M.; Kocarnik, J.M.; May, M.L.; Bhangdia, K.; Crist, A.; Penberthy, L.; Pritchett, N.; Acheson, A.; Deitesfeld, L.; A, B.; et al. The global, regional, and national burden of cancer, 1990–2023, with forecasts to 2050: A systematic analysis for the Global Burden of Disease Study 2023. Lancet 2025, 406, 1565–1586. [Google Scholar] [CrossRef] [PubMed]
- Lyons, V.J.; Helms, A.; Pappas, D. The effect of protein expression on cancer cell capture using the Human Transferrin Receptor (CD71) as an affinity ligand. Anal. Chim. Acta 2019, 1076, 154–161. [Google Scholar] [CrossRef]
- Gennery, A.R. Chapter 5—Autoimmunity in combined immunodeficiency. In Translational Autoimmunity; Rezaei, N., Ed.; Academic Press: Cambridge, MA, USA, 2022; Volume 3, pp. 97–127. [Google Scholar]
- Rodriguez, R.; Schreiber, S.L.; Conrad, M. Persister cancer cells: Iron addiction and vulnerability to ferroptosis. Mol. Cell 2022, 82, 728–740. [Google Scholar] [CrossRef]
- Basuli, D.; Tesfay, L.; Deng, Z.; Paul, B.; Yamamoto, Y.; Ning, G.; Xian, W.; McKeon, F.; Lynch, M.; Crum, C.P. Iron addiction: A novel therapeutic target in ovarian cancer. Oncogene 2017, 36, 4089–4099. [Google Scholar] [CrossRef]
- Gammella, E.; Buratti, P.; Cairo, G.; Recalcati, S. The transferrin receptor: The cellular iron gate. Met. Integr. Biometal Sci. 2017, 9, 1367–1375. [Google Scholar] [CrossRef]
- Kawabata, H. Transferrin and transferrin receptors update. Free. Radic. Biol. Med. 2019, 133, 46–54. [Google Scholar] [CrossRef]
- Wang, S.; He, X.; Wu, Q.; Jiang, L.; Chen, L.; Yu, Y.; Zhang, P.; Huang, X.; Wang, J.; Ju, Z.; et al. Transferrin receptor 1-mediated iron uptake plays an essential role in hematopoiesis. Haematologica 2020, 105, 2071–2082. [Google Scholar] [CrossRef]
- Ned, R.e.M.; Swat, W.; Andrews, N.C. Transferrin receptor 1 is differentially required in lymphocyte development. Blood 2003, 102, 3711–3718. [Google Scholar] [CrossRef]
- Berg, V.; Modak, M.; Brell, J.; Puck, A.; Künig, S.; Jutz, S.; Steinberger, P.; Zlabinger, G.J.; Stöckl, J. Iron Deprivation in Human T Cells Induces Nonproliferating Accessory Helper Cells. ImmunoHorizons 2020, 4, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Aba, Ü.; Maslak, İ.C.; İpşir, C.; Pehlivan, D.; Warnock, N.I.; Tumes, D.J.; Cildir, G.; Erman, B. A Novel Homozygous Germline Mutation in Transferrin Receptor 1 (TfR1) Leads to Combined Immunodeficiency and Provides New Insights into Iron-Immunity Axis. J. Clin. Immunol. 2024, 44, 55. [Google Scholar] [CrossRef]
- von Haehling, S. Iron deficiency in heart failure: Epidemiology, diagnostic criteria and treatment modalities. ESC Heart Fail. 2025, 12, 723–726. [Google Scholar] [CrossRef]
- Jujić, A.; Molvin, J.; Holm Isholth, H.; Dieden, A.; Korduner, J.; Zaghi, A.; Nezami, Z.; Bergmann, A.; Schomburg, L.; Magnusson, M. Association between low selenoprotein P concentrations and anaemia in hospitalized heart failure patients. ESC Heart Fail. 2024, 11, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Kozłowska, B.; Sochanowicz, B.; Kraj, L.; Palusińska, M.; Kołsut, P.; Szymański, Ł.; Lewicki, S.; Śmigielski, W.; Kruszewski, M.; Leszek, P. Expression of Iron Metabolism Proteins in Patients with Chronic Heart Failure. J. Clin. Med. 2022, 11, 837. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhou, Y.; Zhang, C.; Zhai, Z.; Yang, Y.; Liu, X. Transferrin receptor knockdown attenuates atrial fibrillation by inhibiting cardiomyocyte ferroptosis and atrial fibrosis. Exp. Anim. 2025, 74, 348–361. [Google Scholar] [CrossRef]
- Tian, S.; Wang, B.; Ding, Y.; Zhang, Y.; Yu, P.; Chang, Y.-Z.; Gao, G. The role of iron transporters and regulators in Alzheimer’s disease and Parkinson’s disease: Pathophysiological insights and therapeutic prospects. Biomed. Pharmacother. 2024, 179, 117419. [Google Scholar] [CrossRef]
- Zeng, W.; Cai, J.; Zhang, L.; Peng, Q. Iron Deposition in Parkinson’s Disease: A Mini-Review. Cell. Mol. Neurobiol. 2024, 44, 26. [Google Scholar] [CrossRef]
- Pfeifhofer-Obermair, C.; Tymoszuk, P.; Petzer, V.; Weiss, G.; Nairz, M. Iron in the Tumor Microenvironment—Connecting the Dots. Front. Oncol. 2018, 8, 549. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, L.; Ding, J.; Chen, Y. Iron Metabolism in Cancer. Int. J. Mol. Sci. 2018, 20, 95. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Qian, C.; Wang, X.; Qian, Z.-M. Transferrin receptors. Exp. Mol. Med. 2025, 57, 724–732. [Google Scholar] [CrossRef]
- Aisen, P. Transferrin receptor 1. Int. J. Biochem. Cell Biol. 2004, 36, 2137–2143. [Google Scholar] [CrossRef]
- MacKenzie, E.L.; Iwasaki, K.; Tsuji, Y. Intracellular iron transport and storage: From molecular mechanisms to health implications. Antioxid. Redox Signal. 2008, 10, 997–1030. [Google Scholar] [CrossRef]
- Eckenroth, B.E.; Steere, A.N.; Chasteen, N.D.; Everse, S.J.; Mason, A.B. How the binding of human transferrin primes the transferrin receptor potentiating iron release at endosomal pH. Proc. Natl. Acad. Sci. USA 2011, 108, 13089–13094. [Google Scholar] [CrossRef]
- West, A.R.; Oates, P.S. Mechanisms of heme iron absorption: Current questions and controversies. World J. Gastroenterol. 2008, 14, 4101–4110. [Google Scholar] [CrossRef] [PubMed]
- Faubert, B.; Solmonson, A.; DeBerardinis, R.J. Metabolic reprogramming and cancer progression. Science 2020, 368, eaaw5473. [Google Scholar] [CrossRef]
- Ward, P.S.; Thompson, C.B. Metabolic reprogramming: A cancer hallmark even warburg did not anticipate. Cancer Cell 2012, 21, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Manz, D.H.; Blanchette, N.L.; Paul, B.T.; Torti, F.M.; Torti, S.V. Iron and cancer: Recent insights. Ann. N. Y. Acad. Sci. 2016, 1368, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.Y.; Mina, E.; Roetto, A.; Porporato, P.E. Iron: An Essential Element of Cancer Metabolism. Cells 2020, 9, 2591. [Google Scholar] [CrossRef]
- Shen, Y.; Li, X.; Dong, D.; Zhang, B.; Xue, Y.; Shang, P. Transferrin receptor 1 in cancer: A new sight for cancer therapy. Am. J. Cancer Res. 2018, 8, 916–931. [Google Scholar]
- Habashy, H.O.; Powe, D.G.; Staka, C.M.; Rakha, E.A.; Ball, G.; Green, A.R.; Aleskandarany, M.; Paish, E.C.; Douglas Macmillan, R.; Nicholson, R.I.; et al. Transferrin receptor (CD71) is a marker of poor prognosis in breast cancer and can predict response to tamoxifen. Breast Cancer Res. Treat. 2010, 119, 283–293. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, J.; Chen, J.; Zou, M.; Rusidanmu, A.; Yang, R. Blocking transferrin receptor inhibits the growth of lung adenocarcinoma cells in vitro. Thorac. Cancer 2018, 9, 253–261. [Google Scholar] [CrossRef]
- Adachi, M.; Kai, K.; Yamaji, K.; Ide, T.; Noshiro, H.; Kawaguchi, A.; Aishima, S. Transferrin receptor 1 overexpression is associated with tumour de-differentiation and acts as a potential prognostic indicator of hepatocellular carcinoma. Histopathology 2019, 75, 63–73. [Google Scholar] [CrossRef]
- Keer, H.N.; Kozlowski, J.M.; Tsai, Y.C.; Lee, C.; McEwan, R.N.; Grayhack, J.T. Elevated transferrin receptor content in human prostate cancer cell lines assessed in vitro and in vivo. J. Urol. 1990, 143, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.; Li, X.; Yang, L.; Ma, X.; Shen, Y.; Huang, C.; Pan, T.; Cui, J.; Ni, B.; Wang, M. Overexpressed transferrin receptor implied poor prognosis and relapse in gastrointestinal stromal tumors. Front. Oncol. 2023, 13, 1151687. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia-Inducible Factor 1: Control of Oxygen Homeostasis in Health and Disease. Pediatr. Res. 2001, 49, 614–617. [Google Scholar] [CrossRef]
- Lok, C.N.; Ponka, P. Identification of a Hypoxia Response Element in the Transferrin Receptor Gene*. J. Biol. Chem. 1999, 274, 24147–24152. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, L.; Tacchini, L.; Cairo, G. HIF-1-mediated activation of transferrin receptor gene transcription by iron chelation. Nucleic Acids Res. 1999, 27, 4223–4227. [Google Scholar] [CrossRef]
- Tacchini, L.; Bianchi, L.; Bernelli-Zazzera, A.; Cairo, G. Transferrin receptor induction by hypoxia. HIF-1-mediated transcriptional activation and cell-specific post-transcriptional regulation. J. Biol. Chem. 1999, 274, 24142–24146. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.A.; Yu, D.; Zeller, K.I.; Kim, J.W.; Racke, F.; Thomas-Tikhonenko, A.; Dang, C.V. Activation of transferrin receptor 1 by c-Myc enhances cellular proliferation and tumorigenesis. Mol. Cell. Biol. 2006, 26, 2373–2386. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Synthetic Lethal Screening Identifies Compounds Activating Iron-Dependent, Nonapoptotic Cell Death in Oncogenic-RAS-Harboring Cancer Cells. Chem. Biol. 2008, 15, 234–245. [Google Scholar] [CrossRef]
- Chen, C.; Liu, P.; Duan, X.; Cheng, M.; Xu, L.X. Deferoxamine-induced high expression of TfR1 and DMT1 enhanced iron uptake in triple-negative breast cancer cells by activating IL-6/PI3K/AKT pathway. OncoTargets Ther. 2019, 12, 4359–4377. [Google Scholar] [CrossRef] [PubMed]
- Iommarini, L.; Porcelli, A.M.; Gasparre, G.; Kurelac, I. Non-Canonical Mechanisms Regulating Hypoxia-Inducible Factor 1 Alpha in Cancer. Front. Oncol. 2017, 7, 286. [Google Scholar] [CrossRef]
- Malekan, M.; Ebrahimzadeh, M.A.; Sheida, F. The role of Hypoxia-Inducible Factor-1alpha and its signaling in melanoma. Biomed. Pharmacother. 2021, 141, 111873. [Google Scholar] [CrossRef]
- Li, Y.; Sun, X.X.; Qian, D.Z.; Dai, M.S. Molecular Crosstalk Between MYC and HIF in Cancer. Front. Cell Dev. Biol. 2020, 8, 590576. [Google Scholar] [CrossRef]
- Doe, M.R.; Ascano, J.M.; Kaur, M.; Cole, M.D. Myc posttranscriptionally induces HIF1 protein and target gene expression in normal and cancer cells. Cancer Res. 2012, 72, 949–957. [Google Scholar] [CrossRef]
- Perez, G.; Barber, G.P.; Benet-Pages, A.; Casper, J.; Clawson, H.; Diekhans, M.; Fischer, C.; Gonzalez, J.N.; Hinrichs, A.S.; Lee, C.M. The UCSC genome browser database: 2025 update. Nucleic Acids Res. 2025, 53, D1243–D1249. [Google Scholar] [CrossRef]
- Moore, J.E.; Pratt, H.E.; Fan, K.; Phalke, N.; Fisher, J.; Elhajjajy, S.I.; Andrews, G.; Gao, M.; Shedd, N.; Fu, Y. An expanded Registry of candidate cis-Regulatory Elements for studying transcriptional regulation. bioRxiv 2024. [Google Scholar] [CrossRef]
- Rauluseviciute, I.; Riudavets-Puig, R.; Blanc-Mathieu, R.; Castro-Mondragon, J.A.; Ferenc, K.; Kumar, V.; Lemma, R.B.; Lucas, J.; Chèneby, J.; Baranasic, D. JASPAR 2024: 20th anniversary of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2024, 52, D174–D182. [Google Scholar] [CrossRef]
- Bu, X.; Wang, L. Iron metabolism and the tumor microenvironment: A new perspective on cancer intervention and therapy (Review). Int. J. Mol. Med. 2025, 55, 39. [Google Scholar] [CrossRef] [PubMed]
- Ying, J.F.; Lu, Z.B.; Fu, L.Q.; Tong, Y.; Wang, Z.; Li, W.F.; Mou, X.Z. The role of iron homeostasis and iron-mediated ROS in cancer. Am. J. Cancer Res. 2021, 11, 1895–1912. [Google Scholar] [PubMed]
- Zhao, Z. Hydroxyl radical generations form the physiologically relevant Fenton-like reactions. Free. Radic. Biol. Med. 2023, 208, 510–515. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef]
- Brandl, N.; Seitz, R.; Sendtner, N.; Müller, M.; Gülow, K. Living on the Edge: ROS Homeostasis in Cancer Cells and Its Potential as a Therapeutic Target. Antioxidants 2025, 14, 1002. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.; Mondal, Y.; Bharadwaj, K.; Mahajan, M.; Mondal, S.; Sarkar, A. Reactive Oxygen Species (ROS) and Their Profound Influence on Regulating Diverse Aspects of Cancer: A Concise Review. Drug Dev. Res. 2025, 86, e70107. [Google Scholar] [CrossRef] [PubMed]
- Jian, J.; Yang, Q.; Dai, J.; Eckard, J.; Axelrod, D.; Smith, J.; Huang, X. Effects of iron deficiency and iron overload on angiogenesis and oxidative stress-a potential dual role for iron in breast cancer. Free. Radic. Biol. Med. 2011, 50, 841–847. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Qiao, Y.; Zhou, Q.; Wang, Z.; Chen, X.; Liu, D.; Yin, D.; He, M. Iron Overload Damages the Endothelial Mitochondria via the ROS/ADMA/DDAHII/eNOS/NO Pathway. Oxidative Med. Cell. Longev. 2019, 2019, 2340392. [Google Scholar] [CrossRef]
- Mu, W.; Zhou, Z.; Shao, L.; Wang, Q.; Feng, W.; Tang, Y.; He, Y.; Wang, Y. Advances in the relationship between ferroptosis and epithelial–mesenchymal transition in cancer. Front. Oncol. 2023, 13, 1257985. [Google Scholar] [CrossRef]
- Sun, J.L.; Zhang, N.P.; Xu, R.C.; Zhang, G.C.; Liu, Z.Y.; Abuduwaili, W.; Wang, F.; Yu, X.N.; Shi, X.; Song, G.Q.; et al. Tumor cell-imposed iron restriction drives immunosuppressive polarization of tumor-associated macrophages. J. Transl. Med. 2021, 19, 347. [Google Scholar] [CrossRef]
- Liang, W.; Ferrara, N. Iron Metabolism in the Tumor Microenvironment: Contributions of Innate Immune Cells. Front. Immunol. 2021, 11, 626812. [Google Scholar] [CrossRef]
- Jung, M.; Weigert, A.; Mertens, C.; Rehwald, C.; Brüne, B. Iron Handling in Tumor-Associated Macrophages-Is There a New Role for Lipocalin-2? Front. Immunol. 2017, 8, 1171. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; Yuan, Y.; Kuang, Y.; Li, X. Iron Metabolism and Immune Regulation. Front. Immunol. 2022, 13, 816282. [Google Scholar] [CrossRef] [PubMed]
- Teh, M.R.; Frost, J.N.; Armitage, A.E.; Drakesmith, H. Analysis of Iron and Iron-Interacting Protein Dynamics During T-Cell Activation. Front. Immunol. 2021, 12, 714613. [Google Scholar] [CrossRef]
- Sacco, A.; Battaglia, A.M.; Botta, C.; Aversa, I.; Mancuso, S.; Costanzo, F.; Biamonte, F. Iron Metabolism in the Tumor Microenvironment-Implications for Anti-Cancer Immune Response. Cells 2021, 10, 303. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Han, Y.; Li, W.-N.; Xu, R.-H.; Ju, H.-Q. Tumor iron homeostasis and immune regulation. Trends Pharmacol. Sci. 2024, 45, 145–156. [Google Scholar] [CrossRef]
- Pacella, I.; Pinzon Grimaldos, A.; Rossi, A.; Tucci, G.; Zagaglioni, M.; Potenza, E.; Pinna, V.; Rotella, I.; Cammarata, I.; Cancila, V.; et al. Iron capture through CD71 drives perinatal and tumor-associated Treg expansion. JCI Insight 2024, 9, e167967. [Google Scholar] [CrossRef]
- Candelaria, P.V.; Leoh, L.S.; Penichet, M.L.; Daniels-Wells, T.R. Antibodies Targeting the Transferrin Receptor 1 (TfR1) as Direct Anti-cancer Agents. Front. Immunol. 2021, 12, 607692. [Google Scholar] [CrossRef]
- Daniels, T.R.; Bernabeu, E.; Rodríguez, J.A.; Patel, S.; Kozman, M.; Chiappetta, D.A.; Holler, E.; Ljubimova, J.Y.; Helguera, G.; Penichet, M.L. The transferrin receptor and the targeted delivery of therapeutic agents against cancer. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2012, 1820, 291–317. [Google Scholar] [CrossRef]
- Łubgan, D.; Jóźwiak, Z.; Grabenbauer, G.G.; Distel, L.V. Doxorubicin-transferrin conjugate selectively overcomes multidrug resistance in leukaemia cells. Cell. Mol. Biol. Lett. 2009, 14, 113–127. [Google Scholar] [CrossRef]
- Wigner, P.; Zielinski, K.; Labieniec-Watala, M.; Marczak, A.; Szwed, M. Doxorubicin–transferrin conjugate alters mitochondrial homeostasis and energy metabolism in human breast cancer cells. Sci. Rep. 2021, 11, 4544. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, O.; O’Sullivan, J. Iron chelators in cancer therapy. Biometals 2020, 33, 201–215. [Google Scholar] [CrossRef]
- Lui, G.Y.L.; Obeidy, P.; Ford, S.J.; Tselepis, C.; Sharp, D.M.; Jansson, P.J.; Kalinowski, D.S.; Kovacevic, Z.; Lovejoy, D.B.; Richardson, D.R. The Iron Chelator, Deferasirox, as a Novel Strategy for Cancer Treatment: Oral Activity Against Human Lung Tumor Xenografts and Molecular Mechanism of Action. Mol. Pharmacol. 2013, 83, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Daniels, T.R.; Delgado, T.; Rodriguez, J.A.; Helguera, G.; Penichet, M.L. The transferrin receptor part I: Biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin. Immunol. 2006, 121, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Mojarad-Jabali, S.; Mahdinloo, S.; Farshbaf, M.; Sarfraz, M.; Fatahi, Y.; Atyabi, F.; Valizadeh, H. Transferrin receptor-mediated liposomal drug delivery: Recent trends in targeted therapy of cancer. Expert Opin. Drug Deliv. 2022, 19, 685–705. [Google Scholar] [CrossRef]
- Wang, J.; Tian, S.; Petros, R.A.; Napier, M.E.; DeSimone, J.M. The Complex Role of Multivalency in Nanoparticles Targeting the Transferrin Receptor for Cancer Therapies. J. Am. Chem. Soc. 2010, 132, 11306–11313. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Mao, X.; Yang, X.; Zhao, G.; Li, S. New Transferrin Receptor-Targeted Peptide-Doxorubicin Conjugates: Synthesis and In Vitro Antitumor Activity. Molecules 2024, 29, 1758. [Google Scholar] [CrossRef]
- Johnson, M.; El-Khoueiry, A.; Hafez, N.; Lakhani, N.; Mamdani, H.; Rodon, J.; Sanborn, R.E.; Garcia-Corbacho, J.; Boni, V.; Stroh, M.; et al. Phase I, First-in-Human Study of the Probody Therapeutic CX-2029 in Adults with Advanced Solid Tumor Malignancies. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 4521–4530. [Google Scholar] [CrossRef]
- Chen, A.C.; Donovan, A.; Ned-Sykes, R.; Andrews, N.C. Noncanonical role of transferrin receptor 1 is essential for intestinal homeostasis. Proc. Natl. Acad. Sci. USA 2015, 112, 11714–11719. [Google Scholar] [CrossRef]
- Campisi, A.; Bonfanti, R.; Raciti, G.; Bonaventura, G.; Legnani, L.; Magro, G.; Pennisi, M.; Russo, G.; Chiacchio, M.A.; Pappalardo, F.; et al. Gene Silencing of Transferrin-1 Receptor as a Potential Therapeutic Target for Human Follicular and Anaplastic Thyroid Cancer. Mol. Ther. Oncolytics 2020, 16, 197–206. [Google Scholar] [CrossRef]
- Sánchez, M.F.; Tampé, R. Ligand-independent receptor clustering modulates transmembrane signaling: A new paradigm. Trends Biochem. Sci. 2023, 48, 156–171. [Google Scholar] [CrossRef]
- Feng, G.; Arima, Y.; Midorikawa, K.; Kobayashi, H.; Oikawa, S.; Zhao, W.; Zhang, Z.; Takeuchi, K.; Murata, M. Knockdown of TFRC suppressed the progression of nasopharyngeal carcinoma by downregulating the PI3K/Akt/mTOR pathway. Cancer Cell Int. 2023, 23, 185. [Google Scholar] [CrossRef]
- Chan, K.T.; Choi, M.Y.; Lai, K.K.Y.; Tan, W.; Tung, L.N.; Lam, H.Y.; Tong, D.K.H.; Lee, N.P.; Law, S. Overexpression of transferrin receptor CD71 and its tumorigenic properties in esophageal squamous cell carcinoma. Oncol. Rep. 2014, 31, 1296–1304. [Google Scholar] [CrossRef]
- Chen, J.; Fu, Y.; Li, Y.; Weng, S.; Wang, H.; He, J.; Dong, C. Transferrin receptor 1 (TfR1) functions as an entry receptor for scale drop disease virus to invade the host cell via clathrin-mediated endocytosis. J. Virol. 2025, 99, e0067125. [Google Scholar] [CrossRef] [PubMed]
- Vartholomatos, E.; Mantziou, S.; Alexiou, G.A.; Lazari, D.; Sioka, C.; Kyritsis, A.; Markopoulos, G.S. An NF-κB- and Therapy-Related Regulatory Network in Glioma: A Potential Mechanism of Action for Natural Antiglioma Agents. Biomedicines 2022, 10, 935. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, G.; Connor, J.R. A closer look at the role of iron in glioblastoma. Neuro-Oncology 2023, 25, 2136–2149. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Wen, X.; Hong, Y.; Du, H.; Zhang, X.; Song, J.; Yin, Y.; Huang, H.; Shen, G. An anti-transferrin receptor antibody enhanced the growth inhibitory effects of chemotherapeutic drugs on human glioma cells. Int. Immunopharmacol. 2011, 11, 1844–1849. [Google Scholar] [CrossRef]
- Kawak, P.; Sawaftah, N.M.A.; Pitt, W.G.; Husseini, G.A. Transferrin-Targeted Liposomes in Glioblastoma Therapy: A Review. Int. J. Mol. Sci. 2023, 24, 13262. [Google Scholar] [CrossRef]
- Lazari, D.; Alexiou, G.A.; Markopoulos, G.S.; Vartholomatos, E.; Hodaj, E.; Chousidis, I.; Leonardos, I.; Galani, V.; Kyritsis, A.P. N-(p-coumaroyl) serotonin inhibits glioblastoma cells growth through triggering S-phase arrest and apoptosis. J. Neuro-Oncol. 2017, 132, 373–381. [Google Scholar] [CrossRef]
- Vartholomatos, E.; Alexiou, G.A.; Markopoulos, G.S.; Lazari, D.; Tsiftsoglou, O.; Chousidis, I.; Leonardos, I.; Kyritsis, A.P. Deglucohellebrin: A Potent Agent for Glioblastoma Treatment. Anti-Cancer Agents Med. Chem. 2020, 20, 103–110. [Google Scholar] [CrossRef]
- Jian, J.; Yang, Q.; Huang, X. Src regulates Tyr(20) phosphorylation of transferrin receptor-1 and potentiates breast cancer cell survival. J. Biol. Chem. 2011, 286, 35708–35715. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; An, W.; Pang, Z.; Zhao, M.; Xu, A.; Zhao, J. The TFRC as a prognostic biomarker and potential therapeutic target in cervical cancer: A preliminary study. Front. Oncol. 2025, 15, 1523137. [Google Scholar] [CrossRef] [PubMed]
- Vogler, M.; Braun, Y.; Smith, V.M.; Westhoff, M.A.; Pereira, R.S.; Pieper, N.M.; Anders, M.; Callens, M.; Vervliet, T.; Abbas, M.; et al. The BCL2 family: From apoptosis mechanisms to new advances in targeted therapy. Signal Transduct. Target. Ther. 2025, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Kasibhatla, S.; Jessen, K.A.; Maliartchouk, S.; Wang, J.Y.; English, N.M.; Drewe, J.; Qiu, L.; Archer, S.P.; Ponce, A.E.; Sirisoma, N.; et al. A role for transferrin receptor in triggering apoptosis when targeted with gambogic acid. Proc. Natl. Acad. Sci. USA 2005, 102, 12095–12100. [Google Scholar] [CrossRef] [PubMed]
- Delbridge, A.R.D.; Strasser, A. The BCL-2 protein family, BH3-mimetics and cancer therapy. Cell Death Differ. 2015, 22, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Puri, C.; Park, S.J.; Wrobel, L.; Rubinsztein, D.C. Transferrin receptor controls both autophagosome formation and closure via phosphatidylinositol 3-phosphate synthesis. Dev. Cell 2025, 60, 2715–2729.e8. [Google Scholar] [CrossRef]
- Moharir, S.C.; Sirohi, K.; Swarup, G. Regulation of transferrin receptor trafficking by optineurin and its disease-associated mutants. Prog. Mol. Biol. Transl. Sci. 2023, 194, 67–78. [Google Scholar] [CrossRef]
- Mancias, J.D.; Wang, X.; Gygi, S.P.; Harper, J.W.; Kimmelman, A.C. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature 2014, 509, 105–109. [Google Scholar] [CrossRef]
- White, E.; Lattime, E.C.; Guo, J.Y. Autophagy Regulates Stress Responses, Metabolism, and Anticancer Immunity. Trends Cancer 2021, 7, 778–789. [Google Scholar] [CrossRef]
- Russell, R.C.; Guan, K.L. The multifaceted role of autophagy in cancer. EMBO J. 2022, 41, e110031. [Google Scholar] [CrossRef]
- Ren, G.; Zhou, J.; Su, Y.; Yang, Q.; Li, J. TFRC promotes the proliferation, migration, and invasion of osteosarcoma cells by increasing the intracellular iron content and RRM2 expression. Front. Oncol. 2025, 15, 1567216. [Google Scholar] [CrossRef]
- Riggs, K.A.; Hasan, N.; Humphrey, D.; Raleigh, C.; Nevitt, C.; Corbin, D.; Hu, C. Regulation of integrin endocytic recycling and chemotactic cell migration by syntaxin 6 and VAMP3 interaction. J. Cell Sci. 2012, 125, 3827–3839. [Google Scholar] [CrossRef]
- Huang, Y.; Hong, W.; Wei, X. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J. Hematol. Oncol. 2022, 15, 129. [Google Scholar] [CrossRef]
- Kim, H.; Villareal, L.B.; Liu, Z.; Haneef, M.; Falcon, D.M.; Martin, D.R.; Lee, H.J.; Dame, M.K.; Attili, D.; Chen, Y.; et al. Transferrin Receptor-Mediated Iron Uptake Promotes Colon Tumorigenesis. Adv. Sci. 2023, 10, e2207693. [Google Scholar] [CrossRef]
- Jiang, H.; Li, H. Prognostic values of tumoral MMP2 and MMP9 overexpression in breast cancer: A systematic review and meta-analysis. BMC Cancer 2021, 21, 149. [Google Scholar] [CrossRef]
- Bai, C.; Ma, X.; Wang, X.; Chen, X. Correlation between pathological features and protein expressions of TfR1, VEGF and MMP-9 in patients with osteosarcoma. Am. J. Transl. Res. 2022, 14, 4562–4572. [Google Scholar] [PubMed]
- Torti, S.V.; Torti, F.M. Iron and Cancer: 2020 Vision. Cancer Res. 2020, 80, 5435–5448. [Google Scholar] [CrossRef]
- Dürig, J.; Calcagni, M.; Buschmann, J. Transition metals in angiogenesis—A narrative review. Mater. Today Bio 2023, 22, 100757. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.N.; Collisson, E.A.; Mills, G.B.; Shaw, K.R.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M. The cancer genome atlas pan-cancer analysis project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-J.; Pan, L.; Liu, Y.; Rong, Z.; Liu, J.; Liu, F. GEPIA3: Enhanced drug sensitivity and interaction network analysis for cancer research. Nucleic Acids Res. 2025, 53, gkaf423. [Google Scholar] [CrossRef]
- Arneth, B. Tumor Microenvironment. Medicina 2019, 56, 15. [Google Scholar] [CrossRef]
- Voss, K.; Sewell, A.E.; Krystofiak, E.S.; Gibson-Corley, K.N.; Young, A.C.; Basham, J.H.; Sugiura, A.; Arner, E.N.; Beavers, W.N.; Kunkle, D.E.; et al. Elevated transferrin receptor impairs T cell metabolism and function in systemic lupus erythematosus. Sci. Immunol. 2023, 8, eabq0178. [Google Scholar] [CrossRef]
- Savarese, G.; von Haehling, S.; Butler, J.; Cleland, J.G.F.; Ponikowski, P.; Anker, S.D. Iron deficiency and cardiovascular disease. Eur. Heart J. 2023, 44, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Othon-Martínez, D.; Fernandez-Betances, O.A.; Málaga-Espinoza, B.X.; Torres-Perez, M.E.; Cobos, E.; Gutierrez-Martinez, C. Iron and cardiovascular health: A review. J. Investig. Med. Off. Publ. Am. Fed. Clin. Res. 2024, 72, 787–797. [Google Scholar] [CrossRef]
- Lakhal-Littleton, S.; Cleland, J.G.F. Iron deficiency and supplementation in heart failure. Nat. Rev. Cardiol. 2024, 21, 463–486. [Google Scholar] [CrossRef]
- Cheema, B.; Chokshi, A.; Orimoloye, O.; Ardehali, H. Intravenous Iron Repletion for Patients with Heart Failure and Iron Deficiency. JACC 2024, 83, 2674–2689. [Google Scholar] [CrossRef]
- Kumfu, S.; Chattipakorn, S.C.; Chattipakorn, N. Iron overload cardiomyopathy: Using the latest evidence to inform future applications. Exp. Biol. Med. 2022, 247, 574–583. [Google Scholar] [CrossRef]
- Rhee, J.W.; Yi, H.; Thomas, D.; Lam, C.K.; Belbachir, N.; Tian, L.; Qin, X.; Malisa, J.; Lau, E.; Paik, D.T.; et al. Modeling Secondary Iron Overload Cardiomyopathy with Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Cell Rep. 2020, 32, 107886. [Google Scholar] [CrossRef]
- Li, S.; Zhang, X. Iron in Cardiovascular Disease: Challenges and Potentials. Front. Cardiovasc. Med. 2021, 8, 707138. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; El Dahdah, J.; Haroun, E.; Arockiam, A.D.; Safdar, A.; Sorathia, S.; Dong, T.; Griffin, B.; Wang, T.K.M. A Contemporary Review of Clinical Manifestations, Evaluation, and Management of Cardiac Complications of Iron Overload. Hearts 2025, 6, 17. [Google Scholar] [CrossRef]
- Gao, Q.; Zhou, Y.; Chen, Y.; Hu, W.; Jin, W.; Zhou, C.; Yuan, H.; Li, J.; Lin, Z.; Lin, W. Role of iron in brain development, aging, and neurodegenerative diseases. Ann. Med. 2025, 57, 2472871. [Google Scholar] [CrossRef]
- Houldsworth, A. Role of oxidative stress in neurodegenerative disorders: A review of reactive oxygen species and prevention by antioxidants. Brain Commun. 2024, 6, fcad356. [Google Scholar] [CrossRef]
- Fei, Y.; Ding, Y. The role of ferroptosis in neurodegenerative diseases. Front. Cell. Neurosci. 2024, 18, 1655–1661. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Johnsen, K.B.; Kucharz, K.; Lauritzen, M.; Moos, T. Blood-Brain Barrier Transport of Transferrin Receptor-Targeted Nanoparticles. Pharmaceutics 2022, 14, 2237. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Li, H.; Zhang, B.; Li, Y.; Zhu, Z. Targeting Transferrin Receptor 1 for Enhancing Drug Delivery Through the Blood–Brain Barrier for Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 9793. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Thomas, R.; Elgueta, C.; Horl, M.; Osborn, T.; Hallett, P.J.; Bartos, M.; Isacson, O.; Pruszak, J. Comprehensive Cell Surface Antigen Analysis Identifies Transferrin Receptor Protein-1 (CD71) as a Negative Selection Marker for Human Neuronal Cells. Stem Cells 2019, 37, 1293–1306. [Google Scholar] [CrossRef]
- Petralla, S.; Saveleva, L.; Kanninen, K.M.; Oster, J.S.; Panayotova, M.; Fricker, G.; Puris, E. Increased Expression of Transferrin Receptor 1 in the Brain Cortex of 5xFAD Mouse Model of Alzheimer’s Disease Is Associated with Activation of HIF-1 Signaling Pathway. Mol. Neurobiol. 2024, 61, 6383–6394. [Google Scholar] [CrossRef]
- Levi, S.; Ripamonti, M.; Moro, A.S.; Cozzi, A. Iron imbalance in neurodegeneration. Mol. Psychiatry 2024, 29, 1139–1152. [Google Scholar] [CrossRef]
- Chen, Z.-t.; Pan, C.-z.; Ruan, X.-l.; Lei, L.-p.; Lin, S.-m.; Wang, Y.-z.; Zhao, Z.-H. Evaluation of ferritin and TfR level in plasma neural-derived exosomes as potential markers of Parkinson’s disease. Front. Aging Neurosci. 2023, 15, 1216905. [Google Scholar] [CrossRef]
- Cao, J.; Hu, C.; Xu, J.; Han, J.; Zhang, R.; Cao, M.; Yuan, L.; Xu, Z. Aberrant Expression TFR1/CD71 in Gastric Cancer Identifies a Novel Potential Prognostic Marker and Therapeutic Target. Evid.-Based Complement. Altern. Med. Ecam 2022, 2022, 4257342. [Google Scholar] [CrossRef]
- Hou, Y.; Tang, G.; Wang, Q.; Zhou, M.; Xu, R.; Chen, X.; Shi, G.; Wang, Z.; Yan, X.; Zhuang, J.; et al. Transferrin receptor 1 nuclear translocation facilitates tumor progression via p53-mediated chromatin interactions and genome-wide alterations. Signal Transduct. Target. Ther. 2025, 10, 212. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xu, W.Q.; Zhang, W.Q.; Xu, R.C.; Sun, J.L.; Zhang, G.C.; Liu, Z.Y.; Qi, Z.R.; Dong, L.; Weng, S.Q.; et al. Transferrin receptor 1 promotes hepatocellular carcinoma progression and metastasis by activating the mTOR signaling pathway. Hepatol. Int. 2024, 18, 636–650. [Google Scholar] [CrossRef]
- Yang, C.; Li, J.; Guo, Y.; Gan, D.; Zhang, C.; Wang, R.; Hua, L.; Zhu, L.; Ma, P.; Shi, J.; et al. Role of TFRC as a Novel Prognostic Biomarker and in Immunotherapy for Pancreatic Carcinoma. Front. Mol. Biosci. 2022, 9, 756895. [Google Scholar] [CrossRef]
- Hansen, F.J.; Mittelstädt, A.; Clausen, F.-N.; Knoedler, S.; Knoedler, L.; Klöckner, S.; Kuchenreuther, I.; Mazurie, J.; Arnold, L.-S.; Anthuber, A.; et al. CD71 expressing circulating neutrophils serve as a novel prognostic biomarker for metastatic spread and reduced outcome in pancreatic ductal adenocarcinoma patients. Sci. Rep. 2024, 14, 21164. [Google Scholar] [CrossRef] [PubMed]
- Harel, E.; Rubinstein, A.; Nissan, A.; Khazanov, E.; Nadler Milbauer, M.; Barenholz, Y.; Tirosh, B. Enhanced Transferrin Receptor Expression by Proinflammatory Cytokines in Enterocytes as a Means for Local Delivery of Drugs to Inflamed Gut Mucosa. PLoS ONE 2011, 6, e24202. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, R.R.; Bourgonje, A.R.; Hu, S.; Barbieri, R.; Jansen, B.H.; Sinnema, N.; Blokzijl, T.; Taylor, C.T.; Weersma, R.K.; Faber, K.N.; et al. HIF1α-Dependent Induction of TFRC by a Combination of Intestinal Inflammation and Systemic Iron Deficiency in Inflammatory Bowel Disease. Front. Physiol. 2022, 13, 889091. [Google Scholar] [CrossRef]
- Singh, S.; Serwer, L.; DuPage, A.; Elkins, K.; Chauhan, N.; Ravn, M.; Buchanan, F.; Wang, L.; Krimm, M.; Wong, K.; et al. Nonclinical Efficacy and Safety of CX-2029, an Anti-CD71 Probody-Drug Conjugate. Mol. Cancer Ther. 2022, 21, 1326–1336. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Fan, K.; Wang, L.; Ying, X.; Sanders, A.J.; Guo, T.; Xing, X.; Zhou, M.; Du, H.; Hu, Y.; et al. TfR1 binding with H-ferritin nanocarrier achieves prognostic diagnosis and enhances the therapeutic efficacy in clinical gastric cancer. Cell Death Dis. 2020, 11, 92. [Google Scholar] [CrossRef]

| Therapeutic Approach | Example | Mechanism | Development/Status | Key Pros/Cons | Refs |
|---|---|---|---|---|---|
| Monoclonal antibodies/ADCs/Probody–drug conjugates | Anti-CD71 mAbs; CX-2029 (anti-CD71 Probody–MMAE); TFRC gene-silencing approaches | Block CD71 or deliver cytotoxins via CD71-mediated internalization; may reduce iron uptake or kill CD71-high cells | CX-2029: first-in-human Phase I completed; nonclinical efficacy/safety reported | High tumor uptake, Probody masks reduce off-tumor effects; risks of off-tumor binding in proliferative tissues | [68,78,80,137] |
| Transferrin–drug conjugates (Tf–DCs) | Tf–doxorubicin conjugates; TfR-targeted peptide–doxorubicin conjugates | Use endogenous transferrin/CD71 pathway to ferry chemotherapeutics into tumor cells | preclinical and early translational efforts | Leverages physiologic iron pathway; potential competition with endogenous transferrin, heterogeneity of CD71 expression | [69,70,71,77] |
| Iron chelators/ferroptosis-linked strategies | Deferasirox; deferoxamine | Deprive cells of bioavailable iron and/or tilt cells toward ferroptotic vulnerability | Repurposing and combination strategies explored | Conceptually tumor-agnostic; systemic iron depletion risks (anemia, off-target effects) | [72,73] |
| TfR-targeted nanoparticles/liposomes | Tf- liposomes; TfR ligands; H-ferritin nanocarriers | CD71-mediated endocytosis concentrates payloads in tumors and/or across barriers | Preclinical/early clinical feasibility reported for several platforms | Selective uptake; manufacturing, off-tumor uptake | [74,75,76,138] |
| Peptide/aptamer binders to CD71 | Short TfR-targeting peptides | Compact ligands for targeting and internalization via CD71 | Preclinical reports with in vitro efficacy | Small ligands; shorter half-life or weaker affinity than mAbs | [77] |
| CD71 shuttles for brain delivery (BBB crossing) | Bispecific anti-TfR1 ‘shuttles’; TfR-guided carriers | Exploit endothelial CD71 at the BBB | Neurodegeneration drug delivery | CNS access; risk of peripheral sink/anemia | [125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markopoulos, G.S.; Simos, Y.V.; Tsamis, K.I.; Gartzonika, K.; Peschos, D.; Lakkas, L. Beyond Iron: The Roles of CD71 in the Pathophysiology of Cancer—A Comprehensive Review. J. Clin. Med. 2025, 14, 8265. https://doi.org/10.3390/jcm14238265
Markopoulos GS, Simos YV, Tsamis KI, Gartzonika K, Peschos D, Lakkas L. Beyond Iron: The Roles of CD71 in the Pathophysiology of Cancer—A Comprehensive Review. Journal of Clinical Medicine. 2025; 14(23):8265. https://doi.org/10.3390/jcm14238265
Chicago/Turabian StyleMarkopoulos, Georgios S., Yannis V. Simos, Konstantinos I. Tsamis, Konstantina Gartzonika, Dimitrios Peschos, and Lampros Lakkas. 2025. "Beyond Iron: The Roles of CD71 in the Pathophysiology of Cancer—A Comprehensive Review" Journal of Clinical Medicine 14, no. 23: 8265. https://doi.org/10.3390/jcm14238265
APA StyleMarkopoulos, G. S., Simos, Y. V., Tsamis, K. I., Gartzonika, K., Peschos, D., & Lakkas, L. (2025). Beyond Iron: The Roles of CD71 in the Pathophysiology of Cancer—A Comprehensive Review. Journal of Clinical Medicine, 14(23), 8265. https://doi.org/10.3390/jcm14238265







