To Breathe or Not to Breathe: Spontaneous Ventilation During Thoracic Surgery in High-Risk COPD Patients—A Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
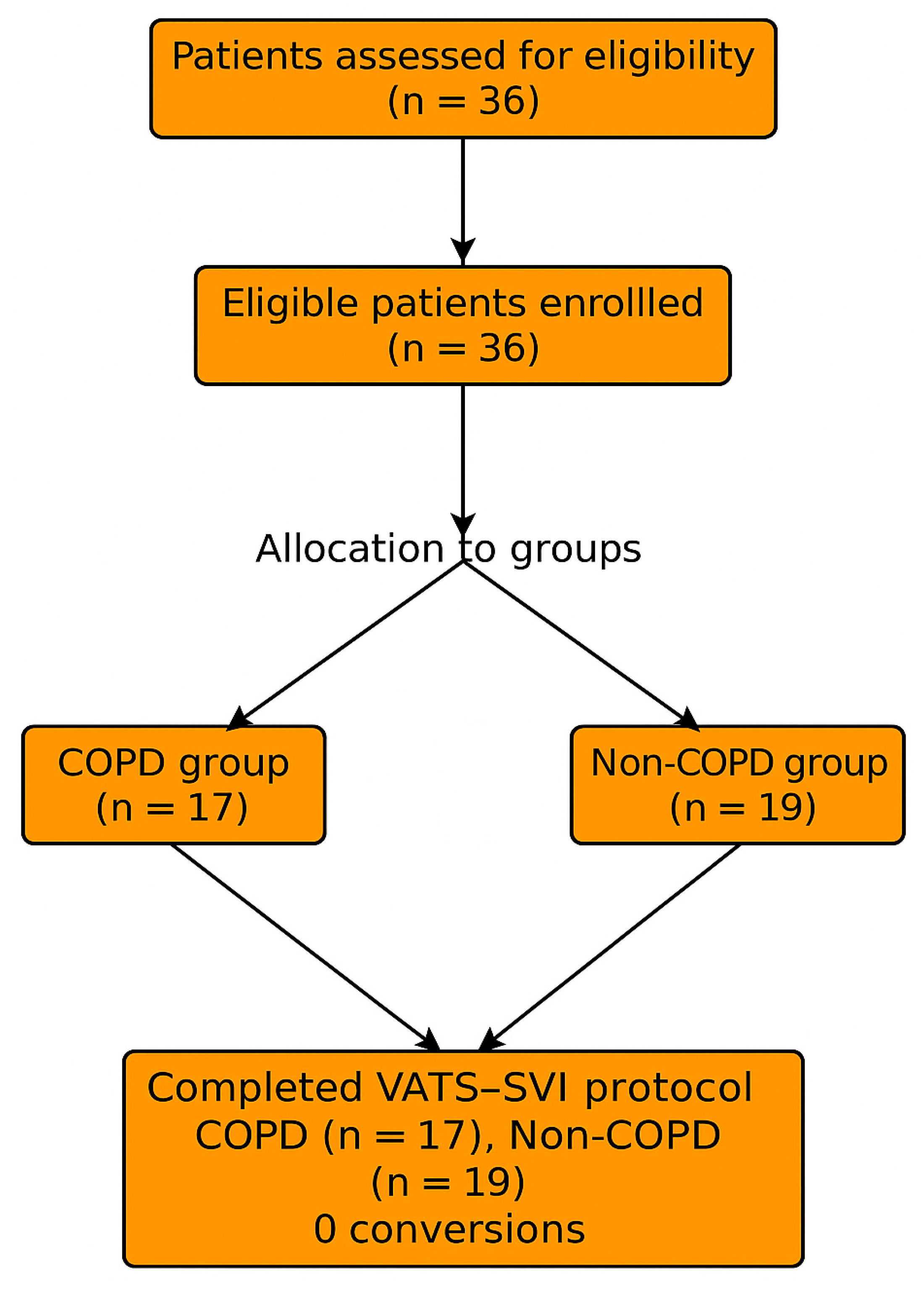
2.1. Study Design and Patient Selection
- Age ≥ 18 years
- ASA physical status I–III
- Lung tumor ≤ 7 cm with N0 or N1 stage (ESTS criteria)
- Feasible for VATS lobectomy with double-lumen intubation and selective one-lung ventilation
- Locally advanced lung cancer (T4 or N2 disease)
- Prior contralateral lobectomy
- Hemodynamic instability, uncontrolled arrhythmia, or severe hypoxemia (PaO2 < 60 mmHg on room air)
- Morbid obesity (BMI > 35 kg/m2)
- Severe pulmonary hypertension (systolic pulmonary artery pressure > 50 mmHg)
- ASA physical status ≥ IV
2.2. Anesthetic Management
2.3. Arterial Blood Gas Sampling
- T1: After radial arterial catheter insertion before induction, on room air (FiO2 = 0.21)
- T2: 15 min after initiation of one-lung spontaneous ventilation and completion of vagal block
- T3: 15 min after completion of lung resection while still under spontaneous ventilation
- T4: 30 min after arrival in the PACU, with supplemental oxygen (FiO2 = 0.5)
2.4. Surgical Technique
3. Results
3.1. Baseline Characteristics
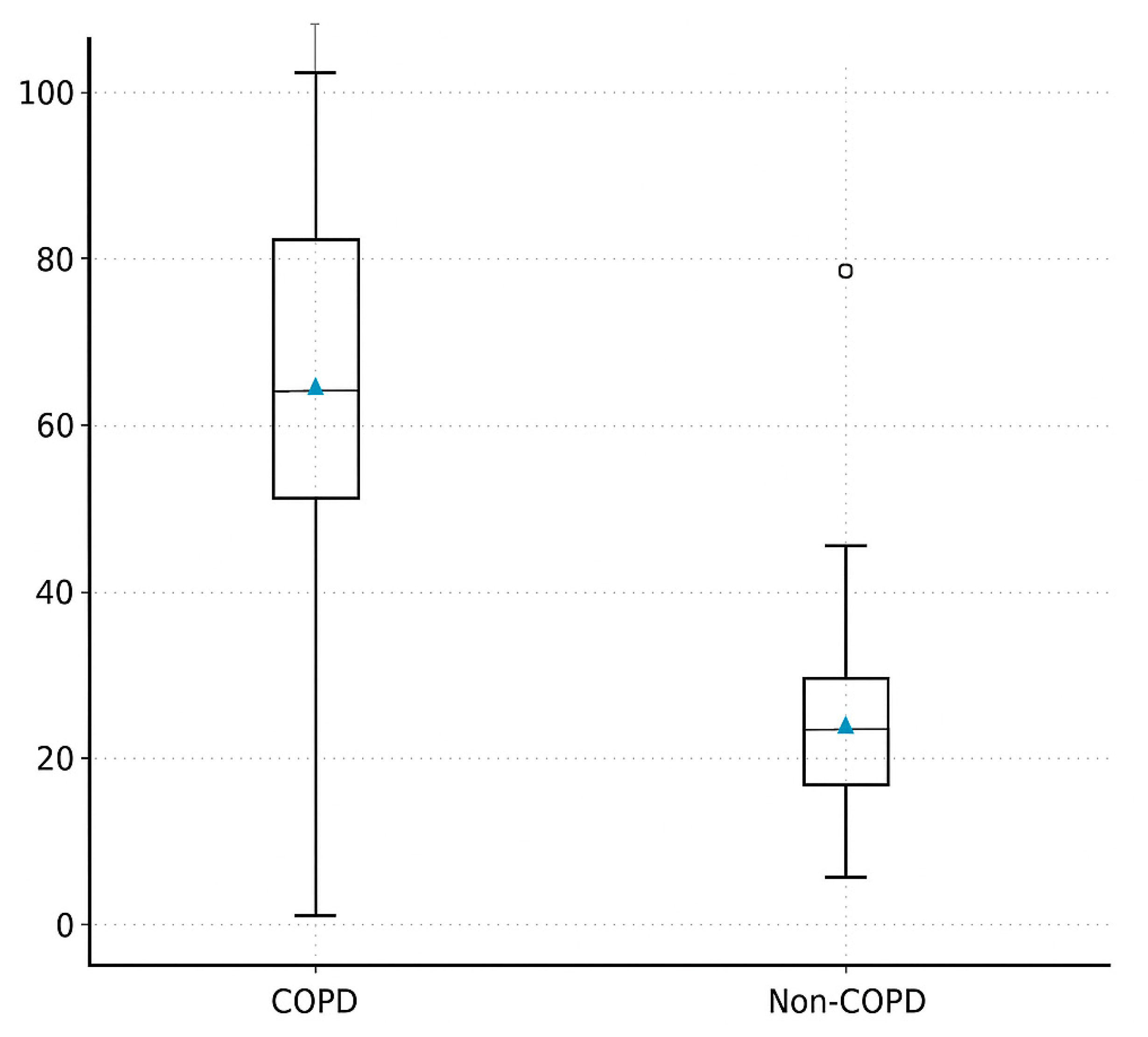
3.2. Primary Outcome Spontaneous Ventilation Fraction (SVI%)
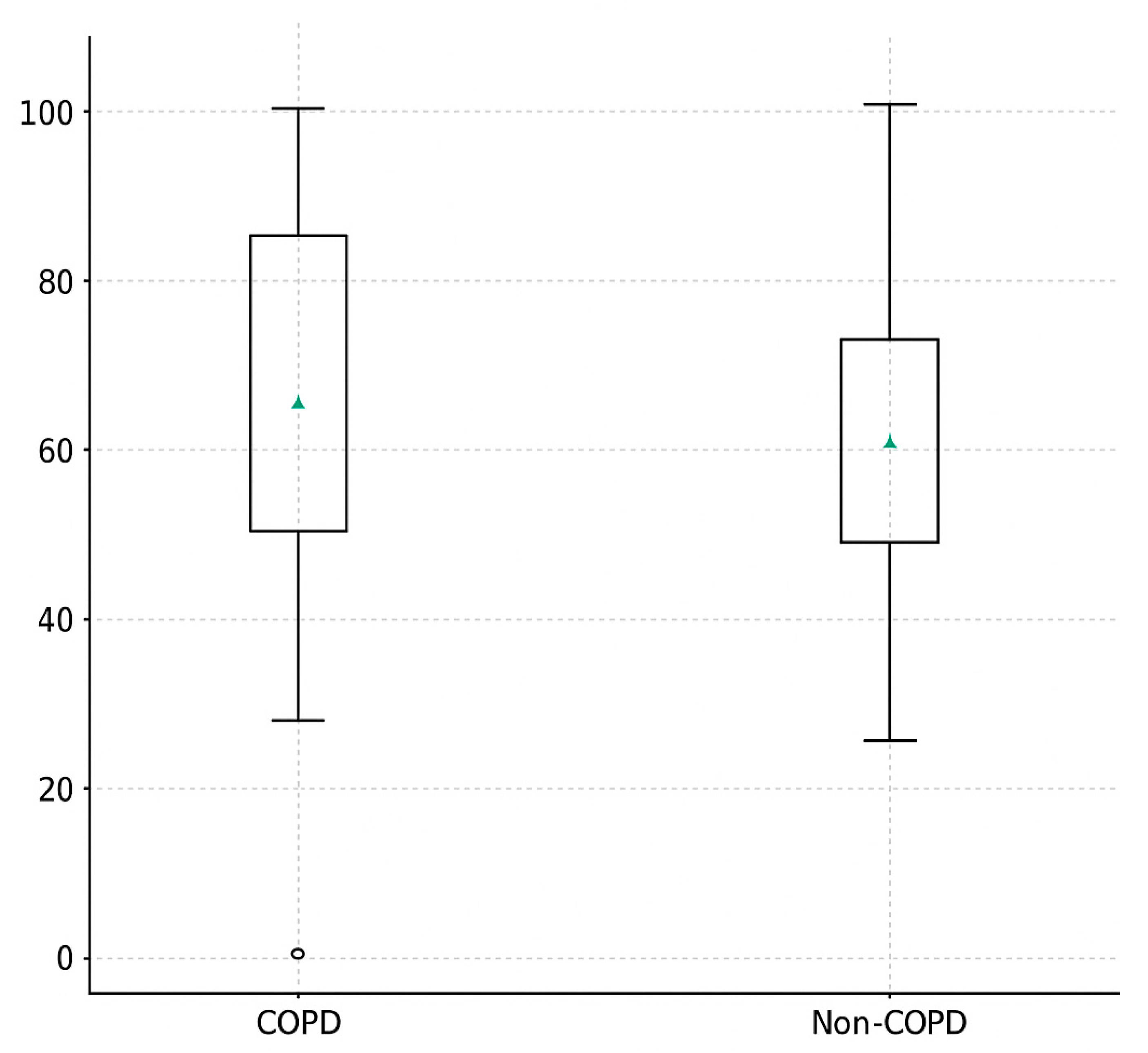
3.3. Duration of Spontaneous Ventilation (Minutes)
3.4. Intraoperative Physiology and Gas Exchange
3.5. Postoperative Outcomes
4. Discussion: Main Findings + Feasibility
4.1. Feasibility and Safety in the Context of Existing Literature
4.2. Physiological Interpretation—Hypercapnia Tolerance and Adaptive Mechanisms
4.3. Protective and Minimally Invasive Ventilation Strategy
4.4. Clinical Implications and Future Directions
4.5. Study Limitations
4.6. Summary of Key Insights
- SVI is feasible and safe in COPD patients during VATS lobectomy.
- COPD patients maintain spontaneous breathing significantly longer, likely due to adaptive hypercapnia tolerance.
- SVI aligns with the concept of lung-protective ventilation and may reduce ventilator-induced lung injury.
- Further large-scale, randomized studies are needed to confirm these findings and standardize patient selection criteria and intraoperative SVI thresholds.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pompeo, E.; Mineo, D.; Rogliani, P.; Sabato, A.F.; Mineo, T.C. Feasibility and Results of Awake Thoracoscopic Resection of Solitary Pulmonary Nodules. Ann. Thorac. Surg. 2004, 78, 1761–1768. [Google Scholar] [CrossRef] [PubMed]
- Lantos, J.; Németh, T.; Barta, Z.; Szabó, Z.; Paróczai, D.; Varga, E.; Hartmann, P. Pathophysiological Advantages of Spontaneous Ventilation. Front. Surg. 2022, 9, 822560. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kiss, G.; Castillo, M. Nonintubated Anesthesia in Thoracic Surgery: General Issues. Ann. Transl. Med. 2015, 3, 110. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Solli, P.; Brandolini, J.; Bertolaccini, L. Tubeless Thoracic Surgery: Ready for Prime Time? J. Thorac. Dis. 2019, 11, 652–656. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, J.S.; Cheng, Y.J.; Hung, M.H.; Tseng, Y.D.; Chen, K.C.; Lee, Y.C. Nonintubated Thoracoscopic Lobectomy for Lung Cancer. Ann. Surg. 2011, 254, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Chen, J.S.; Lin, Y.S.; Tsai, T.M.; Hung, M.H.; Chan, K.C.; Cheng, Y.J. Feasibility and Safety of Nonintubated Thoracoscopic Lobectomy for Geriatric Lung Cancer Patients. Ann. Thorac. Surg. 2013, 95, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Lohser, J.; Slinger, P. Lung Injury After One-Lung Ventilation: A Review of the Pathophysiologic Mechanisms Affecting the Ventilated and the Collapsed Lung. Anesth. Analg. 2015, 121, 302–318. [Google Scholar] [CrossRef] [PubMed]
- Kiss, G.; Castillo, M. Non-Intubated Anesthesia in Thoracic Surgery—Technical Issues. Ann. Transl. Med. 2015, 3, 109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pompeo, E.; Mineo, T.C. Awake Operative Videothoracoscopic Pulmonary Resections. Thorac. Surg. Clin. 2008, 18, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Kozian, A.; Schilling, T.; Schütze, H.; Senturk, M.; Hachenberg, T.; Hedenstierna, G. Ventilatory Protective Strategies during Thoracic Surgery: Effects of Alveolar Recruitment Maneuver and Low-Tidal Volume Ventilation on Lung Density Distribution. Anesthesiology 2011, 114, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rivas, D.; Bonome, C.; Fieira, E.; Aymerich, H.; Fernandez, R.; Delgado, M.; Mendez, L.; de la Torre, M. Non-Intubated Video-Assisted Thoracoscopic Lung Resections: The Future of Thoracic Surgery? Eur. J. Cardiothorac. Surg. 2016, 49, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Sunaga, H.; Blasberg, J.D.; Heerdt, P.M. Anesthesia for Nonintubated Video-Assisted Thoracic Surgery. Curr. Opin. Anaesthesiol. 2017, 30, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Szabo, Z.; Fabo, C.; Oszlanyi, A.; Hawchar, F.; Géczi, T.; Lantos, J.; Furák, J. Anesthetic (R)evolution from the Conventional Concept to the Minimally Invasive Techniques in Thoracic Surgery—Narrative Review. J. Thorac. Dis. 2022, 14, 3045–3060. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Langiano, N.; Fiorelli, S.; Deana, C.; Baroselli, A.; Bignami, E.G.; Matellon, C.; Pompei, L.; Tornaghi, A.; Piccioni, F.; Orsetti, R.; et al. Airway Management in Anesthesia for Thoracic Surgery: A “Real Life” Observational Study. J. Thorac. Dis. 2019, 11, 3257–3269. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lan, L.; Jiang, L.; Duan, C.; Lu, W.; Zhang, C.; Cen, Y.; He, J. A Risk Score for Predicting Postoperative Complications in Non-Intubated Thoracic Surgery. J. Thorac. Dis. 2021, 13, 3960–3968. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Irons, J.F.; Martinez, G. Anaesthetic Considerations for Non-Intubated Thoracic Surgery. J. Vis. Surg. 2016, 2, 61. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fabo, C.; Oszlanyi, A.; Lantos, J.; Rarosi, F.; Horvath, T.; Barta, Z.; Nemeth, T.; Szabo, Z. Non-Intubated Thoracoscopic Surgery—Tips and Tricks from Anesthesiological Aspects: A Mini Review. Front. Surg. 2022, 8, 818456. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hung, W.T.; Hung, M.H.; Wang, M.L.; Cheng, Y.J.; Hsu, H.H.; Chen, J.S. Nonintubated Thoracoscopic Surgery for Lung Tumor: Seven Years’ Experience with 1,025 Patients. Ann. Thorac. Surg. 2019, 107, 1607–1612. [Google Scholar] [CrossRef] [PubMed]
- Anile, M.; Vannucci, J.; Ferrante, F.; Bruno, K.; De Paolo, D.; Bassi, M.; Pugliese, F.; Venuta, F.; NIVATS Interest Group. Non-Intubated Thoracic Surgery: Standpoints and Perspectives. Front. Surg. 2022, 9, 937633. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shao, G.Q.; Pang, D.Z.; Zhang, J.T.; Wang, H.X.; Liuru, T.Y.; Liu, Z.H.; Liang, Y.N.; Liu, J.S. Spontaneous Ventilation Anesthesia Combined with Uniportal and Tubeless Thoracoscopic Sympathectomy in Selected Patients with Primary Palmar Hyperhidrosis. J. Cardiothorac. Surg. 2022, 17, 177. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, J.; Liang, H.; Cui, F.; Liu, H.; Zhu, C.; Liang, W.; He, J.; International Tubeless-Video-Assisted Thoracoscopic Surgery Collaboration. Spontaneous versus Mechanical Ventilation during Video-Assisted Thoracoscopic Surgery for Spontaneous Pneumothorax: A Randomized Trial. J. Thorac. Cardiovasc. Surg. 2022, 163, 1702–1714.e7. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Zhang, Z.; Hu, T.; Qiao, L. Advances in the Use of Non-Intubated Spontaneous-Ventilation Video-Assisted Thoracoscopic Surgery. Front. Surg. 2025, 12, 1584017. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akça, O.; Doufas, A.G.; Morioka, N.; Iscoe, S.; Fisher, J.; Sessler, D.I. Hypercapnia Improves Tissue Oxygenation. Anesthesiology 2002, 97, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liang, H.; Xu, K.; Yang, C.; Liang, L.; Dong, Q.; Yang, H.; Liu, H.; Li, Y.; Patolia, S.; et al. The Strategy of Non-Intubated Spontaneous Ventilation Anesthesia for Upper Tracheal Surgery: A Retrospective Case Series Study. Transl. Lung Cancer Res. 2022, 11, 880–889. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakanishi, R.; Yasuda, M. Awake Thoracoscopic Surgery under Epidural Anesthesia: Is It Really Safe? Chin. J. Cancer Res. 2014, 26, 368–370. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koh, L.Y.; Hwang, N.C. Anesthesia for Nonintubated Video-Assisted Thoracoscopic Surgery. J. Cardiothorac. Vasc. Anesth. 2023, 37, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Nin, N.; Angulo, M.; Briva, A. Effects of Hypercapnia in Acute Respiratory Distress Syndrome. Ann. Transl. Med. 2018, 6, 37. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ni Chonghaile, M.; Higgins, B.; Laffey, J.G. Permissive Hypercapnia: Role in Protective Lung Ventilatory Strategies. Curr. Opin. Crit. Care 2005, 11, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Neto, A.S.; Hemmes, S.N.; Barbas, C.S.; Beiderlinden, M.; Fernandez-Bustamante, A.; Futier, E.; Gajic, O.; El-Tahan, M.R.; Ghamdi, A.A.; Günay, E.; et al. Association between Driving Pressure and Development of Postoperative Pulmonary Complications in Patients Undergoing Mechanical Ventilation for General Anaesthesia: A Meta-Analysis of Individual Patient Data. Lancet Respir. Med. 2016, 4, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Curley, G.F.; Laffey, J.G.; Zhang, H.; Slutsky, A.S. Biotrauma and Ventilator-Induced Lung Injury: Clinical Implications. Chest 2016, 150, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Chen, H.G.; Wu, W.B.; Li, X.J.; Wu, Y.H.; Xu, J.N.; Jia, Y.B.; Zhang, J. Non-Intubated Video-Assisted Thoracoscopic Surgery vs. Intubated Video-Assisted Thoracoscopic Surgery for Thoracic Disease: A Systematic Review and Meta-Analysis of 1,684 Cases. J. Thorac. Dis. 2019, 11, 3556–3568. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Spinelli, E.; Mauri, T.; Beitler, J.R.; Pesenti, A.; Brodie, D. Respiratory Drive in the Acute Respiratory Distress Syndrome: Pathophysiology, Monitoring, and Therapeutic Interventions. Intensive Care Med. 2020, 46, 606–618. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Janík, M.; Juhos, P.; Lučenič, M.; Tarabová, K. Non-Intubated Thoracoscopic Surgery—Pros and Cons. Front. Surg. 2021, 8, 801718. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agar, M.; Gulcek, I.; Kalkan, M.; Ulutas, H.; Celık, M.R.; Aksu, A.; Aydın, S.; Cakmak, M. Current New Approach in Thoracoscopic Surgery: Non-Intubated Uniportal Video-Assisted Thoracoscopic Surgery (NI-UniVATS). Medicina 2025, 61, 641. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deng, H.Y.; Zhu, Z.J.; Wang, Y.C.; Wang, W.P.; Ni, P.Z.; Chen, L.Q. Non-Intubated Video-Assisted Thoracoscopic Surgery under Loco-Regional Anaesthesia for Thoracic Surgery: A Meta-Analysis. Interact. Cardiovasc. Thorac. Surg. 2016, 23, 31–40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Slinger, P.; Campos, J.H. Anesthesia for Thoracic Surgery. In Miller’s Anesthesia, 9th ed.; Gropper, M.A., Ed.; Elsevier: Philadelphia, PA, USA, 2020; pp. 1942–1988. [Google Scholar]
- Mudannayake, R.; Martinez, G.; Bello, I.; Gimenez-Milà, M. Non-Intubated Thoracic Surgery: A Physiological Approach. Arch. Bronconeumol. 2023, 59, 699–701. [Google Scholar] [CrossRef] [PubMed]
- Pompeo, E.; Tacconi, F.; Mineo, T.C. Comparative Results of Non-Resectional Lung Volume Reduction Performed by Awake or Non-Awake Anesthesia. Eur. J. Cardiothorac. Surg. 2011, 39, e51–e58. [Google Scholar] [CrossRef] [PubMed]
- Pompeo, E. From Awake to Minimalist Spontaneous Ventilation Thoracoscopic Lung Surgery: An Ongoing Journey. J. Clin. Med. 2025, 14, 2475. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, B.; Ge, S. Nonintubated Anesthesia for Thoracic Surgery. J. Thorac. Dis. 2014, 6, 1868–1874. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Magouliotis, D.E.; Karamolegkou, A.P.; Zotos, P.A.; Minervini, F.; Cioffi, U.; Scarci, M. Is Less More? A Meta-Analysis of Non-Intubated Versus Intubated VATS for Anatomic Resections in Non-Small Cell Lung Cancer. J. Clin. Med. 2025, 14, 6731. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Furák, J.; Zsoldos, P.; Lantos, J.; Lantos, J.; Rárosi, F.; Szűcs, E.; Fabó, C.; Kecskés, G. Impact of Spontaneous Ventilation with Intubation on Perioperative Results in Uniportal VATS Lobectomy Compared to General Anaesthesia Using a Double-Lumen Tube. J. Thorac. Dis. 2025, 17, 774–783. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eldawlatly, A.; Turkistani, A.; Shelley, B.; El-Tahan, M.; Macfie, A.; Kinsella, J.; Thoracic-Anaesthesia Group Collaborators. Anesthesia for Thoracic Surgery: A Survey of Middle Eastern Practice. Saudi J. Anaesth. 2012, 6, 192–196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pathonsamit, C.; Tantraworasin, A.; Poopipatpab, S.; Laohathai, S. Perioperative Outcomes of Non-Intubated versus Intubated Video-Assisted Thoracoscopic Surgery in Different Thoracic Procedures: A Propensity Score-Matched Analysis. BMC Anesthesiol. 2022, 22, 154. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pompeo, E. State of the Art and Perspectives in Non-Intubated Thoracic Surgery. Ann. Transl. Med. 2014, 2, 106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bluth, T.; Serpa Neto, A.; Schultz, M.J.; Pelosi, P.; Gama de Abreu, M.; PROBESE Collaborative Group; Bobek, I.; Canet, J.C.; Cinnella, G.; de Baerdemaeker, L.; et al. Effect of Intraoperative High Positive End-Expiratory Pressure (PEEP) with Recruitment Maneuvers vs. Low PEEP on Postoperative Pulmonary Complications in Obese Patients: A Randomized Clinical Trial. JAMA 2019, 321, 2292–2305. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Donnell, D.E.; Ora, J.; Webb, K.A.; Laveneziana, P.; Jensen, D. Mechanisms of Activity-Related Dyspnea in Pulmonary Diseases. Respir. Physiol. Neurobiol. 2009, 167, 116–132. [Google Scholar] [CrossRef] [PubMed]
| Parameter | COPD (n = 17) | Non-COPD (n = 19) | p Value |
|---|---|---|---|
| Age (years) | 68.4 ± 6.9 | 67.8 ± 7.1 | 0.78 |
| Sex (M/F) | 12/5 | 13/6 | 0.84 |
| BMI (kg/m2) | 27.1 ± 4.6 | 26.3 ± 4.2 | 0.56 |
| ASA II/III (%) | 47/53 | 53/47 | 0.64 |
| Smoking history (%) | 88 | 63 | 0.09 |
| FEV1 (% pred) | 61 ± 9 | 83 ± 8 | <0.001 *** |
| FVC (% pred) | 72 ± 10 | 95 ± 7 | <0.001 *** |
| FEV1/FVC (%) | 53.8 [47.5–59.9] | 82.4 [78.5–85.2] | <0.001 *** |
| Comorbidity Category | Variable | COPD (n = 17) | Non-COPD (n = 19) | p Value |
|---|---|---|---|---|
| Cardiovascular | Hypertension | 13 (76%) | 12 (63%) | 0.42 |
| Coronary artery disease | 8 (47%) | 6 (32%) | 0.36 | |
| Arrhythmia (AF or other) | 4 (24%) | 3 (16%) | 0.55 | |
| Heart failure (NYHA II–III) | 3 (18%) | 1 (5%) | 0.23 | |
| Metabolic | Diabetes mellitus type II | 5 (29%) | 4 (21%) | 0.57 |
| Dyslipidemia | 6 (35%) | 5 (26%) | 0.53 | |
| Obesity (BMI ≥ 30 kg/m2) | 3 (18%) | 2 (11%) | 0.61 | |
| Pulmonary | Asthma or chronic bronchitis | 5 (29%) | 2 (11%) | 0.17 |
| Pulmonary hypertension | 3 (18%) | 0 (0%) | 0.06 | |
| History of prior thoracic surgery | 2 (12%) | 1 (5%) | 0.44 | |
| Renal/Hepatic | Chronic kidney disease | 2 (12%) | 1 (5%) | 0.44 |
| Chronic liver disease | 1 (6%) | 0 (0%) | 0.29 | |
| Other | Smoking (current/former) | 15 (88%) | 12 (63%) | 0.09 |
| ASA physical status ≥ III | 9 (53%) | 9 (47%) | 0.64 |
| Variable | COPD | Non-COPD | p Value |
|---|---|---|---|
| Spontaneous ventilation time (min) | 82 ± 14 | 58 ± 16 | <0.001 *** |
| Spontaneous ventilation fraction (%) | 80 [70–90] | 60 [45–80] | 0.11 |
| MAP (mmHg) | 82 ± 9 | 84 ± 8 | 0.48 |
| HR (bpm) | 76 ± 10 | 78 ± 12 | 0.52 |
| ETCO2 (mmHg) | 49 [46–53] | 44 [41–47] | 0.06 |
| PaO2 (mmHg) | 108 ± 15 | 111 ± 14 | 0.42 |
| Conversion to controlled ventilation (%) | 0 | 0 | — |
| Time Point | COPD PaCO2 (mmHg) | Non-COPD PaCO2 | pH COPD | pH Non-COPD |
|---|---|---|---|---|
| T1 (Pre-op) | 44.8 ± 5.2 | 39.6 ± 4.8 | 7.41 ± 0.03 | 7.43 ± 0.03 |
| T2 (During SVI) | 52.6 ± 6.7 | 46.3 ± 5.1 | 7.36 ± 0.04 | 7.39 ± 0.03 |
| T3 (Post-resection) | 55.8 ± 7.2 | 48.7 ± 5.5 | 7.34 ± 0.05 | 7.37 ± 0.04 |
| T4 (PACU) | 47.3 ± 5.9 | 42.5 ± 4.9 | 7.38 ± 0.03 | 7.40 ± 0.04 |
| Outcome | COPD | Non-COPD | p Value |
|---|---|---|---|
| ICU stay (days) | 1.1 ± 0.4 | 1.0 ± 0.5 | 0.48 |
| Total hospital stay (days) | 5.8 ± 1.7 | 5.6 ± 1.5 | 0.72 |
| Postoperative complications (%) | 12% | 11% | 0.67 |
| Reintubation (%) | 0 | 0 | — |
| 30-day mortality (%) | 0 | 0 | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szarvas, M.; Fabo, C.; Demeter, G.; Oszlanyi, A.; Vaida, S.; Furak, J.; Szabo, Z. To Breathe or Not to Breathe: Spontaneous Ventilation During Thoracic Surgery in High-Risk COPD Patients—A Feasibility Study. J. Clin. Med. 2025, 14, 8244. https://doi.org/10.3390/jcm14228244
Szarvas M, Fabo C, Demeter G, Oszlanyi A, Vaida S, Furak J, Szabo Z. To Breathe or Not to Breathe: Spontaneous Ventilation During Thoracic Surgery in High-Risk COPD Patients—A Feasibility Study. Journal of Clinical Medicine. 2025; 14(22):8244. https://doi.org/10.3390/jcm14228244
Chicago/Turabian StyleSzarvas, Matyas, Csongor Fabo, Gabor Demeter, Adam Oszlanyi, Stefan Vaida, Jozsef Furak, and Zsolt Szabo. 2025. "To Breathe or Not to Breathe: Spontaneous Ventilation During Thoracic Surgery in High-Risk COPD Patients—A Feasibility Study" Journal of Clinical Medicine 14, no. 22: 8244. https://doi.org/10.3390/jcm14228244
APA StyleSzarvas, M., Fabo, C., Demeter, G., Oszlanyi, A., Vaida, S., Furak, J., & Szabo, Z. (2025). To Breathe or Not to Breathe: Spontaneous Ventilation During Thoracic Surgery in High-Risk COPD Patients—A Feasibility Study. Journal of Clinical Medicine, 14(22), 8244. https://doi.org/10.3390/jcm14228244






