Smoking Amplifies Comorbidity-Associated Risk in Orthopaedic Surgery: A Multiplicative Interaction
Abstract
1. Introduction
2. Methods
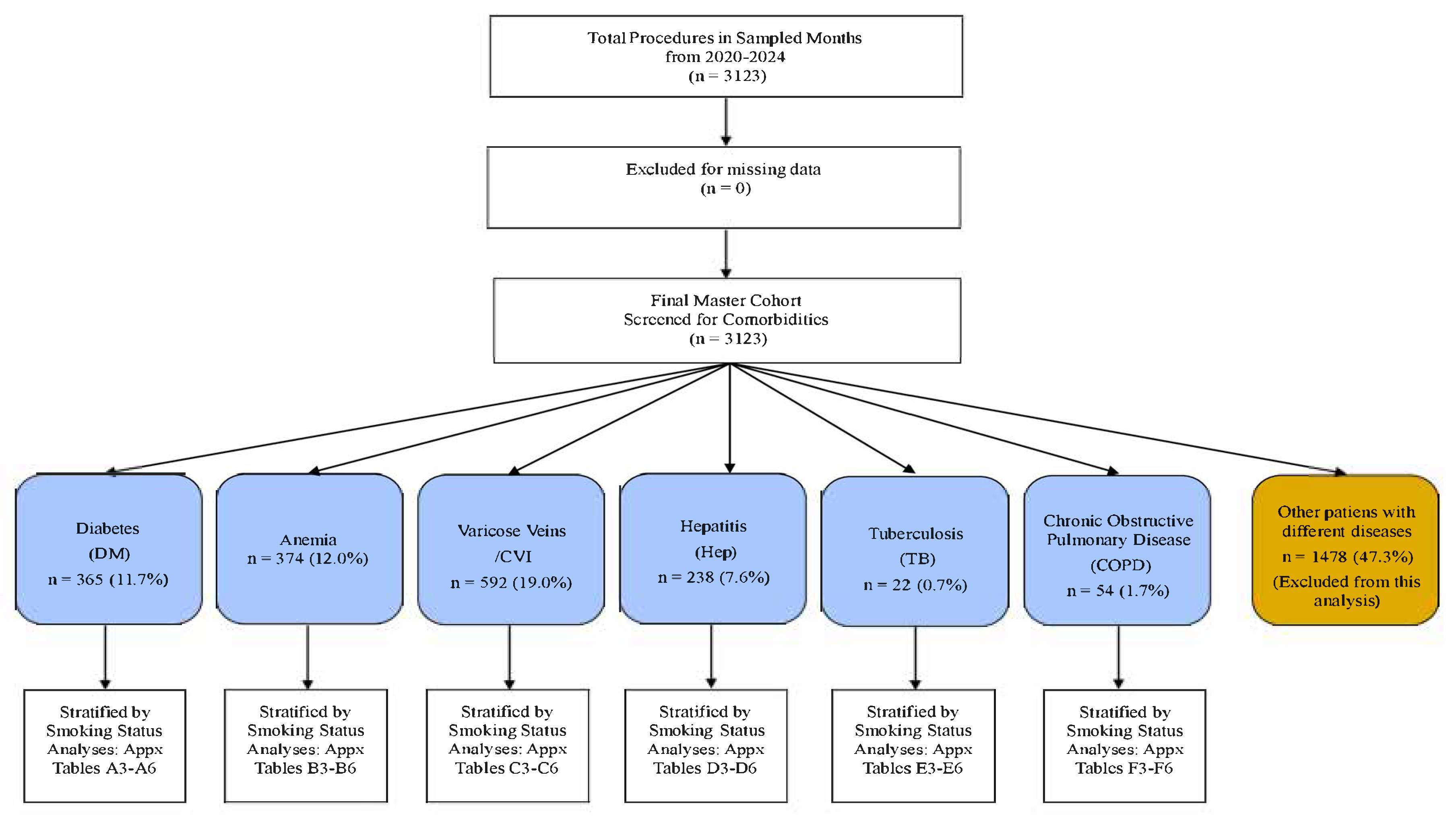
2.1. Study Design and Population
2.2. Patient Stratification and Variables
- Active Smoker: Any documented note of current tobacco use. Data on smoking intensity (e.g., cigarettes per day, pack-years) were not consistently available in the clinical records and were therefore not included in this analysis. A 6-month cutoff was not applied, as this level of temporal detail was not consistently recorded. The classification relied on the clinician’s global assessment (e.g., “smoker,” “active smoker”).
- Former Smoker: A documented history of smoking but note of abstinence (e.g., “former smoker,” “quit smoking”). Specific duration of abstinence or pack-year history were not available.
- Non-Smoker: No documented history of tobacco use.
2.3. Comorbidity Definitions Were Rigorously Applied
- Diabetes Mellitus (DM): n = 365; documented diagnosis or use of hypoglycaemic agents.
- Anaemia: n = 374; defined as a preoperative Hb level of <12 g/dL in women or <13 g/dL in men. A preoperative hemoglobin level was part of the standard institutional protocol for surgical clearance. The ‘available data’ refers to the results of this routinely performed test, which was available for the vast majority of patients. Patients were included in the anemia cohort if their recorded pre-op Hb met the criteria.
- Hepatic Dysfunction: n = 238; documented diagnosis of hepatitis, cirrhosis, or steatosis; or ALT/AST >1.5 × upper limit of normal.
- Chronic Venous Disease (CVD): n = 592; documented chronic venous insufficiency, varicose veins, or history of venous ulceration.
- COPD: n = 54; documented diagnosis [16].
- History of TB: n = 22; documented history of treated tuberculosis.
2.4. Orthopaedic Surgical Procedures
- Trauma and Fracture Fixation: Intramedullary nailing, open reduction and internal fixation (ORIF) of fractures (e.g., proximal femur, tibia).
- Arthroplasty: Primary total hip, knee, and shoulder arthroplasty.
- Spinal Surgery: Instrumented fusion.
2.5. Outcome Measures
- Preoperative Readiness: Hb, platelet count, INR, albumin, CRP, ASA score.
- Intraoperative Outcomes: Estimated blood loss, transfusion requirement, and a composite “hostile field” outcome. This composite was defined pragmatically for this study as the occurrence of ≥2 of the following: estimated blood loss EBL >95th percentile for the procedure type, intraoperative transfusion, or a qualitative surgeon note of “friable tissues,” “poor bone quality,” or “persistent oozing” as manually extracted from the operative reports.
- Postoperative Orthopaedic-Specific Outcomes (Primary): The incidence of Periprosthetic Joint Infection (PJI) as primary outcome, diagnosis based on clinical and laboratory criteria per surgeon note and diagnosed according to the 2018 International Consensus Meeting (ICM) criteria [22] as documented in the clinical record. Different outcomes were assessed based on data available from the primary surgical hospitalization and any subsequent inpatient readmissions to our institution. Follow-up data from outpatient clinic visits or non-surgical readmissions were not systematically captured in this retrospective analysis. Therefore, the reported complication rates likely represent a minimum estimate, particularly for later-presenting outcomes. They included Non-union (defined as a lack of radiographic bridging at a minimum of 6 months postoperatively, as described in radiology reports), implant failure, reoperation/revision surgery, and 30-day mortality. The lack of a standardized, long-term follow-up protocol is a limitation, and outcomes are based on the available clinical documentation. Estimated blood loss (EBL), transfusion requirement, and a composite “hostile surgical field” outcome. This composite was defined as the occurrence of two or more of the following objectively measurable or clearly documented events: (1) EBL greater than the 95th percentile for the specific procedure type, (2) requirement for an intraoperative blood transfusion, or (3) a qualitative surgeon note of “friable tissues” or “persistent oozing” that was manually extracted from the operative reports. While the “hostile field” introduces the potential for ascertainment bias, the composite outcome primarily relied on the objective measures of EBL and transfusion.
2.6. Definition of Key Variables
- Thrombocytopenia: Defined as a platelet count of <150 K/µL.
- Hemodynamic Instability: Defined intraoperatively as a systolic blood pressure <90 mmHg or a mean arterial pressure <65 mmHg, or the requirement for a vasopressor bolus or infusion to maintain pressure, as recorded in the anesthesia report.
- Absolute Risk Difference (ARD): The ARD and its 95% confidence interval presented in Table 1 were calculated directly from the raw risk proportions in the exposed and unexposed groups.
2.7. Statistical Analysis
3. Results
3.1. Master Cohort Overview
3.2. Synergistic Impact on Preoperative Physiology
3.3. Intraoperative Challenges: The “Hostile Field”
3.4. Postoperative Orthopaedic-Specific Outcomes
3.5. Trends in Smaller Cohorts (Descriptive Analysis)
4. Discussion
4.1. Pathophysiological Correlation to Orthopaedic Failure
4.2. Clinical Implications: A Call for “Integrated Physiological Prehabilitation”
- The Anemic Smoker: Correction of anemia (Hb > 10 g/dL) with IV iron, EPO, or transfusion is strongly recommended before considering surgery [32].
4.3. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aOR | Adjusted Odds Ratio |
| ALT | Alanine Aminotransferase |
| AP | Attributable Proportion |
| ASA | American Society of Anesthesiologists (Physical Status Classification System) |
| AST | Aspartate Aminotransferase |
| CI | Confidence Interval |
| COPD | Chronic Obstructive Pulmonary Disease |
| CRP | C-Reactive Protein |
| CVD | Chronic Venous Disease |
| DM | Diabetes Mellitus |
| EBL | Estimated Blood Loss |
| EPO | Erythropoietin |
| Hb | Hemoglobin |
| HbA1c | Hemoglobin A1c |
| INR | International Normalized Ratio |
| IV | Intravenous |
| PJI | Periprosthetic Joint Infection |
| RERI | Relative Excess Risk due to Interaction |
| SD | Standard Deviation |
| TB | Tuberculosis |
References
- Einhorn, T.A. The cell and molecular biology of fracture healing. Clin. Orthop. Relat. Res. 1998, 355, S7–S21. [Google Scholar] [CrossRef]
- Gristina, A.G. Biomaterial-centered infection: Microbial adhesion versus tissue integration. Science 1987, 237, 1588–1595. [Google Scholar] [CrossRef]
- Jupiter, J.B.; Ring, D.C.; Rosen, H. The complications and difficulties of management of nonunion in the severely obese. J. Orthop. Trauma 1995, 9, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Frisch, N.B.; Courtney, P.M.; Della Valle, C.J. Perioperative smoking cessation in orthopedic surgery: A review of current evidence. JBJS Rev. 2015, 3, e1. [Google Scholar]
- Snell-Bergeon, J.K.; Wadwa, R.P. Hypoglycemia, diabetes, and cardiovascular disease. Diabetes Technol. Ther. 2012, 14 (Suppl. S1), S51–S58. [Google Scholar] [CrossRef]
- Halm, E.A.; Wang, J.J.; Boockvar, K.; Penrod, J.; Silberzweig, S.B.; Magaziner, J.; Koval, K.J.; Siu, A.L. The effect of perioperative anemia on clinical and functional outcomes in patients with hip fracture. J. Orthop. Trauma. 2004, 18, 369–374. [Google Scholar] [CrossRef]
- US Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General; US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health: Atlanta, GA, USA, 2014.
- World Health Organization. WHO Global Report on Trends in Prevalence of Tobacco Use 2000–2030, 4th ed.; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Sørensen, L.T. Wound healing and infection in surgery: The pathophysiological impact of smoking, smoking cessation, and nicotine replacement therapy: A systematic review. Ann. Surg. 2012, 255, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.M.; Villebro, N.; Pedersen, T.; Tønnesen, H. Effect of preoperative smoking intervention on postoperative complications: A randomised clinical trial. Lancet 2002, 359, 114–117. [Google Scholar] [CrossRef]
- Sørensen, L.T. Wound healing and infection in surgery: The clinical impact of smoking and smoking cessation: A systematic review and meta-analysis. Arch. Surg. 2012, 147, 373–383. [Google Scholar] [CrossRef]
- Knops, S.P.; Tonnesen, H.; Møller, A.M. The effect of preoperative smoking cessation and smoking dose on postoperative complications: A systematic review and meta-analysis. J. Clin. Anesth. 2023, 90, 111222. [Google Scholar]
- Martin, E.T.; Kaye, K.S.; Knott, C.; Nguyen, H.; Santarossa, M.; Evans, R.; Bertran, E.; Jaber, L. Diabetes and Risk of Surgical Site Infection: A Systematic Review and Meta-analysis. Infect. Control Hosp. Epidemiol. 2016, 37, 88–99. [Google Scholar] [CrossRef]
- GBD 2013 Risk Factors Collaborators; Forouzanfar, M.H.; Alexander, L.; Anderson, H.R.; Bachman, V.F.; Biryukov, S.; Brauer, M.; Burnett, R.; Casey, D.; Coates, M.; et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 2287–2323. [Google Scholar] [CrossRef]
- GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1659–1724. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2024 Report). 2024. Available online: https://goldcopd.org/2024-gold-report (accessed on 12 November 2025).
- Arcavi, L.; Benowitz, N.L. Cigarette smoking and infection. Arch. Intern. Med. 2004, 164, 2206–2216. [Google Scholar] [CrossRef] [PubMed]
- Delamaire, M.; Maugendre, D.; Moreno, M.; Le Goff, M.C.; Allannic, H.; Genetet, B. Impaired leucocyte functions in diabetic patients. Diabet. Med. 1997, 14, 29–34. [Google Scholar] [CrossRef]
- Trevisan, C.; Alessi, A.; Girotti, G.; Zanforlini, B.M.; Bertocco, A.; Mazzochin, M.; Zoccarato, F.; Piovesan, F.; Dianin, M.; Giannini, S.; et al. The Impact of Smoking on Bone Metabolism, Bone Mineral Density and Vertebral Fractures in Postmenopausal Women. J. Clin. Densitom. 2020, 23, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Tande, A.J.; Patel, R. Prosthetic joint infection. Clin. Microbiol. Rev. 2014, 27, 302–345. [Google Scholar] [CrossRef]
- Lanoix, J.P.; Gaudry, S.; Flicoteaux, R.; Ruimy, R.; Wolff, M. Tuberculosis in the intensive care unit: A descriptive analysis in a low-burden country. Int. J. Tuberc. Lung Dis. 2014, 18, 581–587. [Google Scholar] [CrossRef]
- Parvizi, J.; Tan, T.L.; Goswami, K.; Higuera, C.; Della Valle, C.; Chen, A.F.; Shohat, N. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J. Arthroplast. 2018, 33, 1309–1314.e2. [Google Scholar] [CrossRef]
- Knol, M.J.; van der Tweel, I.; Grobbee, D.E.; Numans, M.E.; Geerlings, M.I. Estimating interaction on an additive scale between continuous determinants in a logistic regression model. Int. J. Epidemiol. 2007, 36, 1111–1118. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, M.; Bi, Y.; Xu, M.; Xu, Y.; Li, M.; Wang, T.; Huang, F.; Xu, B.; Zhang, J.; et al. Active smoking, passive smoking, and risk of nonalcoholic fatty liver disease (NAFLD): A population-based study in China. J. Epidemiol. 2013, 23, 115–121. [Google Scholar] [CrossRef]
- Arnson, Y.; Shoenfeld, Y.; Amital, H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J. Autoimmun. 2010, 34, J258–J265. [Google Scholar] [CrossRef]
- Raikin, S.M.; Landsman, J.C.; Alexander, V.A.; Froimson, M.I.; Plaxton, N.A. Effect of nicotine on the rate and strength of long bone fracture healing. Clin. Orthop. Relat. Res. 1998, 353, 231–237. [Google Scholar] [CrossRef]
- Kayal, R.A.; Tsatsas, D.; Bauer, M.A.; Allen, B.; Al-Sebaei, M.O.; Kakar, S.; Leone, C.W.; Morgan, E.F.; Gerstenfeld, L.C.; Einhorn, T.A.; et al. Diminished bone formation during diabetic fracture healing is related to the premature resorption of cartilage associated with increased osteoclast activity. J. Bone Miner. Res. 2007, 22, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Mina, D.S.; Clarke, H.; Ritvo, P.; Leung, Y.; Matthew, A.; Katz, J.; Trachtenberg, J.; Alibhai, S. Effect of total-body prehabilitation on postoperative outcomes: A systematic review and meta-analysis. Physiotherapy 2018, 100, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Qaseem, A.; Snow, V.; Fitterman, N.; Hornbake, E.R.; Lawrence, V.A.; Smetana, G.W.; Weiss, K.; Owens, D.K.; Aronson, M.; Barry, P.; et al. Risk assessment for and strategies to reduce perioperative pulmonary complications for patients undergoing noncardiothoracic surgery: A guideline from the American College of Physicians. Ann. Intern. Med. 2006, 144, 575–580. [Google Scholar] [CrossRef]
- Thomsen, T.; Villebro, N.; Møller, A.M. Interventions for preoperative smoking cessation. Cochrane Database Syst. Rev. 2014, 2014, CD002294. [Google Scholar] [CrossRef]
- American Diabetes Association. 16. Diabetes Care in the Hospital: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47 (Suppl. S1), S295–S306. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Acheson, A.G.; Auerbach, M.; Besser, M.; Habler, O.; Kehlet, H.; Liumbruno, G.M.; Lasocki, S.; Meybohm, P.; Baikady, R.R.; et al. International consensus statement on the peri-operative management of anaemia and iron deficiency. Anaesthesia 2017, 72, 233–247. [Google Scholar] [CrossRef]
- Tripodi, A.; Mannucci, P.M. The coagulopathy of chronic liver disease. N. Engl. J. Med. 2011, 365, 147–156. [Google Scholar] [CrossRef]
- O’Leary, J.G.; Greenberg, C.S.; Patton, H.M.; Caldwell, S.H. AGA Clinical Practice Update: Coagulation in Cirrhosis. Gastroenterology 2019, 157, 34–43.e1. [Google Scholar] [CrossRef] [PubMed]
- American Society of Anesthesiologists Task Force on Perioperative Blood Management. Practice Guidelines for Perioperative Blood Management: An Updated Report by the American Society of Anesthesiologists Task Force on Perioperative Blood Management. Anesthesiology 2015, 122, 241–275. [Google Scholar] [CrossRef] [PubMed]
| Comorbidity (Cohort Size) | Primary Outcome | Smoking Status | n/N (%) | Adjusted Odds Ratio (aOR) (95% CI) † | p-Value | Absolute Risk Difference, % (ARD, 95% CI) | p for Interaction ‡ |
|---|---|---|---|---|---|---|---|
| Diabetes Mellitus (n = 365) | Non-Union | Active Smoker | 5/58 (8.6%) | 3.0 (1.1–8.2) | 0.03 | +6.0% (0.2–11.8) | <0.05 |
| Non-Smoker | 8/240 (3.3%) | Ref. | |||||
| Periprosthetic Joint Infection (PJI) | Active Smoker | 5/61 (8.2%) | 3.1 (1.1–8.9) | 0.04 | +5.4% (0.3–10.5) | <0.05 | |
| Non-Smoker | 7/250 (2.8%) | Ref. | |||||
| Revision Surgery | Active Smoker | 7/58 (12.1%) | 2.7 (1.2–6.1) | 0.02 | +7.1% (1.2–13.0) | <0.05 | |
| Non-Smoker | 12/240 (5.0%) | Ref. | |||||
| Hepatic Dysfunction (n = 238) | Wound Haematoma | Active Smoker | 7/48 (14.6%) | 3.1 (1.3–7.4) | 0.01 | +9.4% (1.8–17.0) | 0.02 |
| Non-Smoker | 8/153 (5.2%) | Ref. | |||||
| Periprosthetic Joint Infection (PJI) | Active Smoker | 5/48 (10.4%) | 2.9 (1.1–7.9) | 0.03 | +6.6% (0.5–12.7) | 0.03 | |
| Non-Smoker | 6/158 (3.8%) | Ref. | |||||
| Anaemia (Severe, Hb < 8 g/dL) (n = 35) | 30-Day Mortality § | Active Smoker | 2/5 (40.0%) | 8.9 (1.8–43.1) | <0.01 | +39.4% (10.0–68.8) | <0.01 |
| Non-Smoker | 1/30 (3.3%) | Ref. | |||||
| Chronic Venous Disease (n = 592) | Reoperation | Active Smoker | 10/83 (12.05%) | 2.8 (1.1–7.1) | 0.02 | +7.6% (1.5–13.7) | N/S |
| Non-smoker | 18/384 (4.69%) | Ref. | - | - | - |
| Characteristic | Group | Diabetes Mellitus (DM) | Anemia | Hepatic Dysfunction | Chronic Venous Disease (CVD) |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, years (Mean ± SD) | Active Smokers | 61.9 ± 13.2 | 53.4 ± 16.2 | 54.9 ± 16.7 | 54.6 ± 16.2 |
| Non-Smokers | 73.9 ± 9.7 | 71.6 ± 14.8 | 72.1 ± 14.6 | 71.9 ± 14.6 | |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | |
| Male Sex, n (%) | Active Smokers | 36 (62.1%) | 40 (69.0%) | 40 (72.7%) | 58 (69.9%) |
| Non-Smokers | 84 (35.0%) | 77 (32.1%) | 52 (32.9%) | 133 (34.6%) | |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | |
| Preoperative Physiological Markers | |||||
| Anemia, n (%) † | Active Smokers | 48 (85.2%) | 58 (100.0%) | 32 (64.0%) | 43 (35.5%) |
| Non-Smokers | 177 (74.8%) | 230 (95.8%) | 116 (73.4%) | 86 (22.3%) | |
| p-value | 0.10 | 0.23 | 0.42 | <0.01 | |
| Thrombocytopenia, n (%) | Active Smokers | 13 (22.4%) | 6 (14.3%) | 6 (15.0%) | 15 (12.4%) |
| Non-Smokers | 22 (9.1%) | 54 (22.5%) | 24 (16.9%) | 57 (14.8%) | |
| p-value | 0.02 | 0.30 | 0.83 | 0.52 | |
| Comorbidity Cohort | Outcome | Relative Excess Risk Due to Interaction (RERI) (95% CI) | Attributable Proportion (AP) (95% CI) |
|---|---|---|---|
| Diabetes Mellitus | Non-Union | 2.66 | 0.50 |
| Periprosthetic Joint Infection (PJI) | 5.04 | 0.65 | |
| Hepatic Dysfunction | Wound Haematoma | 2.1 | 0.68 |
| Anaemia (Severe) | 30-Day Mortality | Very high | Very high |
| Comorbidity Cohort | Outcome | Former Smokers n/N (%) | Active Smokers aOR (95% CI) † | Former Smokers aOR (95% CI) † vs. Non-Smokers |
|---|---|---|---|---|
| Diabetes Mellitus | Non-Union | 2/22 (9.1%) | 3.0 (1.1–8.2) | 2.1 (0.8–5.5) |
| PJI | 1/22 (4.5%) | 3.1 (1.1–8.9) | 1.8 (0.6–5.4) | |
| Revision Surgery | 3/22 (13.6%) | 2.7 (1.2–6.1) | 2.3 (1.0–5.3) | |
| Anemia | PJI | 2/22 (9.1%) | 2.2 (0.4–13.5) | 1.9 (0.7–5.1) |
| Hepatic Dysfunction | Wound Haematoma | 2/15 (13.3%) | 3.1 (1.3–7.4) | 2.5 (0.9–6.8) |
| PJI | 1/15 (6.7%) | 2.9 (1.1–7.9) | 1.9 (0.6–6.0) | |
| Chronic Venous Disease | Reoperation | 3/35 (8.6%) | 2.8 (1.1–7.1) | 1.9 (0.8–4.5) |
| COPD | Prolonged Hospitalization | 3/8 (37.5%) | 2.4 (0.7–8.6) | 2.0 (0.6–6.5) |
| Tuberculosis | Any Septic Complication | 1/4 (25.0%) | 4.1 (0.4–42.2) | 2.5 (0.5–12.0) |
| Indicator | Active Smokers (n = 58) | Non-Smokers (n = 240) | p-Value |
|---|---|---|---|
| Hemodynamic Instability | 50.0% (29/58) | 30.0% (72/240) | 0.006 |
| Intraoperative Transfusion Requirement | 34.5% (20/58) | 25.0% (60/240) | 0.12 |
| COMPOSITE: Hostile Surgical Field (≥2 Indicator) * | 55.2% (32/58) | 38.8% (93/240) | 0.02 |
| Indicator | Active Smokers (n = 58) | Non-Smokers (n = 240) | p-Value |
|---|---|---|---|
| Hemodynamic Instability | 48.3% (28/58) | 32.1% (77/240) | 0.02 |
| Intraoperative Transfusion Requirement | 58.6% (34/58) | 45.8% (110/240) | 0.08 |
| COMPOSITE: Hostile Surgical Field (≥2 Indicators) * | 51.7% (30/58) | 36.7% (88/240) | 0.03 |
| Indicator | Active Smokers (n = 55) | Non-Smokers (n = 158) | p-Value |
|---|---|---|---|
| Hemodynamic Instability | 41.8% (23/55) | 28.5% (45/158) | 0.06 |
| Intraoperative Transfusion Requirement | 38.2% (21/55) | 25.3% (40/158) | 0.07 |
| COMPOSITE: Hostile Surgical Field (≥2 Indicators) * | 40.0% (22/55) | 26.6% (42/158) | 0.06 |
| Indicator | Active Smokers (n = 83) | Non-Smokers (n = 384) | p-Value |
|---|---|---|---|
| Hemodynamic Instability | 38.6% (32/83) | 25.8% (99/384) | 0.02 |
| Intraoperative Transfusion Requirement | 30.1% (25/83) | 22.4% (86/384) | 0.13 |
| COMPOSITE: Hostile Surgical Field (≥2 Indicators) * | 33.7% (28/83) | 23.2% (89/384) | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ianoși, E.S.; Roșu, D.-M.; Solyom, A.; Grigorescu, B.L.; Vultur, M.; Ianoși, M.B. Smoking Amplifies Comorbidity-Associated Risk in Orthopaedic Surgery: A Multiplicative Interaction. J. Clin. Med. 2025, 14, 8217. https://doi.org/10.3390/jcm14228217
Ianoși ES, Roșu D-M, Solyom A, Grigorescu BL, Vultur M, Ianoși MB. Smoking Amplifies Comorbidity-Associated Risk in Orthopaedic Surgery: A Multiplicative Interaction. Journal of Clinical Medicine. 2025; 14(22):8217. https://doi.org/10.3390/jcm14228217
Chicago/Turabian StyleIanoși, Edith Simona, Daria-Maria Roșu, Arpad Solyom, Bianca Liana Grigorescu, Mara Vultur, and Maria Beatrice Ianoși. 2025. "Smoking Amplifies Comorbidity-Associated Risk in Orthopaedic Surgery: A Multiplicative Interaction" Journal of Clinical Medicine 14, no. 22: 8217. https://doi.org/10.3390/jcm14228217
APA StyleIanoși, E. S., Roșu, D.-M., Solyom, A., Grigorescu, B. L., Vultur, M., & Ianoși, M. B. (2025). Smoking Amplifies Comorbidity-Associated Risk in Orthopaedic Surgery: A Multiplicative Interaction. Journal of Clinical Medicine, 14(22), 8217. https://doi.org/10.3390/jcm14228217






