De-Novo Stress Urinary Incontinence After Apical Prolapse Surgery: Potential Link with the Zone of Critical Elasticity
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, J.M.; Vaughan, C.P.; Goode, P.S.; Redden, D.T.; Burgio, K.L.; Richter, H.E.; Markland, A.D.D. Prevalence and trends of symptomatic pelvic floor disorders in U.S. women. Obstet. Gynaecol. 2014, 123, 141–148. [Google Scholar] [CrossRef]
- MacLennan, A.H.; Taylor, A.W.; Wilson, D.H.; Wilson, D. The prevalence of pelvic floor disorders and their relationship to gender, age, parity and mode of delivery. BJOG 2000, 107, 1460–1470. [Google Scholar] [CrossRef]
- Tegerstedt, G.; Maehle-Schmidt, M.; Nyrén, O.; Hammarström, M. Prevalence of symptomatic pelvic organ prolapse in a Swedish population. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2005, 16, 497–503. [Google Scholar] [CrossRef]
- Hove, M.C.P.S.-T.; Pool-Goudzwaard, A.L.; Eijkemans, M.J.C.; Steegers-Theunissen, R.P.M.; Burger, C.W.; Vierhout, M.E. The prevalence of pelvic organ prolapse symptoms and signs and their relation with bladder and bowel disorders in a general female population. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2009, 20, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Espuña-Pons, M.; Diez-Itza, I.; Anglès-Acedo, S.; Covernton, P.J.O.; on behalf of GISPEM Group. Cough stress tests to diagnose stress urinary incontinence in women with pelvic organ prolapse with indication for surgical treatment. Neurourol. Urodyn. 2020, 39, 819–825. [Google Scholar] [CrossRef]
- Burrows, L.J.; Meyn, L.A.; Walters, M.D.; Weber, A.M. Pelvic symptoms in women with pelvic organ prolapse. Obstet. Gynecol. 2004, 104 Pt 1, 982–988. [Google Scholar] [CrossRef]
- Haylen, B.T.; Maher, C.F.; Barber, M.D.; Camargo, S.; Dandolu, V.; Digesu, A.; Goldman, H.B.; Huser, M.; Milani, A.L.; Moran, P.A.; et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic organ prolapse (POP). Int. Urogynecol. J. 2016, 27, 165–194. [Google Scholar] [CrossRef]
- Meschia, M.; Pifarotti, P.; Spennacchio, M.; Buonaguidi, A.; Gattei, U.; Somigliana, E. A randomized comparison of tension-free vaginal tape and endopelvic fascia plication in women with genital prolapse and occult stress urinary incontinence. Am. J. Obstet. Gynecol. 2004, 190, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Engh, A.M.E.; Ekeryd, A.; Magnusson, Å.; Olsson, I.; Otterlind, L.; Tobiasson, G. Can de novo stress incontinence after anterior wall repair be predicted? Acta Obstet. Gynecol. Scand. 2011, 90, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Jelovsek, J.E.M.; Chagin, K.; Brubaker, L.; Rogers, R.G.; Richter, H.E.; Arya, L.; Barber, M.D.M.; Shepherd, J.P.M.; Nolen, T.L.D.; Norton, P.; et al. A model for predicting the risk of de novo stress urinary incontinence in women undergoing pelvic organ prolapse surgery. Obstet. Gynecol. 2014, 123 Pt 1, 279–287. [Google Scholar] [CrossRef]
- Brubaker, L.; Cundiff, G.; Fine, P.; Nygaard, I.; Richter, H.; Visco, A.; Zyczynski, H.; Brown, M.B.; Weber, A. A randomized trial of colpopexy and urinary reduction efforts (CARE): Design and methods. Control. Clin. Trials 2003, 24, 629–642. [Google Scholar] [CrossRef]
- Wei, J.; Nygaard, I.; Richter, H.; Brown, M.; Barber, M.; Kenton, K.; Nager, C.; Schaffer, J.; Visco, A.; Weber, A.; et al. Outcomes following vaginal prolapse repair and mid urethral sling (OPUS) trial--design and methods. Clin. Trials 2009, 6, 162–171. [Google Scholar] [CrossRef]
- Khayyami, Y.; Elmelund, M.; Lose, G.; Klarskov, N. De novo urinary incontinence after pelvic organ prolapse surgery-a national database study. Int. Urogynecol. J. 2020, 31, 305–308. [Google Scholar] [CrossRef]
- Baessler, K.; Christmann-Schmid, C.; Maher, C.; Haya, N.; Crawford, T.J.; Brown, J. Surgery for women with pelvic organ prolapse with or without stress urinary incontinence. Cochrane Database Syst. Rev. 2018, 8, CD013108. [Google Scholar] [CrossRef]
- Ross, J.H.; Carter-Brooks, C.M.; Ruppert, K.M.; Giugale, L.E.; Shepherd, J.P.; Zyczynski, H.M. Assessing the Performance of the De Novo Postoperative Stress Urinary Incontinence Calculator. Female Pelvic Med. Reconstr. Surg. 2021, 27, 23–27. [Google Scholar] [CrossRef]
- Sabadell, J.; Salicrú, S.; Montero-Armengol, A.; Rodriguez-Mias, N.; Gil-Moreno, A.; Poza, J.L. External validation of de novo stress urinary incontinence prediction model after vaginal prolapse surgery. Int. Urogynecol. J. 2019, 30, 1719–1723. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Lee, Y.; Suh, D.H.; Lee, S.; Jeon, M.J. External validation of the de novo stress urinary incontinence prediction model after pelvic organ prolapse surgery in Korean women: A retrospective cohort study. BMC Womens Health 2023, 23, 656. [Google Scholar] [CrossRef] [PubMed]
- Kasturi, S.; Diaz, S.I.; McDermott, C.D.; Woodman, P.J.; Bump, R.C.; Terry, C.L.; Hale, D.S. De novo stress urinary incontinence after negative prolapse reduction stress testing for total vaginal mesh procedures: Incidence and risk factors. Am. J. Obstet. Gynecol. 2011, 205, 487.e1–487.e4. [Google Scholar] [CrossRef] [PubMed]
- Yoshio, Y.; Hayashi, T.; Tokiwa, S.; Sawada, Y.; Okada, Y.; Achila, B.; Kitagawa, Y.; Nomura, J. Predictive urodynamic factors for de novo stress urinary incontinence after laparoscopic sacrocolpopexy for pelvic organ prolapse. Low. Urin. Tract Symptoms 2021, 13, 498–504. [Google Scholar] [CrossRef]
- Falah-Hassani, K.; Reeves, J.; Shiri, R.; Hickling, D.; McLean, L. The pathophysiology of stress urinary incontinence: A systematic review and meta-analysis. Int. Urogynecol. J. 2021, 32, 1607, Erratum in Int. Urogynecol. J. 2021, 32, 501–552. [Google Scholar]
- Enhorning, G.E. A concept of urinary continence. Urol. Int. 1976, 31, 3–5. [Google Scholar] [CrossRef]
- DeLancey, J.O. Structural support of the urethra as it relates to stress urinary incontinence: The hammock hypothesis. Am. J. Obstet. Gynecol. 1994, 170, 1713–1720; discussion 1720–1723. [Google Scholar] [CrossRef] [PubMed]
- Petros, P.E.; Ulmsten, U.I. An integral theory of female urinary incontinence. Experimental and clinical considerations. Acta Obstet. Gynecol. Scand. Suppl. 1990, 153, 7–31. [Google Scholar] [CrossRef]
- Petros, P.E.P.; Ulmsten, U. Role of the pelvic floor in bladder neck opening and closure I: Muscle forces. Int. Urogynecol. J. Pelvic Floor Dysfunct. 1997, 8, 74–80. [Google Scholar] [CrossRef]
- Petros, P.; Gunnemann, A.; Liedl, B. Use of Martius flaps in complex female urethral surgery and the tethered vagina syndrome. Central Eur. J. Urol. 2014, 67, 208–209. [Google Scholar] [CrossRef]
- LeClaire, E.L.; Mukati, M.S.; Juarez, D.; White, D.; Quiroz, L.H. Is de novo stress incontinence after sacrocolpopexy related to anatomical changes and surgical approach? Int. Urogynecol. J. 2014, 25, 1201–1206. [Google Scholar] [CrossRef]
- Kato, K.; Yoshimura, Y.; Narushima, M.; Suzuki, S.; Hattori, R. “Central Road” cystoscopic finding: The road to worsened incontinence following laparoscopic sacrocolpopexy. IJU Case Rep. 2020, 3, 204–206. [Google Scholar] [CrossRef]
- Nomura, Y.; Okada, Y.; Hiramatsu, A.; Matsubara, E.; Kato, K.; Yoshimura, Y. A new method of adjusting mesh tension using cystoscopy during laparoscopic sacrocolpopexy. Int. Urogynecol. J. 2021, 32, 3089–3093. [Google Scholar] [CrossRef]
- Olsen, A.; Smith, V.; Bergstrom, J.; Colling, J.; Clark, A. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet. Gynecol. 1997, 89, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Fialkow, M.F.; Newton, K.M.; Lentz, G.M.; Weiss, N.S. Lifetime risk of surgical management for pelvic organ prolapse or urinary incontinence. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2008, 19, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Kuribayashi, M.; Kitagawa, Y.; Narimoto, K.; Urata, S.; Kawaguchi, S.; Namiki, M. Predictor of de novo stress urinary incontinence following TVM procedure: A further analysis of preoperative voiding function. Int. Urogynecol. J. 2013, 24, 407–411. [Google Scholar] [CrossRef]
- Visco, A.G.; Brubaker, L.; Nygaard, I.; Richter, H.E.; Cundiff, G.; Fine, P.; Zyczynski, H.; Brown, M.B.; Weber, A.M.; Network, P.F.D. The role of preoperative urodynamic testing in stress-continent women undergoing sacrocolpopexy: The Colpopexy and Urinary Reduction Efforts (CARE) randomized surgical trial. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2008, 19, 607–614. [Google Scholar] [CrossRef]
- Wen, L.; Shek, K.L.; Dietz, H.P. Changes in urethral mobility and configuration after prolapse repair. Ultrasound Obstet. Gynecol. 2019, 53, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Alas, A.N.; Chinthakanan, O.; Espaillat, L.; Plowright, L.; Davila, G.W.; Aguilar, V.C. De novo stress urinary incontinence after pelvic organ prolapse surgery in women without occult incontinence. Int. Urogynecol. J. 2017, 28, 583–590. [Google Scholar] [CrossRef] [PubMed]
- El Hamamsy, D.; Fayyad, A.M. New onset stress urinary incontinence following laparoscopic sacrocolpopexy and its relation to anatomical outcomes. Int. Urogynecol. J. 2015, 26, 1041–1045. [Google Scholar] [CrossRef]
- Christiansen, U.J.; Hansen, M.J.; Lauszus, F.F. Hysterectomy is not associated with de-novo urinary incontinence: A ten-year cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 215, 175–179. [Google Scholar] [CrossRef]
- Lo, T.-S.; Karim, N.B.; Nawawi, E.A.; Wu, P.-Y.; Nusee, Z. Predictors for de novo stress urinary incontinence following extensive pelvic reconstructive surgery. Int. Urogynecol. J. 2015, 26, 1313–1319. [Google Scholar] [CrossRef]
- Kim, Y.; Rowley, J.E.; Ortega, M.V.; James, K.E.; Von Bargen, E. Incidence of de novo stress urinary incontinence following minimally invasive sacrocolpopexy. Int. Urogynecol. J. 2023, 34, 1599–1605. [Google Scholar] [CrossRef] [PubMed]
- Davenport, M.T.; Sokol, E.R.; Comiter, C.V.; Elliott, C.S. Does the Degree of Cystocele Predict De Novo Stress Urinary Incontinence After Prolapse Repair? Further Analysis of the Colpopexy and Urinary Reduction Efforts Trial. Female Pelvic Med. Reconstr. Surg. 2018, 24, 292–294. [Google Scholar] [CrossRef]
- Wei, J.T.; Nygaard, I.; Richter, H.E.; Nager, C.W.; Barber, M.D.; Kenton, K.; Amundsen, C.L.; Schaffer, J.; Meikle, S.F.; Spino, C. A midurethral sling to reduce incontinence after vaginal prolapse repair. N. Engl. J. Med. 2012, 366, 2358–2367. [Google Scholar] [CrossRef]
- Karjalainen, P.K.; Tolppanen, A.-M.; Wihersaari, O.; Nieminen, K.; Mattsson, N.K.; Jalkanen, J.T. Changes in Stress Urinary Incontinence Symptoms after Pelvic Organ Prolapse Surgery: A Nationwide Cohort Study (FINPOP). Int. Urogynecol. J. 2024, 35, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Ugianskiene, A.; Kjærgaard, N.; Lindquist, A.S.I.; Larsen, T.; Glavind, K. Retrospective study on de novo postoperative urinary incontinence after pelvic organ prolapse surgery. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 219, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Hafidh, B.A.; Chou, Q.; Khalil, M.M.; Al-Mandeel, H. De novo stress urinary incontinence after vaginal repair for pelvic organ prolapse: One-year follow-up. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 168, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Kummeling, M.T.; Rietbergen, J.B.; Withagen, M.I.; Mannaerts, G.H.; van der Weiden, R.M. Sequential urodynamic assessment before and after laparoscopic sacrocolpopexy. Acta Obstet. Gynecol. Scand. 2013, 92, 172–177. [Google Scholar] [CrossRef]
- Oyama, K.; Ikeda, S.; Yuda, M. Does Concurrent Burch Colposuspension Reduce Postoperative Stress Urinary Incontinence in Laparoscopic Sacrocolpopexy? An Interim Analysis. J. Minim. Invasive Gynecol. 2025, 32, 807–814. [Google Scholar] [CrossRef]
- Başer, E.; Seçkin, K.D.; kadïroğullari, P.; Kiyak, H. The effect of sacrospinous ligament fixation during vaginal hysterectomy on postoperative de novo stress incontinence occurrence: A prospective study with 2-year follow-up. Turk. J. Med. Sci. 2020, 50, 978–984. [Google Scholar] [CrossRef]
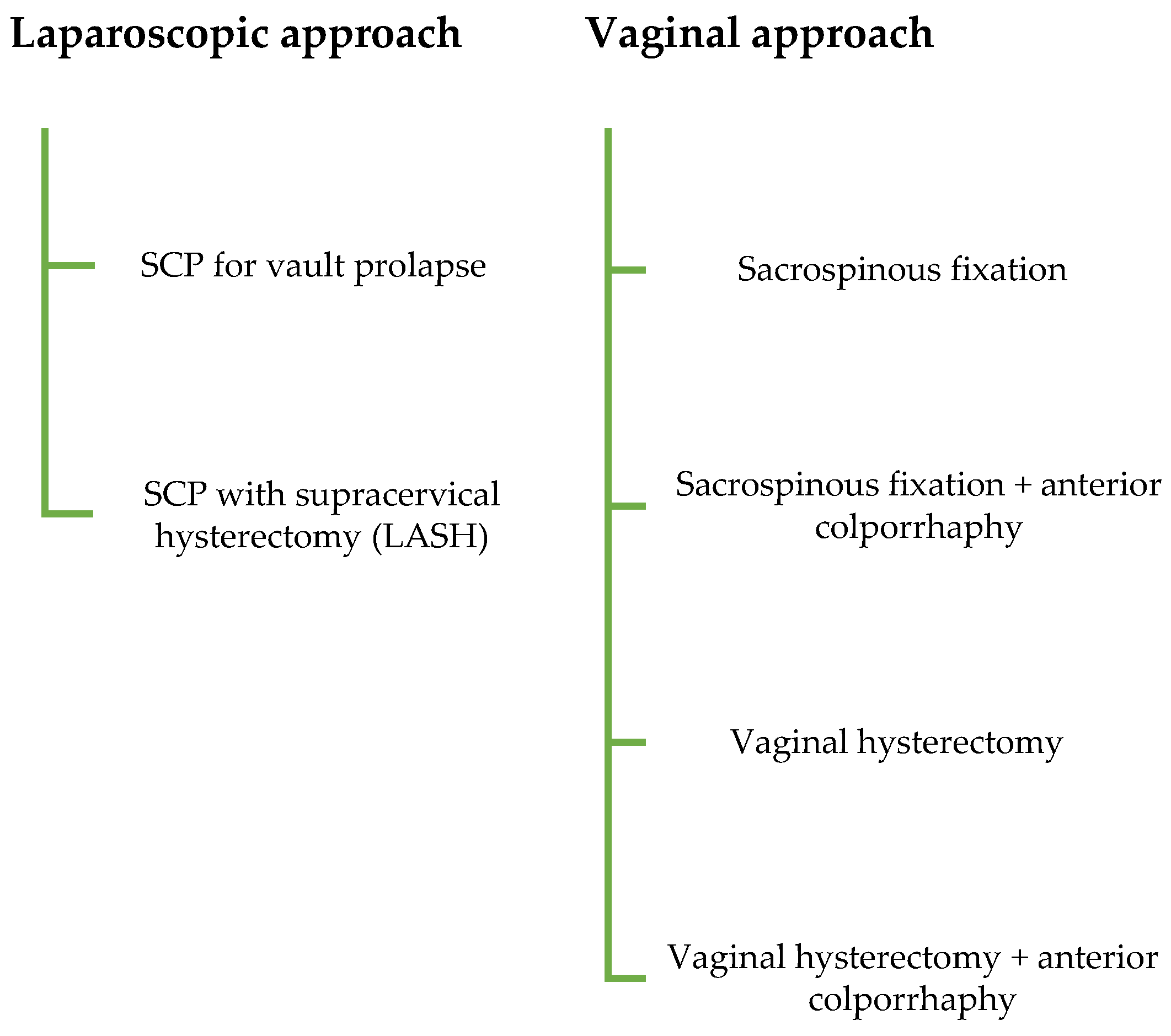
| Variable | Descriptive Statistics * |
|---|---|
| Age (years) | 71.2 (60.2–88.7) |
| Parity | 2 (0–6) |
| POP-Q Stage 3 or 4 | 62 (30.5) |
| Cystocele | |
| Stage I | 18 (10.3) |
| Stage II | 102 (58.6) |
| Stage III and over | 54 (31) |
| OAB Symptoms preoperatively | |
| No | 92 (44.9) |
| Yes | 113 (55.1) |
| OAB-Wet | 27 (23.9) |
| OAB-Dry | 86 (76.1) |
| Previous abdominal surgery (n) | 1 (0–7) |
| Previous hysterectomy | |
| Yes | 75 (36.4) |
| No | 131 (63.6) |
| Previous POP surgery | |
| Yes | 53 (25.7) |
| No | 153 (74.3) |
| Previous urinary incontinence surgery | |
| Yes | 7 (3.4) |
| No | 199 (96.6) |
| BMI (kg/m2) | 26.2 (17.4–41.9) |
| Length of hospital stay (days) | 5 (2–11) |
| ASA Classification | 2 (1–3) |
| De novo SUI | |
| Yes | 18 (8.7) |
| No | 188 (91.3) |
| Follow-up postoperatively (Day) | 67.8 (27–398) |
| Variable | Descriptive Statistics | p * | |
|---|---|---|---|
| SUI | No SUI | ||
| Age (years) | 68.0 (61.4–81.6) | 71.3 (60.2–88.7) | 0.350 |
| Parity | 2 (1–3) | 2 (0–6) | 0.330 |
| POP-Q Stage 3 or 4 | 0.430 | ||
| Yes | 7 (11.3) | 55 (88.7) | |
| No | 11 (7.8) | 130 (92.2) | |
| Cystocele | 0.402 | ||
| Stage I | 1 (5.6) | 17 (94.4) | |
| Stage II | 8 (7.8) | 94 (92.2) | |
| Stage III and over | 6 (11.1) | 48 (88.9) | |
| OAB Symptoms preoperatively | 0.954 | ||
| Wet | 2 (7.4) | 25 (92.6) | |
| Dry | 8 (7.6) | 78 (90.7) | |
| None | 8 (8.7) | 84 (83.9) | |
| Previous abdominal surgery (n) | 0.50 (0–3) | 1(0–7) | 0.370 |
| BMI (kg/m2) | 26.81 (22.0–31.8) | 25.97 (17.4–41.9) | 0.287 |
| ASA Classification | 2 (2–3) | 2 (1–3) | 0.968 |
| Previous hysterectomy | 0.037 | ||
| Yes | 11 (14.7) | 64 (85.3) | |
| No | 7 (5.3) | 124 (94.7) | |
| Previous POP surgery | 0.008 | ||
| Yes | 10 (18.9) | 43 (81.1) | |
| No | 8 (13.4) | 145 (94.8) | |
| Previous urinary incontinence surgery | 0.478 | ||
| Yes | 1 (14.3) | 6 (85.7) | |
| No | 17 (8.5) | 182 (91.5) | |
| Length of hospital stay (days) | 5 (3–8) | 5 (2–11) | 0.756 |
| Follow-up postoperatively (Day) | 61 (36–192) | 69 (27–398) | 0.068 |
| Study Group | <0.001 | ||
| Sacrospinous fixation | 0 (0) | 22 (100) | |
| Sacrospinous fixation + anterior colporrhaphy | 3 (8.6) | 32 (91.4) | |
| SCP with supracervical hysterectomy (LASH) | 1 (5.6) | 17 (94.4) | |
| SCP for vault prolapse | 11 (27.5) | 29 (72.5) | |
| Vaginal hysterectomy | 0 (0) | 6 (100) | |
| Vaginal hysterectomy + anterior colporrhaphy | 3 (3.5) | 82 (96.5) | |
| Risk Factor (Ref. Category) | OR | %95 Confidence Interval | p * |
|---|---|---|---|
| Surgical technique | 0.016 | ||
| Vaginal hysterectomy + anterior colporrhaphy (ref) | 1.00 | - | - |
| SCP for vault prolapse | 10.368 | 2.70–39.79 | 0.001 |
| SCP with supracervical hysterectomy (LASH) | 1.608 | 0.15–16.40 | 0.68 |
| Sacrospinous fixation + anterior colporrhaphy | 2.562 | 0.49–13.36 | 0.26 |
| Sacrospinous fixation | ~0 | - | - |
| Vaginal hysterectomy | ~0 | - | - |
| Cystocele stage | 0.699 | ||
| Stage I (ref) | 1.00 | - | - |
| Stage II | 1.45 | 0.17–12.32 | 0.735 |
| Stage III and over | 2.13 | 0.23–18.95 | 0.500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Degirmenci, Y.; Efe Sayın, C.; Shehaj, I.; Schmidt, M.W.; Klamminger, G.G. De-Novo Stress Urinary Incontinence After Apical Prolapse Surgery: Potential Link with the Zone of Critical Elasticity. J. Clin. Med. 2025, 14, 8153. https://doi.org/10.3390/jcm14228153
Degirmenci Y, Efe Sayın C, Shehaj I, Schmidt MW, Klamminger GG. De-Novo Stress Urinary Incontinence After Apical Prolapse Surgery: Potential Link with the Zone of Critical Elasticity. Journal of Clinical Medicine. 2025; 14(22):8153. https://doi.org/10.3390/jcm14228153
Chicago/Turabian StyleDegirmenci, Yaman, Ceren Efe Sayın, Ina Shehaj, Mona Wanda Schmidt, and Gilbert Georg Klamminger. 2025. "De-Novo Stress Urinary Incontinence After Apical Prolapse Surgery: Potential Link with the Zone of Critical Elasticity" Journal of Clinical Medicine 14, no. 22: 8153. https://doi.org/10.3390/jcm14228153
APA StyleDegirmenci, Y., Efe Sayın, C., Shehaj, I., Schmidt, M. W., & Klamminger, G. G. (2025). De-Novo Stress Urinary Incontinence After Apical Prolapse Surgery: Potential Link with the Zone of Critical Elasticity. Journal of Clinical Medicine, 14(22), 8153. https://doi.org/10.3390/jcm14228153





