Selective Removal of Neutrophil Extracellular Traps (NETs) Combined with Ex Vivo Lung Perfusion (EVLP): Current Evidence and Future Perspectives
Abstract
1. Introduction
2. Scientific Background
3. Current State of Research and Future Perspectives
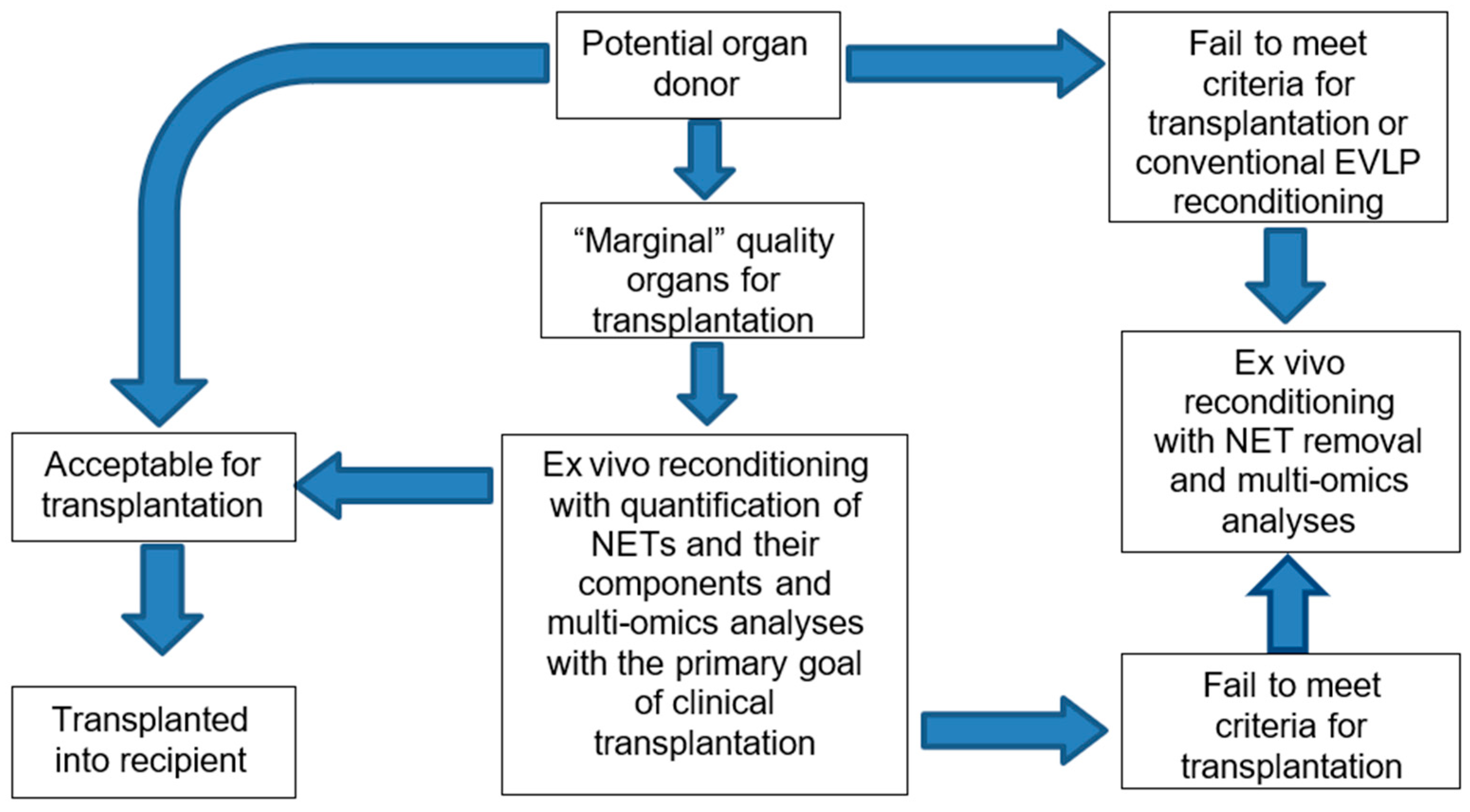
4. Marginal Donor Lungs and Potential to “Safely” Push the Limits
5. Specifics in Allograft Assessment During and After Selective NET Removal
5.1. Conventional Physiological Evaluation
5.2. Histopathological and Molecular Analysis
5.3. Digital Imaging
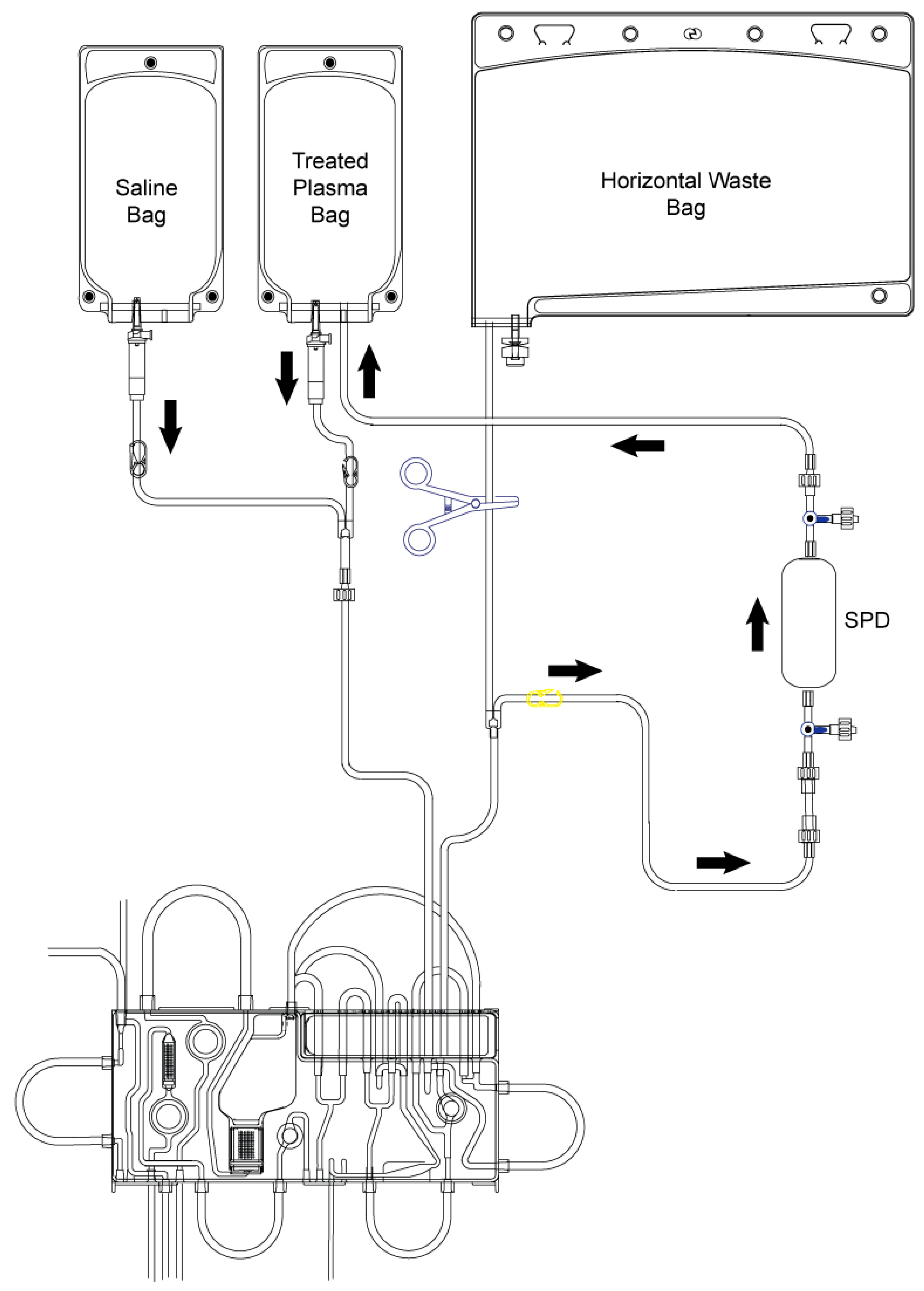
6. Technical Aspects of Selective NET Removal
Ventilation Parameters on EVLP
7. Translation into Clinical Practice
8. Safety, Regulatory and Ethical Considerations
9. Translation to Other Organs in Transplantation
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Popov, A.F.; Sabashnikov, A.; Patil, N.P.; Zeriouh, M.; Mohite, P.N.; Zych, B.; Saez, D.G.; Schmack, B.; Ruhparwar, A.; Dohmen, P.M.; et al. Ex vivo lung perfusion—State of the art in lung donor pool expansion. Med. Sci. Monit. Basic Res. 2015, 21, 9–14. [Google Scholar] [CrossRef]
- Fisher, S.; O’Connor, J.; Redmond, K. Contributions, bias, and research gaps in large animal ex vivo lung perfusion models. A systematic review. JHLT Open 2025, 10, 100356. [Google Scholar] [CrossRef]
- Nakata, K.; Gao, Q.; Kahan, R.; Kesseli, S.J.; Hughes, B.; Alderete, I.; DeLaura, I.F.; Anwar, I.J.; Aardema, C., Jr.; Jarrett, E.; et al. Dual ex vivo lung perfusion via pulmonary and bronchial arteries in a porcine model. JTCVS Tech. 2025, 33, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Fawad, M.; Elde, S.; Heng, E.; Garrison, A.; Choi, A.Y.; Simmons, J.; McNulty, J.P.; Chadwick, B.; Ruaengsri, C.; et al. Preservation of bronchial artery circulation on ex-vivo lung perfusion. Sci. Rep. 2025, 15, 23354. [Google Scholar] [CrossRef]
- McCaig, A.; Sage, A.; Keshavjee, S.; Liu, M. Biomarkers for human donor lung assessment during ex vivo lung perfusion. J. Heart Lung Transplant. 2025. [Google Scholar] [CrossRef]
- Eppinger, M.J.; Ward, P.A.; Bolling, S.F.; Deeb, G.M. Regulatory effects of interleukin-10 on lung ischemia-reperfusion injury. J. Thorac. Cardiovasc. Surg. 1996, 112, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Machuca, T.N.; Cypel, M.; Bonato, R.; Yeung, J.C.; Chun, Y.M.; Juvet, S.; Guan, Z.; Hwang, D.M.; Chen, M.; Saito, T.; et al. Safety and Efficacy of Ex Vivo Donor Lung Adenoviral IL-10 Gene Therapy in a Large Animal Lung Transplant Survival Model. Hum. Gene Ther. 2017, 28, 757–765. [Google Scholar] [CrossRef]
- Mesaki, K.; Juvet, S.; Yeung, J.; Guan, Z.; Wilson, G.W.; Hu, J.; Davidson, A.R.; Kleinstiver, B.P.; Cypel, M.; Liu, M.; et al. Immunomodulation of the donor lung with CRISPR-mediated activation of IL-10 expression. J. Heart Lung Transpl. 2023, 42, 1363–1377. [Google Scholar] [CrossRef] [PubMed]
- Messer, M.P.; Kellermann, P.; Weber, S.J.; Hohmann, C.; Denk, S.; Klohs, B.; Schultze, A.; Braumüller, S.; Huber-Lang, M.S.; Perl, M. Silencing of fas, fas-associated via death domain, or caspase 3 differentially affects lung inflammation, apoptosis, and development of trauma-induced septic acute lung injury. Shock 2013, 39, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Del Sorbo, L.; Costamagna, A.; Muraca, G.; Rotondo, G.; Civiletti, F.; Vizio, B.; Bosco, O.; Martin Conte, E.L.; Frati, G.; Delsedime, L.; et al. Intratracheal Administration of Small Interfering RNA Targeting Fas Reduces Lung Ischemia-Reperfusion Injury. Crit. Care Med. 2016, 44, e604–e613. [Google Scholar] [CrossRef]
- Fisher, A.; Andreasson, A.; Chrysos, A.; Lally, J.; Mamasoula, C.; Exley, C.; Wilkinson, J.; Qian, J.; Watson, G.; Lewington, O.; et al. An observational study of Donor Ex Vivo Lung Perfusion in UK lung transplantation: DEVELOP-UK. Health Technol. Assess. 2016, 20, 1–276. [Google Scholar] [CrossRef]
- Li, S.S.; Funamoto, M.; Singh, R.; Rabi, S.A.; Kreso, A.; Michel, E.; Langer, N.B.; Osho, A.A. Outcomes of donation after circulatory death (DCD) and ex-vivo lung perfusion (EVLP) lung transplantation. J. Heart Lung Transplant. 2025, 44, 721–733. [Google Scholar] [CrossRef]
- Keshavjee, S.; Sage, A.T.; Borrillo, T.; Yeung, J.C.; Piyasena, D.; Wakeam, E.; Donahoe, L.; Waddell, T.K.; de Perrot, M.; Pierre, A.; et al. 1000 Cases of Ex Vivo Lung Perfusion for Lung Transplantation: A Single Centre Experience. J. Thorac. Cardiovasc. Surg. 2025. [Google Scholar] [CrossRef]
- Braithwaite, S.A.; Blankman, P.; van der Kaaij, N.P. New Techniques for the Optimization of Donor Lungs/Hearts. Anesthesiol. Clin. 2025, 43, 243–265. [Google Scholar] [CrossRef]
- Bonneau, S.; Landry, C.; Bégin, S.; Adam, D.; Villeneuve, L.; Clavet-Lanthier, M.É.; Dasilva, A.; Charles, E.; Dumont, B.L.; Neagoe, P.E.; et al. Correlation between Neutrophil Extracellular Traps (NETs) Expression and Primary Graft Dysfunction Following Human Lung Transplantation. Cells 2022, 11, 3420. [Google Scholar] [CrossRef] [PubMed]
- Mittendorfer, M.; Pierre, L.; Huzevka, T.; Schofield, J.; Abrams, S.T.; Wang, G.; Toh, C.H.; Bèchet, N.B.; Caprnja, I.; Kjellberg, G.; et al. Restoring discarded porcine lungs by ex vivo removal of neutrophil extracellular traps. J. Heart Lung Transplant. 2024, 43, 1919–1929. [Google Scholar] [CrossRef]
- Caldarone, L.; Mariscal, A.; Sage, A.; Khan, M.; Juvet, S.; Martinu, T.; Zamel, R.; Cypel, M.; Liu, M.; Palaniyar, N.; et al. Neutrophil extracellular traps in ex vivo lung perfusion perfusate predict the clinical outcome of lung transplant recipients. Eur. Respir. J. 2019, 53, 1801736. [Google Scholar] [CrossRef]
- Zhou, X.; Jin, J.; Lv, T.; Song, Y. A Narrative Review: The Role of NETs in Acute Respiratory Distress Syndrome/Acute Lung Injury. Int. J. Mol. Sci. 2024, 25, 1464. [Google Scholar] [CrossRef]
- Nakata, K.; Alderete, I.S.; Hughes, B.A.; Hartwig, M.G. Ex vivo lung perfusion: Recent advancements and future directions. Front. Immunol. 2025, 16, 1513546. [Google Scholar] [CrossRef] [PubMed]
- Filipczak, N.; Yalamarty, S.S.K.; Li, X.; Pathrikar, T.V.; Pinapati, R.; Vanjara, B.; Torchilin, V. Neutrophil extracellular traps: Formation, pathological roles, and nanoparticle-based therapeutic targeting strategies. J. Control. Release 2025, 387, 114220. [Google Scholar] [CrossRef] [PubMed]
- Jarzebska, N.; Rodionov, R.N.; Voit-Bak, K.; Straube, R.; Mücke, A.; Tselmin, S.; Rettig, R.; Julius, U.; Siow, R.; Gräßler, J.; et al. Neutrophil Extracellular Traps (NETs) as a Potential Target for Anti-Aging: Role of Therapeutic Apheresis. Horm. Metab. Res. 2025. [Google Scholar] [CrossRef]
- Yaykasli, K.O.; Schauer, C.; Muñoz, L.E.; Mahajan, A.; Knopf, J.; Schett, G.; Herrmann, M. Neutrophil Extracellular Trap-Driven Occlusive Diseases. Cells 2021, 10, 2208. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, Y.; Yang, X.; Yan, C.; Feng, Y. Recent Insights into Neutrophil Extracellular Traps in Cardiovascular Diseases. J. Clin. Med. 2022, 11, 6662. [Google Scholar] [CrossRef]
- Masucci, M.T.; Minopoli, M.; Del Vecchio, S.; Carriero, M.V. The Emerging Role of Neutrophil Extracellular Traps (NETs) in Tumor Progression and Metastasis. Front. Immunol. 2020, 11, 1749. [Google Scholar] [CrossRef]
- Sayah, D.M.; Mallavia, B.; Liu, F.; Ortiz-Muñoz, G.; Caudrillier, A.; DerHovanessian, A.; Ross, D.J.; Lynch, J.P., 3rd; Saggar, R.; Ardehali, A.; et al. Neutrophil extracellular traps are pathogenic in primary graft dysfunction after lung transplantation. Am. J. Respir. Crit. Care Med. 2015, 191, 455–463. [Google Scholar] [CrossRef]
- Chen, L.; Chen, C.; Cheng, Y.; Zhou, J.; Ge, F.; Jiang, H.; Qiao, G.; Liu, F. Short-term and long-term outcomes of lung transplantation from marginal donors: A single-center retrospective study. J. Thorac. Dis. 2024, 16, 8656–8668. [Google Scholar] [CrossRef]
- Reul, R.M.; Loor, G.; Garcha, P.S.; Goss, J.A.; Rana, A.A. Allograft discard risk index for lung transplantation. J. Heart Lung Transplant. 2021, 40, 1658–1667. [Google Scholar] [CrossRef] [PubMed]
- Stefanuto, P.H.; Romano, R.; Rees, C.A.; Nasir, M.; Thakuria, L.; Simon, A.; Reed, A.K.; Marczin, N.; Hill, J.E. Volatile organic compound profiling to explore primary graft dysfunction after lung transplantation. Sci. Rep. 2022, 12, 2053. [Google Scholar] [CrossRef] [PubMed]
- Tatham, K.C.; O’Dea, K.P.; Romano, R.; Donaldson, H.E.; Wakabayashi, K.; Patel, B.V.; Thakuria, L.; Simon, A.R.; Sarathchandra, P.; Harefield POPSTAR investigators; et al. Intravascular donor monocytes play a central role in lung transplant ischaemia-reperfusion injury. Thorax 2018, 73, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Thakuria, L.; Packwood, K.; Firouzi, A.; Rogers, P.; Soresi, S.; Habibi-Parker, K.; Lyster, H.; Zych, B.; Garcia-Saez, D.; Mohite, P.; et al. A pharmacokinetic analysis of posaconazole oral suspension in the serum and alveolar compartment of lung transplant recipients. Int. J. Antimicrob. Agents 2016, 47, 69–76. [Google Scholar] [CrossRef]
- Seaby, E.G.; Gilbert, R.D. Thrombotic microangiopathy following haematopoietic stem cell transplant. Pediatr. Nephrol. 2018, 33, 1489–1500. [Google Scholar] [CrossRef]
- Dengu, F.; Abbas, H.; Schofield, J.; Morovat, R.; Quaglia, A.; Pamart, D.; Candiracci, J.; Micaller, J.; Herzog, M.; Wang, G.; et al. Removal of circulating nucleosomes/neutrophil extracellular traps (NETs) reduces ex-situ reperfusion injury in porcine DCD livers preserved with normothermic machine perfusion (NMP). In Proceedings of the International Liver Transplantation Society Annual Congress 2023, Rotterdam, The Netherlands, 3–6 May 2023. [Google Scholar]
- Abbas, H.; Dengu Fungai Sadik, H.; Ceresa, C.; Aswani, A.; Friend, P. Circulating neutrophil extracellular traps (NETs) in deceased donors prior to retrieval and during normothermic perfusion—An opportunity for donor liver optimisation in-situ, ex-situ and beyond. Transplantation 2024, 108. [Google Scholar]
- Abbas, H.; Dengu, F.; Schofield, J.; Wang, G.; Morovat, A.; Quaglia, A.; Abrams, S.; Toh, C.H.; Aswani, A.; Friend, P. Optimisation of donation after circulatory death livers—Removal of extracellular histones during normothermic perfusion and following whole blood reperfusion in an ex-situ porcine model. Transplantation 2024, 108. [Google Scholar]
- Moore, D.H.; Shah, N.L. Reducing Cell-Free DNA and Neutrophil Extracellular Traps May Improve Outcomes of Orthotopic Liver Transplantation. Liver Transpl. 2018, 24, 1649–1650. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qin, X.; Lei, Z.; Chai, H.; Wu, Z. Diphenyleneiodonium ameliorates acute liver rejection during transplantation by inhibiting neutrophil extracellular traps formation in vivo. Transpl. Immunol. 2021, 68, 101434. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qin, X.; Lei, Z.; Chai, H.; Huang, Z.; Wu, Z. Tetramethylpyrazine inhibits neutrophil extracellular traps formation and alleviates hepatic ischemia/reperfusion injury in rat liver transplantation. Exp. Cell Res. 2021, 406, 112719. [Google Scholar] [CrossRef]
- Tian, H.; Xiong, Y.; Zhan, J.; Lu, Z.; Zhang, Y.; Leng, Y.; Huang, Q.; Xia, Z. Inhibition of Macrophage ARID3A Alleviates Myocardial Ischemia-Reperfusion Injury After Heart Transplantation by Reducing THBS1/CD47 Signaling-Mediated Neutrophil Extracellular Traps Formation. Adv. Sci. 2025, e09952. [Google Scholar] [CrossRef]
- Pei, J.; Weng, H.; Peng, J.; Wu, M.; Zhan, X.; Zhu, G.; Wang, D.; Pan, X.; An, N. Identification of potential targets regulating neutrophil extracellular traps in acute rejection of kidney transplantation based on transcriptomics and animal experiments. Int. Immunopharmacol. 2025, 147, 114008. [Google Scholar] [CrossRef]


| Platform | Outcome |
|---|---|
| Extended preservation | Prolonged preservation feasible using optimization of perfusion and ventilation strategies and prone positioning |
| Marginal donors | Use of A2A receptor antagonists, hydrogen sulfide, methylprednisolone and perfluorocarbon-based oxygen carriers (PFCOCs) improves outcome |
| Specific lung injury | Use of antibiotics (sepsis), surfactant (aspiration), ultraviolet C and photodynamic therapy (hepatitis C) could restore lung function |
| Dual EVLP | Use of antibiotics (sepsis), surfactant (aspiration), ultraviolet C and photodynamic therapy (hepatitis C) could restore lung function |
| EVLP biomarkers | Cytokines, cell death, and endothelial-related molecules may predict survival and be used to monitor various therapeutics for donor lung repair |
| Therapy delivery | IL-10 overexpression delivered via an adenovirus vector and silencing of Fas using small interfering RNA to reduce IRI and inflammation, respectively |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabashnikov, A.; Agrawal, S.; Zych, B.; Krasivskyi, I.; Abbas, S.H.; Fungai, D.; Williams, T.; Thakuria, L.; Aswani, A.; Osman, M.; et al. Selective Removal of Neutrophil Extracellular Traps (NETs) Combined with Ex Vivo Lung Perfusion (EVLP): Current Evidence and Future Perspectives. J. Clin. Med. 2025, 14, 8136. https://doi.org/10.3390/jcm14228136
Sabashnikov A, Agrawal S, Zych B, Krasivskyi I, Abbas SH, Fungai D, Williams T, Thakuria L, Aswani A, Osman M, et al. Selective Removal of Neutrophil Extracellular Traps (NETs) Combined with Ex Vivo Lung Perfusion (EVLP): Current Evidence and Future Perspectives. Journal of Clinical Medicine. 2025; 14(22):8136. https://doi.org/10.3390/jcm14228136
Chicago/Turabian StyleSabashnikov, Anton, Sanjay Agrawal, Bartlomiej Zych, Ihor Krasivskyi, Syed Hussain Abbas, Dengu Fungai, Thomas Williams, Louit Thakuria, Andrew Aswani, Mohamed Osman, and et al. 2025. "Selective Removal of Neutrophil Extracellular Traps (NETs) Combined with Ex Vivo Lung Perfusion (EVLP): Current Evidence and Future Perspectives" Journal of Clinical Medicine 14, no. 22: 8136. https://doi.org/10.3390/jcm14228136
APA StyleSabashnikov, A., Agrawal, S., Zych, B., Krasivskyi, I., Abbas, S. H., Fungai, D., Williams, T., Thakuria, L., Aswani, A., Osman, M., Monteagudo-Vela, M., Gerovasili, V., & Reed, A. (2025). Selective Removal of Neutrophil Extracellular Traps (NETs) Combined with Ex Vivo Lung Perfusion (EVLP): Current Evidence and Future Perspectives. Journal of Clinical Medicine, 14(22), 8136. https://doi.org/10.3390/jcm14228136





