Evolution of Liver Fibrosis in Romanian HCV Patients Following Treatment with Direct-Acting Antivirals
Abstract
1. Introduction
2. Materials and Methods
- Inclusion criteria were as follows:
- i.
- Adult patients with viremic HCV infection, regardless of the degree of fibrosis, who underwent DAA therapy in our clinic and achieved SVR.
- ii.
- Patients who signed informed consent.
- Exclusion criteria were as follows:
- i.
- Patients under the age of 18.
- ii.
- Patients who refused to sign or were unable to sign the informed consent for participation in the study.
- iii.
- Patients meeting exclusion criteria for starting antiviral therapy (active neoplasms or decompensated cirrhosis Child B or C who received antiviral therapy in a gastroenterology clinic).
- iv.
- Patients who did not achieve SVR after the first course of DAA and were subsequently treated with Sofosbuvir/Velpatasvir/Voxilaprevir as well as patients who obtained SVR and were reinfected with HCV and underwent a second course of DAA.
- v.
- Patients who interrupted the DAA course due to adverse effects.
- vi.
- Patients who did not present in 2025 for the hepatic fibrosis evaluation visit.
- vii.
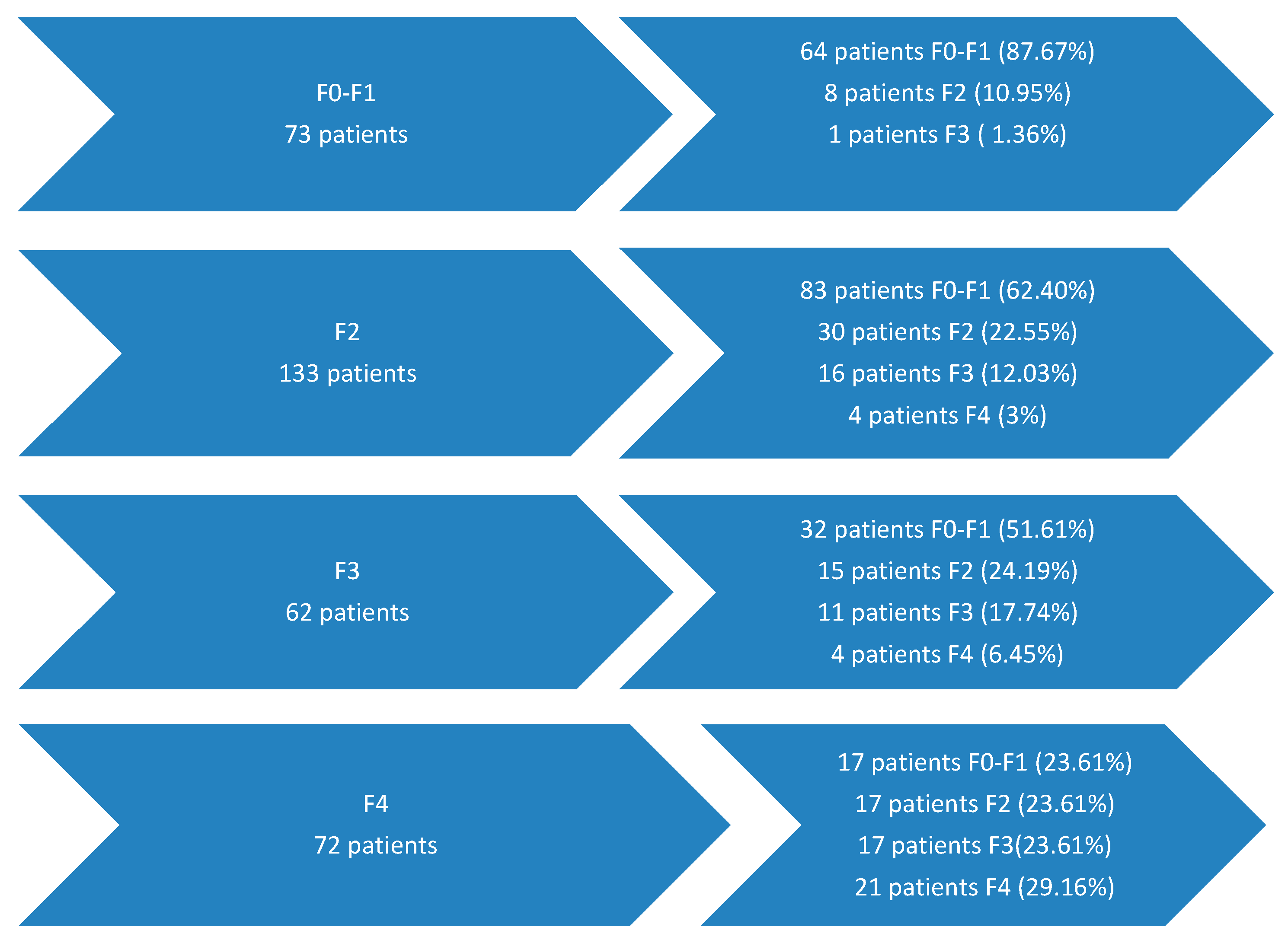
- Patients with initial fibrosis F0–F1 (73 patients), 64 of whom maintained their fibrosis after antiviral treatment and 9 patients who worsened it possibly due to factors independent of HCV.
- (1)
- Group A—181 patients who experienced improvement in hepatic fibrosis in 2025 compared to initial fibrosis;
- (2)
- Group B—86 patients who had stable fibrosis or experienced worsening of hepatic fibrosis (except patients with initial fibrosis F0–F1).
3. Results
- -
- Females predominated in both groups (p = 0.1705, without relevant statistical significance) (Table 1).
- -
- The average age in group A was 60.2 years, while in group B, it was 59.96 years, without relevant statistical significance (t-test, p = 0.8645) (Table 1).
- -
- At the initial assessment, the two groups did not present statistically significant differences in the means of the biochemical parameters ALT, AST, total and direct bilirubin, serum creatinine, serum glucose, serum albumin, the prothrombin concentration, and the INR. The average platelet count, total leucocyte count, and hemoglobin are lower in group B, statistically significant, compared to group A (Table 2). At the initiation of therapy, hepatic fibrosis predominantly in both groups was F2 (Table 3).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Hepatitis Report 2024. Available online: https://www.who.int/publications/i/item/9789240091672 (accessed on 25 August 2025).
- Centrul Național de Supraveghere și Control al Bolilor Transmisibile. Hepatita Virală Tip B și C—Analiza Rezultatelor Studiului Sero-Epidemiologic de Prevalență, România, Anii 2022–2023. Available online: https://www.cnscbt.ro/index.php/analiza-date-supraveghere/hepatita-virala-tip-b-si-c (accessed on 25 August 2025).
- Balta, A.A.S.; Ignat, M.D.; Barbu, R.E.; Dumitru, C.; Radaschin, D.S.; Bulza, V.; Mateescu Costin, S.A.; Pleșea-Condratovici, C.; Baroiu, L. Impact of Direct-Acting Antivirals on Extrahepatic Manifestations in Chronic Hepatitis C: A Narrative Review with a Hermeneutic Approach. Healthcare 2025, 13, 1953. [Google Scholar] [CrossRef]
- World Health Organization. Hepatitis C; WHO: Geneva, Switzerland, 2023; Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed on 25 August 2025).
- World Health Organization. Global Health Sector Strategy on Viral Hepatitis 2016–2021. Available online: https://www.bing.com/search?q=5.+World+Health+Organization.+Global+Health+Sector+Strategy+on+Viral+Hepatitis+2016–2021%3A+Towards+Ending+Viral+Hepatitis.+WHO%2C+June+2016%2C+pp.+1–56&cvid=41472bfe1fff44168cd6d7f0852a9897&gs_lcrp=EgRlZGdlKgYIABBFGDkyBggAEEUYOTIHCAEQ6wcYQNIBBzYzMWowajmoAgiwAgE&FORM=ANAB01&PC=NMTS (accessed on 25 August 2025).
- Friedman, S.L. Evolving challenges in hepatic fibrosis. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 425–436. [Google Scholar] [CrossRef]
- Hernandez-Gea, V.; Friedman, S.L. Pathogenesis of liver fibrosis. Annu. Rev. Pathol. 2011, 6, 425–456. [Google Scholar] [CrossRef]
- World Health Organization. Global Hepatitis Report 2017; WHO: Geneva, Switzerland, 2017; Available online: https://www.who.int/publications/i/item/9789241565455 (accessed on 25 August 2025).
- Pinzani, M. Pathophysiology of liver fibrosis. Dig. Dis. 2015, 33, 492–497. [Google Scholar] [CrossRef]
- Gressner, O.A.; Weiskirchen, R.; Gressner, A.M. Biomarkers of liver fibrosis: Clinical translation of molecular pathogenesis or based on liver-dependent malfunction tests. Clin. Chim. Acta 2007, 381, 107–113. [Google Scholar] [CrossRef]
- Henderson, N.C.; Iredale, J.P. Liver fibrosis: Cellular mechanisms of progression and resolution. Clin. Sci. 2007, 112, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Gressner, A.M.; Weiskirchen, R.; Breitkopf, K.; Dooley, S. Roles of TGF-β in hepatic fibrosis. Front. Biosci. 2002, 7, D793–D807. [Google Scholar] [CrossRef] [PubMed]
- Gressner, A.M.; Weiskirchen, R. The tightrope of therapeutic suppression of active transforming growth factor-beta: High enough to fall deeply? J. Hepatol. 2003, 39, 856–859. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.L. Global control of hepatitis C: Where challenge meets opportunity. Nat. Med. 2013, 19, 850–858. [Google Scholar] [CrossRef]
- Seki, E.; Brenner, D.A. Recent advancement of molecular mechanisms of liver fibrosis. J. Hepatobiliary Pancreat. Sci. 2015, 22, 512–518. [Google Scholar] [CrossRef]
- Zoubek, M.E.; Trautwein, C.; Strnad, P. Reversal of liver fibrosis: From fiction to reality. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 129–141. [Google Scholar] [CrossRef]
- Troeger, J.S.; Mederacke, I.; Gwak, G.Y.; Dapito, D.H.; Mu, X.; Hsu, C.C.; Pradere, J.P.; Friedman, R.A.; Schwabe, R.F. Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology 2012, 143, 1073–1083.e22. [Google Scholar] [CrossRef]
- Povero, D.; Busletta, C.; Novo, E.; Di Bonzo, L.V.; Cannito, S.; Paternostro, C.; Parola, M. Liver fibrosis: A dynamic and potentially reversible process. Histol. Histopathol. 2010, 25, 1075–1091. [Google Scholar]
- Khan, S.M.D.; Saxena, R.M. Regression of hepatic fibrosis and evolution of cirrhosis: A concise review. Adv. Anat. Pathol. 2021, 28, 408–414. [Google Scholar] [CrossRef]
- Hedenstierna, M.; Nangarhari, A.; El-Sabini, A.; Weiland, O.; Aleman, S. Cirrhosis, high age and high body mass index are risk factors for persisting advanced fibrosis after sustained virological response in chronic hepatitis C. J. Viral Hepat. 2018, 25, 802–810. [Google Scholar] [CrossRef]
- Manns, M.P.; McHutchison, J.G.; Gordon, S.C.; Rustgi, V.K.; Shiffman, M.; Reindollar, R.; Goodman, Z.D.; Koury, K.; Ling, M.; Albrecht, J.K. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomised trial. Lancet 2001, 358, 958–965. [Google Scholar] [CrossRef]
- McHutchison, J.G.; Gordon, S.C.; Schiff, E.R.; Shiffman, M.L.; Lee, W.M.; Rustgi, V.K.; Goodman, Z.D.; Ling, M.H.; Cort, S.; Albrecht, J.K. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N. Engl. J. Med. 1998, 339, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Khalili, K.; Nguyen, G.C. Non-invasive diagnosis of advanced fibrosis and cirrhosis. World J. Gastroenterol. 2014, 20, 16820–16830. [Google Scholar] [CrossRef]
- AASLD-IDSA. HCV Guidance: Recommendations for Testing, Management, and Treating Hepatitis C. When and in Whom to Initiate HCV Therapy. Available online: https://www.hcvguidelines.org/ (accessed on 25 August 2025).
- Chou, R.; Wasson, N. Blood tests to diagnose fibrosis or cirrhosis in patients with chronic hepatitis C virus infection: A systematic review. Ann. Intern. Med. 2013, 158, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Rockey, D.C.; Friedman, S.L. Fibrosis regression after eradication of hepatitis C virus: From bench to bedside. Gastroenterology 2021, 160, 1502–1520. [Google Scholar] [CrossRef] [PubMed]
- Elsharkawy, A.; Samir, R.; El-Kassas, M. Fibrosis regression following hepatitis C antiviral therapy. World J. Hepatol. 2022, 14, 1120–1130. [Google Scholar] [CrossRef] [PubMed]
- Degos, F.; Perez, P.; Roche, B.; Mahmoudi, A.; Asselineau, J.; Voitot, H.; Bedossa, P. FIBROSTIC study group: Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: A multicenter prospective study (the FIBROSTIC study). J. Hepatol. 2010, 53, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Leuștean, A.; Popescu, C.; Nichita, L.; Tilișcan, C.; Aramă, V. Dynamics of APRI and FIB-4 in HCV cirrhotic patients who achieved SVR after DAA therapy. Exp. Ther. Med. 2021, 21, 99. [Google Scholar] [CrossRef] [PubMed]
- Leuștean, A.; Olariu, M.C.; Mihai, N.; Tilișcan, C.; Molagic, V.; Duport-Dodot, I.; Stratan, L.M.; Aramă, S.S.; Aramă, V. Regression of liver fibrosis in HCV cirrhotic patients treated with interferon-free therapies. Farmacia 2024, 72, 826–831. [Google Scholar] [CrossRef]
- Tawazun Health. Interpretation of FibroScan. Available online: https://tawazunhealth.com/blog/the-path-to-liver-health-understanding-liver-stiffness/ (accessed on 11 May 2025).
- Ridgeway, G.; McCaffrey, D.; Morral, A.; Griffin, B.A.; Burgette, L.; R Twang (c) Twang: Toolkit for Weighting and Analysis of Nonequivalent Groups. R Package, Version 1.5. 2017. Available online: https://CRAN.R-project.org/package=twang (accessed on 10 November 2025).
- Poynard, T.; McHutchison, J.; Manns, M.; Trepo, C.; Lindsay, K.; Goodman, Z.; Ling, M.H.; Albrecht, J. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology 2002, 122, 1303–1313. [Google Scholar] [CrossRef]
- George, S.L.; Bacon, B.R.; Brunt, E.M.; Mihindukulasuriya, K.L.; Hoffmann, J.; Di Bisceglie, A.M. Clinical, virologic, histologic, and biochemical outcomes after successful HCV therapy: A 5-year follow-up of 150 patients. Hepatology 2009, 49, 729–738. [Google Scholar] [CrossRef]
- Abu-Freha, N.; Abu-Kosh, O.; Yardeni, D.; Ashur, Y.; Abu-Arar, M.; Yousef, B.; Monitin, S.; Weissmann, S.; Etzion, O. Liver fibrosis regression and associated factors in HCV patients treated with direct-acting antiviral agents. Life 2023, 13, 1872. [Google Scholar] [CrossRef]
- Yoo, H.W.; Park, J.Y.; Kim, S.G.; Jung, Y.K.; Lee, S.H.; Kim, M.Y.; Jun, D.W.; Jang, J.Y.; Lee, J.W.; Kwon, O.S. Regression of liver fibrosis and hepatocellular carcinoma development after HCV eradication with oral antiviral agents. Sci. Rep. 2022, 12, 193. [Google Scholar] [CrossRef]
- Trivedi, H.D.; Curry, M.P.; Lai, M. Reply to: The presence of diabetes impacts liver fibrosis and steatosis by transient elastography in a primary care population. Ann. Hepatol. 2021, 25, 100347. [Google Scholar] [CrossRef]
- Sato, S.; Kawai, H.; Sato, S.; Iwasaki, H.; Omori, M.; Kita, Y.; Ikeda, Y.; Awatsu, T.; Murata, A.; Taniguchi, G.; et al. Hypertension and diabetes mellitus are associated with high FIB-4 index in a health checkup examination cohort without known liver disease. BMC Gastroenterol. 2022, 22, 478. [Google Scholar] [CrossRef]
- Ignat, M.D.; Balta, A.A.S.; Barbu, R.E.; Draganescu, M.L.; Nechita, L.; Voinescu, D.C.; Nechita, A.; Stefanopol, I.A.; Busila, C.; Baroiu, L. Antiviral Therapy of Chronic Hepatitis B Virus between Present and Future. J. Clin. Med. 2024, 13, 2055. [Google Scholar] [CrossRef] [PubMed]
- Balta, A.A.S.; Ignat, M.D.; Barbu, R.E.; Baroiu, L.; Moroianu, L.A.; Lutenco, V.; Bulza, V.; Patriciu, M.; Dumitru, C.; Debita, M. HBV, HCV, and HDV Triple-Infection—A Therapeutic Challenge. Diseases 2025, 13, 168. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Wong, S.; Karim, M.E.; Manges, A.R.; Makuza, J.D.; Bartlett, S.R.; Velásquez García, H.A.; Luster, D.; Adu, P.A.; Binka, M.; et al. Treatment of HCV with direct-acting antivirals on reducing mortality related to extrahepatic manifestations: A large population-based study in British Columbia, Canada. Lancet Reg. Health–Am. 2023, 29, 100658. [Google Scholar] [CrossRef]
- Moon, A.M.; Green, P.K.; Rockey, D.C.; Berry, K.; Ioannou, G.N. Hepatitis C eradication with direct-acting anti-virals reduces the risk of variceal bleeding. Aliment. Pharmacol. Ther. 2020, 51, 364–373. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Duong, H.; Luster, M.; Kanwal, F.; Hill, D.D.; Burroughs, M.; Hernandez, C.; Haber, B.A.; Larsen, L.M.; Marcinak, J.F.; et al. Risk of Hepatocellular Cancer in U.S. Patients with Compensated Cirrhosis Treated with Direct-Acting Antivirals Versus Interferon. Aliment. Pharmacol. Ther. 2025, 61, 1226–1237. [Google Scholar] [CrossRef] [PubMed]
| Group A | Group B | ||||
|---|---|---|---|---|---|
| Mean (SD) | Count, % | Mean (SD) | Count, % | ||
| Patient age at the initiation of antiviral therapy (years) | 60.2 (10.94) | 59.96 (10.27) | |||
| Patients’ sex | Masculine | 63 (34.80%) | 22 (25.58%) | ||
| Feminine | 118 (65.19%) | 64 (74.41%) | |||
| N = 181 (Group A) | N = 86 (Group B) | p (t-Test) | |||||
|---|---|---|---|---|---|---|---|
| Av. | SD | Median | Av. | SD | Median | ||
| Leukocytes (N/µL) | 6.95 | 1.85 | 6.60 | 6.23 | 1.84 | 6.03 | 0.0032 |
| Hb (g/dL) | 14.55 | 1.43 | 14.60 | 13.79 | 1.99 | 14 | 0.0004 |
| Platelets (N/µL) | 214.77 | 67.95 | 204 | 202.41 | 73.15 | 199 | 0.0014 |
| ALT (U/L) | 85.56 | 72.01 | 60.50 | 84.44 | 62.25 | 70.25 | 0.9015 |
| AST (U/L) | 68.47 | 57.57 | 49 | 74.42 | 53.59 | 63.95 | 0.4206 |
| Total bilirubin (mg/dL) | 0.78 | 0.32 | 0.70 | 0.83 | 0.37 | 0.75 | 0.2581 |
| Direct bilirubin (mg/dL) | 0.3 | 0.15 | 0.27 | 0.35 | 0.27 | 0.29 | 0.0533 |
| Prothrombin concentration (%) | 94.66 | 15.12 | 93.7 | 91.51 | 17.95 | 93.35 | 0.1360 |
| Serum albumin (g/dL) | 4.55 | 0.34 | 4.54 | 4.47 | 0.27 | 4.49 | 0.0568 |
| Serum creatinine (mg/dL) | 0.84 | 0.18 | 0.81 | 0.83 | 0.21 | 0.81 | 0.6883 |
| Serum glucose (mg/dL) | 113.52 | 19.09 | 104.1 | 116.31 | 42.12 | 105.2 | 0.4561 |
| INR | 1.02 | 0.92 | 1 | 1.02 | 0.14 | 1.02 | 1 |
| RNAHCV (UI/mL) | 1,742,977.95 | 2,499,433.34 | 22,200,000 | 1,892,322.73 | 2,534,584.15 | 926,270.5 | 0.6501 |
| Group A (181 Patients) | Group B (86 Patients) | p-Value | ||||
|---|---|---|---|---|---|---|
| No | % | No | % | |||
| Initial stage of fibrosis in patients from the studied groups | F0–F1 | 0 | 0.0% | 0 | 0% | 1 |
| F2 | 83 | 45.85% | 50 | 58.13% | 0.0811 | |
| F3 | 47 | 25.96% | 15 | 17.44% | 0.1658 | |
| F4 | 51 | 28.17% | 21 | 24.41% | 0.6175 | |
| N = 181 (Group A) | N = 86 (Group B) | p (t-Test) | |||||
|---|---|---|---|---|---|---|---|
| Av. | SD | Median | Av. | SD | Median | ||
| Leukocytes (N/µL) | 6.93 | 2.22 | 6.5 | 6.66 | 1.57 | 6.65 | 0.3118 |
| Platelets (N/µL) | 220.64 | 60.85 | 219.5 | 204.85 | 62.26 | 194 | 0.0503 |
| Hb (g/dL) | 14.42 | 1.16 | 14.50 | 14.13 | 1.20 | 1396 | 0.0602 |
| ALT(U/L) | 22.44 | 9.82 | 19.75 | 27.43 | 12.55 | 26.7 | 0.0005 |
| AST(U/L) | 24.54 | 8.09 | 22.15 | 28.87 | 9.17 | 27.2 | 0.0001 |
| Total bilirubin (mg/dL) | 0.67 | 0.33 | 0.59 | 0.74 | 0.41 | 0.63 | 0.1362 |
| Direct bilirubin (mg/dL) | 0.20 | 0.09 | 0.19 | 0.26 | 0.19 | 0.21 | 0.0005 |
| Prothrombin concentration (%) | 97.77 | 17.24 | 98.2 | 94.34 | 20.9 | 94.6 | 0.1579 |
| Serum albumin (g/dL) | 4.53 | 0.34 | 4.54 | 4.46 | 0.27 | 4.49 | 0.0952 |
| Serum creatinine (mg/dL) | 0.87 | 0.19 | 0.85 | 0.84 | 0.23 | 0.80 | 0.2618 |
| Serum glucose (mg/dL) | 108.56 | 19.09 | 104.1 | 118.16 | 42.12 | 105.2 | 0.0109 |
| INR | 1.01 | 0.11 | 1 | 1.05 | 0.15 | 1.03 | 0.0146 |
| N = 181 (Group A) | N = 86 (Group B) | p (t-Test) | |||||
|---|---|---|---|---|---|---|---|
| Av. | SD | Median | Av. | SD | Median | ||
| Leukocytes (×106/µL) | 6.56 | 2.02 | 6.2 | 7.1 | 1.92 | 6.95 | 0.0391 |
| Platelets (×103/µL) | 219.35 | 65.92 | 216 | 190.43 | 63.15 | 195.5 | 0.0008 |
| Hb (g/dL) | 14.11 | 1.19 | 14 | 14.04 | 1.66 | 13.9 | 0.6943 |
| ALT (U/L) | 21.26 | 8.85 | 19.3 | 26.77 | 9.62 | 26.2 | <0.0001 |
| AST (U/L) | 24.12 | 6.66 | 22.8 | 26.88 | 7.67 | 27.2 | 0.0029 |
| Total bilirubin (mg/dL) | 0.71 | 0.39 | 0.6 | 0.72 | 0.44 | 0.6 | 0.8512 |
| Direct bilirubin (mg/dL) | 0.21 | 0.09 | 0.19 | 0.23 | 0.16 | 0.18 | 0.1933 |
| Prothrombin concentration (%) | 99.0 | 15.06 | 97.05 | 89.6 | 15 | 88.45 | <0.0001 |
| Serum albumin | 4.58 | 0.29 | 4.62 | 4.56 | 0.33 | 4.58 | 0.6152 |
| Serum creatinine (mg/dL) | 0.85 | 0.16 | 0.83 | 0.85 | 0.24 | 0.79 | 1 |
| Serum glucose | 107.1 | 20.19 | 102.6 | 127.95 | 59.28 | 108.85 | <0.0001 |
| INR | 1.01 | 0.08 | 1.01 | 1.06 | 0.1 | 1.05 | <0.0001 |
| AFP (ng/mL) | 3.05 | 2.18 | 2.43 | 3.75 | 1.98 | 3.23 | 0.0180 |
| Amylase (U/L) | 91.38 | 39.54 | 84.1 | 88.23 | 44.18 | 84.6 | <0.0001 |
| N = 181 (Group A) | N = 86 (Group B) | p (t-Test) | |||||
|---|---|---|---|---|---|---|---|
| Av. | SD | Median | Av. | SD | Median | ||
| Leukocytes (×106/µL) | 6.52 | 1.93 | 5.75 | 6.85 | 1.78 | 6.75 | 0.0156 |
| Platelets (×103/µL) | 210.28 | 67.9 | 216 | 214.21 | 70.68 | 207.5 | 0.6631 |
| Hb (g/dL) | 14.1 | 1.43 | 14.05 | 14.06 | 1.05 | 13.85 | 0.8172 |
| ALT (U/L) | 25.55 | 8.99 | 22.65 | 27.53 | 11.97 | 25.55 | 0.1334 |
| AST (U/L) | 26.35 | 7.86 | 26 | 29.02 | 11.01 | 28.35 | 0.0242 |
| Total bilirubin (mg/dL) | 0.78 | 0.39 | 0.7 | 0.6 | 0.16 | 0.54 | 0.0001 |
| Direct bilirubin (mg/dL) | 0.25 | 0.09 | 0.22 | 0.24 | 0.18 | 0.20 | 0.5733 |
| Prothrombin concentration (%) | 100.32 | 15.5 | 97 | 99.67 | 11.52 | 98.6 | 0.7296 |
| Serum albumin (g/dL) | 4.62 | 0.36 | 4.65 | 4.62 | 0.34 | 4.61 | 1 |
| Serum creatinine (mg/dL) | 0.89 | 0.19 | 0.87 | 0.77 | 0.14 | 0.78 | <0.0001 |
| Serum glucose (mg/dL) | 113.77 | 37.1 | 110 | 118.67 | 25.51 | 116.3 | 0.2696 |
| INR | 1.01 | 0.12 | 1.02 | 1.01 | 0.01 | 1.01 | 1 |
| AFP (ng/mL) | 3.12 | 1.66 | 2.8 | 3.37 | 1.7 | 2.76 | 0.2549 |
| Amylase (U/L) | 90.17 | 34.24 | 79.1 | 93.46 | 38 | 85.15 | 0.4797 |
| Group A (181 Patients) | Group B (86 Patients) | p-Value | ||||
|---|---|---|---|---|---|---|
| No | % | No | % | |||
| Fibrosis stage-1–8 years after antiviral therapy in patients from the studied groups | F0–F1 | 132 | 72.92% | 0 | 0% | <0.0001 |
| F2 | 32 | 17.67% | 30 | 34.88% | 0.0031 | |
| F3 | 17 | 9.39% | 27 | 31.39% | <0.0001 | |
| F4 | 0 | 0% | 29 | 33.72% | <0.0001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balta, A.A.S.; Uibariu Barbu, R.E.; Baroiu, L.; Bulza, V.; Bujoreanu, F.; Moroianu, M.; Ignat, M.D.; Cambrea, S.C.; Dumea, E.; Stoian, V. Evolution of Liver Fibrosis in Romanian HCV Patients Following Treatment with Direct-Acting Antivirals. J. Clin. Med. 2025, 14, 8112. https://doi.org/10.3390/jcm14228112
Balta AAS, Uibariu Barbu RE, Baroiu L, Bulza V, Bujoreanu F, Moroianu M, Ignat MD, Cambrea SC, Dumea E, Stoian V. Evolution of Liver Fibrosis in Romanian HCV Patients Following Treatment with Direct-Acting Antivirals. Journal of Clinical Medicine. 2025; 14(22):8112. https://doi.org/10.3390/jcm14228112
Chicago/Turabian StyleBalta, Alexia Anastasia Stefania, Raisa Eloise Uibariu Barbu, Liliana Baroiu, Valentin Bulza, Florin Bujoreanu, Marius Moroianu, Mariana Daniela Ignat, Simona Claudia Cambrea, Elena Dumea, and Valerian Stoian. 2025. "Evolution of Liver Fibrosis in Romanian HCV Patients Following Treatment with Direct-Acting Antivirals" Journal of Clinical Medicine 14, no. 22: 8112. https://doi.org/10.3390/jcm14228112
APA StyleBalta, A. A. S., Uibariu Barbu, R. E., Baroiu, L., Bulza, V., Bujoreanu, F., Moroianu, M., Ignat, M. D., Cambrea, S. C., Dumea, E., & Stoian, V. (2025). Evolution of Liver Fibrosis in Romanian HCV Patients Following Treatment with Direct-Acting Antivirals. Journal of Clinical Medicine, 14(22), 8112. https://doi.org/10.3390/jcm14228112






