Gut Microbiota Alterations in Heart Failure Patients: Insights from a Systematic Review
Abstract
1. Introduction
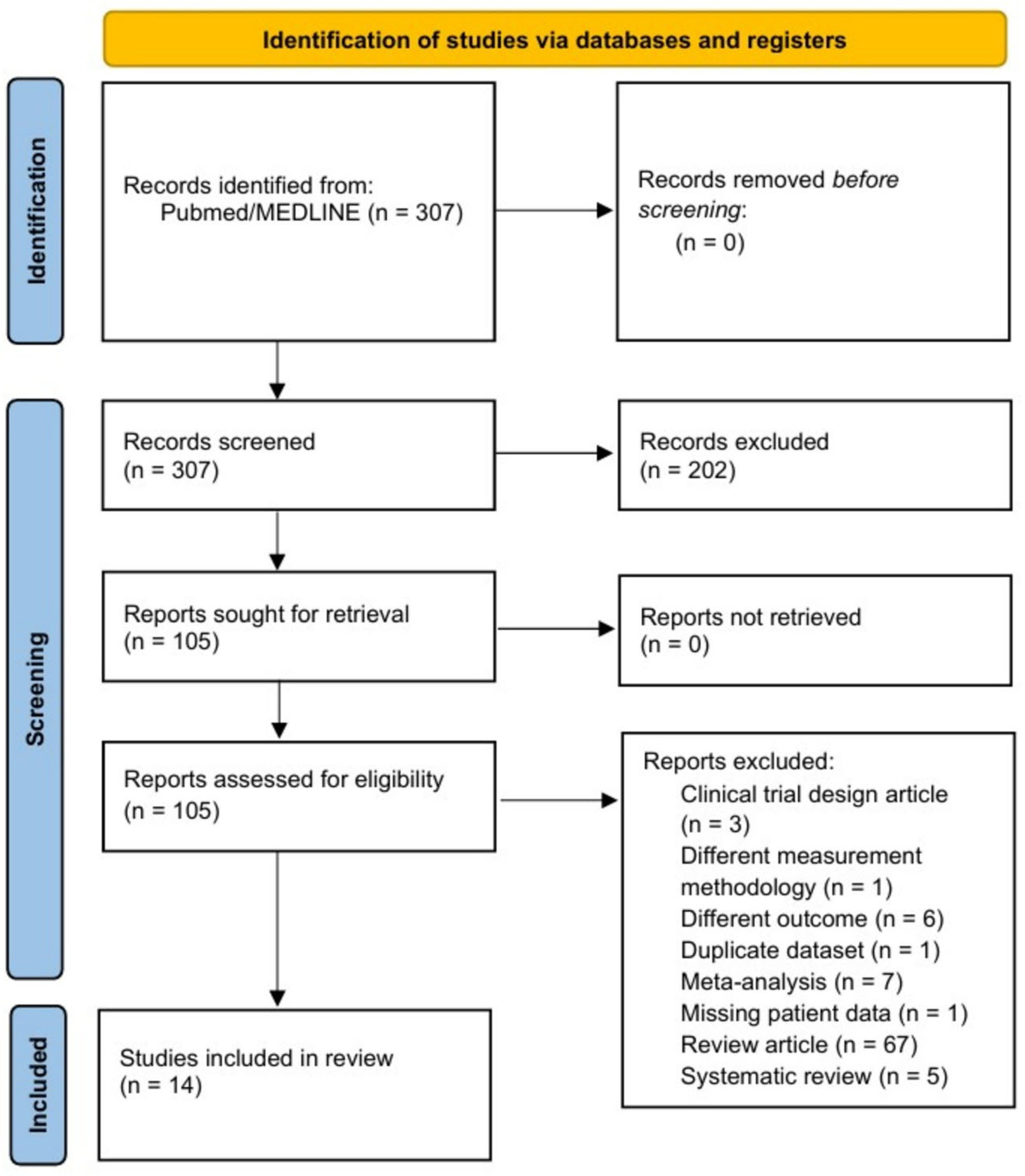
2. Methods
3. Results
3.1. Characteristics of the Participants and Studies Included in the Review
3.2. Methodology of the Studies Included in the Review
3.3. Quality Assessment
3.4. Alpha and Beta Diversity in HF Versus the Control Group
3.5. Differences in Relative Abundance of the Intestinal Microbiota in HF Versus the Control Group
4. Discussion
4.1. Inflammation and Intestinal Microbiota in Heart Failure
4.2. Metabolites and Inflammation Derived from the Different Bacterial Genera in Heart Failure
4.3. Metagenomic Functional Insights in Heart Failure
4.4. Dietary Interventions for the Intestinal Microbiota in Heart Failure
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HR | Hazard ratio |
| HF | Heart failure |
| IS | Indoxyl sulfate |
| IL-6 | Interleukin 6 |
| LVEF | Left ventricular ejection fraction |
| LPS | Lipopolysaccharide |
| NF-kB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| RoB | Risk of bias |
| SCFA | Short-chain fatty acids |
| NHE3 | Sodium-Hydrogen exchanger 3 |
| TLRs | Toll-like receptors |
| TGF-β1 | Transforming growth factor beta 1 |
| TMA | Trimethylamine |
| TMAO | Trimethylamine N-oxide |
| TNF-α | Tumor necrosis factor alpha |
References
- Bozkurt, B.; Coats, A.J.S.; Tsutsui, H.; Abdelhamid, C.M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal Definition and Classification of Heart Failure: A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. Eur. J. Heart Fail. 2021, 23, 352–380. [Google Scholar] [PubMed]
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global Burden of Heart Failure: A Comprehensive and Updated Review of Epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287. [Google Scholar] [CrossRef]
- Albert, C.; Estep, J.D. Economic Impact of Chronic Heart Failure Management in Today’s Cost-Conscious Environment. Card. Electrophysiol. Clin. 2019, 11, 1–9. [Google Scholar] [CrossRef]
- Kwok, C.S.; Abramov, D.; Parwani, P.; Ghosh, R.K.; Kittleson, M.; Ahmad, F.Z.; Al Ayoubi, F.; Van Spall, H.G.C.; Mamas, M.A. Cost of Inpatient Heart Failure Care and 30-Day Readmissions in the United States. Int. J. Cardiol. 2021, 329, 115–122. [Google Scholar] [CrossRef]
- Dixit, K.; Chaudhari, D.; Dhotre, D.; Shouche, Y.; Saroj, S. Restoration of Dysbiotic Human Gut Microbiome for Homeostasis. Life Sci. 2021, 278, 119622. [Google Scholar] [CrossRef]
- Yang, X.; Xie, L.; Li, Y.; Wei, C. More than 9,000,000 Unique Genes in Human Gut Bacterial Community: Estimating Gene Numbers inside a Human Body. PLoS ONE 2009, 4, e6074. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Microbiology: Diversity of the Human Intestinal Microbial Flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Huttenhower, C.; Gevers, D.; Knight, R.; Abubucker, S.; Badger, J.H.; Chinwalla, A.T.; Creasy, H.H.; Earl, A.M.; Fitzgerald, M.G.; Fulton, R.S.; et al. Structure, Function and Diversity of the Healthy Human Microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Fadhillah, F.S.; Kona’atul, H.; Juniarto, A.Z.; Sobirin, M.A.; Maharani, N.; Pramono, A. Diet and the Gut Microbiota Profiles in Individuals at Risk of Chronic Heart Failure—A Review on the Asian Population. Asia Pac. J. Clin. Nutr. 2025, 34, 141–152. [Google Scholar]
- Florek, K.; Komorowska, K.; Ptak, J.; Jarocki, M.; Gontarczyk, J.; Mania, R.; Boluk, A.; Żurawska-Płaksej, E.; Łaczmański, Ł.; Sokolski, M. Gut Microbiota’s Role in Heart Failure. Heart Fail. Rev. 2025, ahead of print. [Google Scholar] [CrossRef]
- Vich Vila, A.; Collij, V.; Sanna, S.; Sinha, T.; Imhann, F.; Bourgonje, A.R.; Mujagic, Z.; Jonkers, D.M.A.E.; Masclee, A.A.M.; Fu, J.; et al. Impact of Commonly Used Drugs on the Composition and Metabolic Function of the Gut Microbiota. Nat. Commun. 2020, 11, 362. [Google Scholar] [CrossRef]
- Cienkowski, K.; Cienkowska, A.; Kupczynska, K.; Bielecka-Dabrowa, A. The Role of Gut Microbiota and Its Metabolites in Patients with Heart Failure. Biomedicines 2024, 12, 894. [Google Scholar] [CrossRef]
- Nagatomo, Y.; Tang, W.H.W. Intersections between Microbiome and Heart Failure: Revisiting the Gut Hypothesis. J. Card. Fail. 2015, 21, 973–980. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Carra, M.C.; Romandini, P.; Romandini, M. Risk of Bias Evaluation of Cross-Sectional Studies: Adaptation of the Newcastle-Ottawa Scale. J. Periodontal Res. 2025, ahead of print. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Participants. JAMA 2025, 333, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Pasini, E.; Aquilani, R.; Testa, C.; Baiardi, P.; Angioletti, S.; Boschi, F.; Verri, M.; Dioguardi, F. Pathogenic Gut Flora in Patients with Chronic Heart Failure. JACC Heart Fail. 2016, 4, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Kummen, M.; Mayerhofer, C.C.K.; Vestad, B.; Broch, K.; Awoyemi, A.; Storm-Larsen, C.; Ueland, T.; Yndestad, A.; Hov, J.R.; Trøseid, M. Gut Microbiota Signature in Heart Failure Defined from Profiling of 2 Independent Cohorts. J. Am. Coll. Cardiol. 2018, 71, 1184–1186. [Google Scholar] [CrossRef]
- Hayashi, T.; Yamashita, T.; Takahashi, T.; Tabata, T.; Watanabe, H.; Gotoh, Y.; Shinohara, M.; Kami, K.; Tanaka, H.; Matsumoto, K.; et al. Uncovering the Role of Gut Microbiota in Amino Acid Metabolic Disturbances in Heart Failure Through Metagenomic Analysis. Front. Cardiovasc. Med. 2021, 8, 789325. [Google Scholar] [CrossRef]
- Cui, X.; Ye, L.; Li, J.; Jin, L.; Wang, W.; Li, S.; Bao, M.; Wu, S.; Li, L.; Geng, B.; et al. Metagenomic and Metabolomic Analyses Unveil Dysbiosis of Gut Microbiota in Chronic Heart Failure Patients. Sci. Rep. 2018, 8, 635. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cai, Z.; Ferrari, M.W.; Liu, Y.; Li, C.; Zhang, T.; Lyu, G. The Correlation between Gut Microbiota and Serum Metabolomic in Elderly Patients with Chronic Heart Failure. Mediat. Inflamm. 2021, 2021, 5587428. [Google Scholar] [CrossRef]
- Huang, Z.; Mei, X.; Jiang, Y.; Chen, T.; Zhou, Y. Gut Microbiota in Heart Failure Patients with Preserved Ejection Fraction (GUMPTION Study). Front. Cardiovasc. Med. 2022, 8, 803744. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Du, D.; Fu, T.; Han, Y.; Li, P.; Ju, H. Alterations of the Gut Microbiota in Patients with Severe Chronic Heart Failure. Front. Microbiol. 2022, 12, 813289. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, B.; Sun, Y.; Deng, H.; Wang, H.; Qiao, Z. Alteration of the Gut Microbiota and Metabolite Phenylacetylglutamine in Patients with Severe Chronic Heart Failure. Front. Cardiovasc. Med. 2023, 9, 1076806. [Google Scholar] [CrossRef]
- Peng, J.; Gong, H.; Lyu, X.; Liu, Y.; Li, S.; Tan, S.; Dong, L.; Zhang, X. Characteristics of the Fecal Microbiome and Metabolome in Older Patients with Heart Failure and Sarcopenia. Front. Cell Infect. Microbiol. 2023, 13, 1127041. [Google Scholar] [CrossRef]
- Kamo, T.; Akazawa, H.; Suda, W.; Saga-Kamo, A.; Shimizu, Y.; Yagi, H.; Liu, Q.; Nomura, S.; Naito, A.T.; Takeda, N.; et al. Dysbiosis and Compositional Alterations with Aging in the Gut Microbiota of Patients with Heart Failure. PLoS ONE 2023, 13, 1127041. [Google Scholar] [CrossRef]
- Katsimichas, T.; Ohtani, T.; Motooka, D.; Tsukamoto, Y.; Kioka, H.; Nakamoto, K.; Konishi, S.; Chimura, M.; Sengoku, K.; Miyawaki, H.; et al. Non-Ischemic Heart Failure with Reduced Ejection Fraction Is Associated with Altered Intestinal Microbiota. Circ. J. 2018, 82, 1640–1650. [Google Scholar] [CrossRef]
- Hayashi, T.; Yamashita, T.; Watanabe, H.; Kami, K.; Yoshida, N.; Tabata, T.; Emoto, T.; Sasaki, N.; Mizoguchi, T.; Irino, Y.; et al. Gut Microbiome and Plasma Microbiome-Related Metabolites in Patients with Decompensated and Compensated Heart Failure. Circ. J. 2018, 83, 182–192. [Google Scholar] [CrossRef]
- Beale, A.L.; O’Donnell, J.A.; Nakai, M.E.; Nanayakkara, S.; Vizi, D.; Carter, K.; Dean, E.; Ribeiro, R.V.; Yiallourou, S.; Carrington, M.J.; et al. The Gut Microbiome of Heart Failure with Preserved Ejection Fraction. J. Am. Heart Assoc. 2021, 10, e020654. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.F.; Caparrós-Martin, J.A.; Gray, N.; Lodge, S.; Wist, J.; Lee, S.; O’Gara, F.; Shah, A.; Ward, N.C.; Dwivedi, G. Insights into the Associations between the Gut Microbiome, Its Metabolites, and Heart Failure. Am. J. Physiol. Heart Circ. Physiol. 2023, 325, H1325–H1336. [Google Scholar] [CrossRef]
- Luedde, M.; Winkler, T.; Heinsen, F.A.; Rühlemann, M.C.; Spehlmann, M.E.; Bajrovic, A.; Lieb, W.; Franke, A.; Ott, S.J.; Frey, N. Heart Failure Is Associated with Depletion of Core Intestinal Microbiota. ESC Heart Fail. 2017, 4, 282–290. [Google Scholar] [CrossRef]
- Mayerhofer, C.C.K.; Kummen, M.; Holm, K.; Broch, K.; Awoyemi, A.; Vestad, B.; Storm-Larsen, C.; Seljeflot, I.; Ueland, T.; Bohov, P.; et al. Low Fibre Intake Is Associated with Gut Microbiota Alterations in Chronic Heart Failure. ESC Heart Fail. 2020, 7, 456–466. [Google Scholar] [CrossRef]
- Modrego, J.; Ortega-Hernández, A.; Goirigolzarri, J.; Restrepo-Córdoba, M.A.; Bäuerl, C.; Cortés-Macías, E.; Sánchez-González, S.; Esteban-Fernández, A.; Pérez-Villacastín, J.; Collado, M.C.; et al. Gut Microbiota and Derived Short-Chain Fatty Acids Are Linked to Evolution of Heart Failure Patients. Int. J. Mol. Sci. 2023, 24, 13892. [Google Scholar] [CrossRef]
- Schwinger, R.H.G. Pathophysiology of Heart Failure. Cardiovasc. Diagn. Ther. 2021, 11, 263–276. [Google Scholar] [CrossRef]
- Amir, O.; Rogowski, O.; David, M.; Lahat, N.; Wolff, R.; Lewis, B.S. Circulating Interleukin-10: Association with Higher Mortality in Systolic Heart Failure Patients with Elevated Tumor Necrosis Factor-Alpha. Isr. Med. Assoc. J. 2010, 12, 158–162. [Google Scholar] [PubMed]
- Kunst, C.; Schmid, S.; Michalski, M.; Tümen, D.; Buttenschön, J.; Müller, M.; Gülow, K. The Influence of Gut Microbiota on Oxidative Stress and the Immune System. Biomedicines 2023, 11, 1388. [Google Scholar] [CrossRef]
- Mostafavi Abdolmaleky, H.; Zhou, J.R. Gut Microbiota Dysbiosis, Oxidative Stress, Inflammation, and Epigenetic Alterations in Metabolic Diseases. Antioxidants 2024, 13, 985. [Google Scholar] [CrossRef] [PubMed]
- Fountoulakis, P.N.; Theofilis, P.; Vlachakis, P.K.; Karakasis, P.; Pamporis, K.; Sagris, M.; Dimitroglou, Y.; Tsioufis, P.; Oikonomou, E.; Tsioufis, K.; et al. Gut Microbiota in Heart Failure—The Role of Inflammation. Biomedicines 2025, 13, 911. [Google Scholar] [CrossRef]
- Takala, J. Determinants of Splanchnic Blood Flow. Br. J. Anaesth. 1996, 77, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Sandek, A.; Bjarnason, I.; Volk, H.D.; Crane, R.; Meddings, J.B.; Niebauer, J.; Kalra, P.R.; Buhner, S.; Herrmann, R.; Springer, J.; et al. Studies on Bacterial Endotoxin and Intestinal Absorption Function in Patients with Chronic Heart Failure. Int. J. Cardiol. 2012, 157, 80–85. [Google Scholar] [CrossRef]
- Polsinelli, V.B.; Sinha, A.; Shah, S.J. Visceral Congestion in Heart Failure: Right Ventricular Dysfunction, Splanchnic Hemodynamics, and the Intestinal Microenvironment. Curr. Heart Fail. Rep. 2017, 14, 519–528. [Google Scholar] [CrossRef]
- Rezabakhsh, A.; Habtemariam, S.; Parvizi, R.; Meddahi-Pellé, A.; Ruiz, V.R.; Pavon-Djavid, G.; Barzgari, A. The Gut-Heart Axis: A Correlation between Paneth Cells’ Dysfunction, Microbiome Dysbiosis, and Cardiovascular Diseases. Cell Commun. Signal 2025, 23, 347. [Google Scholar] [CrossRef]
- Branchereau, M.; Burcelin, R.; Heymes, C. The Gut Microbiome and Heart Failure: A Better Gut for a Better Heart. Rev. Endocr. Metab. Disord. 2019, 20, 407–414. [Google Scholar] [CrossRef]
- Madan, S.; Mehra, M.R. Gut Dysbiosis and Heart Failure: Navigating the Universe Within. Eur. J. Heart Fail. 2020, 22, 629–637. [Google Scholar] [CrossRef]
- Schwandner, R.; Dziarski, R.; Wesche, H.; Rothe, M.; Kirschning, C.J. Peptidoglycan- and Lipoteichoic Acid-Induced Cell Activation Is Mediated by Toll-like Receptor 2. J. Biol. Chem. 1999, 274, 17406–17409. [Google Scholar] [CrossRef] [PubMed]
- Soriani, M.; Santi, I.; Taddei, A.; Rappuoli, R.; Grandi, G.; Telford, J.L. Group B Streptococcus Crosses Human Epithelial Cells by a Paracellular Route. J. Infect. Dis. 2006, 193, 241–250. [Google Scholar] [CrossRef]
- Sumitomo, T. Streptococcus pyogenes Translocates across an Epithelial Barrier. Nihon Saikingaku Zasshi 2017, 72, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.; Gautier, T.; Masson, D.; Bouhemad, B.; Guinot, P.G. Endotoxemia in Acute Heart Failure and Cardiogenic Shock: Evidence, Mechanisms and Therapeutic Options. J. Clin. Med. 2023, 12, 2579. [Google Scholar] [CrossRef] [PubMed]
- Asgharzadeh, F.; Bargi, R.; Hosseini, M.; Farzadnia, M.; Khazaei, M. Cardiac and Renal Fibrosis and Oxidative Stress Balance in Lipopolysaccharideinduced Inflammation in Male Rats. ARYA Atheroscler. 2018, 14, 71–77. [Google Scholar]
- Niebauer, J.; Volk, H.D.; Kemp, M.; Dominguez, M.; Schumann, R.R.; Rauchhaus, M.; Poole-Wilson, P.A.; Andrew, J.; Coats, S.; Anker, S.D. Endotoxin and Immune Activation in Chronic Heart Failure: A Prospective Cohort Study. Lancet 1999, 353, 1838–1842. [Google Scholar] [CrossRef] [PubMed]
- Sandek, A.; Bauditz, J.; Swidsinski, A.; Buhner, S.; Weber-Eibel, J.; von Haehling, S.; Schroedl, W.; Karhausen, T.; Doehner, W.; Rauchhaus, M.; et al. Altered Intestinal Function in Patients with Chronic Heart Failure. J. Am. Coll. Cardiol. 2007, 50, 1561–1569. [Google Scholar] [CrossRef]
- Zhen, J.; Zhou, Z.; He, M.; Han, H.X.; Lv, E.H.; Wen, P.B.; Liu, X.; Wang, Y.T.; Cai, X.C.; Tian, J.Q.; et al. The Gut Microbial Metabolite Trimethylamine N-Oxide and Cardiovascular Diseases. Front. Endocrinol. 2023, 14, 1085041. [Google Scholar] [CrossRef]
- Dalla Via, A.; Gargari, G.; Taverniti, V.; Rondini, G.; Velardi, I.; Gambaro, V.; Visconti, G.L.; De Vitis, V.; Gardana, C.; Ragg, E.; et al. Urinary TMAO Levels Are Associated with the Taxonomic Composition of the Gut Microbiota and with the Choline TMA-Lyase Gene (CutC) Harbored by Enterobacteriaceae. Nutrients 2019, 12, 62. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Zhang, H.; Xiang, Q.; Hu, H.; Zhai, C.; Xu, S.; Tian, H. Role of Trimethylamine N-Oxide in Heart Failure. Rev. Cardiovasc. Med. 2024, 25, 240. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, Z.; Yan, J.; Liu, H.; Liu, Q.; Deng, Y.; Ou, C.; Chen, M. Gut Microbe-Derived Metabolite Trimethylamine N-Oxide Induces Cardiac Hypertrophy and Fibrosis. Lab. Investig. 2019, 99, 346–357. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Wang, Z.; Fan, Y.; Levison, B.; Hazen, J.E.; Donahue, L.M.; Wu, Y.; Hazen, S.L. Prognostic Value of Elevated Levels of Intestinal Microbe-Generated Metabolite Trimethylamine-N-Oxide in Patients with Heart Failure: Refining the Gut Hypothesis. J. Am. Coll. Cardiol. 2014, 64, 1908–1914. [Google Scholar] [CrossRef]
- Li, W.; Huang, A.; Zhu, H.; Liu, X.; Huang, X.; Huang, Y.; Cai, X.; Lu, J.; Huang, Y. Gut Microbiota-Derived Trimethylamine N-Oxide Is Associated with Poor Prognosis in Patients with Heart Failure. Med. J. Aust. 2020, 213, 374–379. [Google Scholar] [CrossRef]
- Jarmukhanov, Z.; Mukhanbetzhanov, N.; Kozhakhmetov, S.; Nurgaziyev, M.; Sailybayeva, A.; Bekbossynova, M.; Kushugulova, A. The Association between the Gut Microbiota Metabolite Trimethylamine N-Oxide and Heart Failure. Front. Microbiol. 2024, 15, 1440241. [Google Scholar] [CrossRef]
- Imazu, M.; Takahama, H.; Shindo, K.; Hasegawa, T.; Kanzaki, H.; Anzai, T.; Asanuma, H.; Morita, T.; Asakura, M.; Kitakaze, M. A Pathophysiological Role of Plasma Indoxyl Sulfate in Patients with Heart Failure. Int. J. Gerontol. 2017, 11, 62–66. [Google Scholar] [CrossRef]
- Imazu, M.; Fukuda, H.; Kanzaki, H.; Amaki, M.; Hasegawa, T.; Takahama, H.; Hitsumoto, T.; Tsukamoto, O.; Morita, T.; Ito, S.; et al. Plasma Indoxyl Sulfate Levels Predict Cardiovascular Events in Patients with Mild Chronic Heart Failure. Sci. Rep. 2020, 10, 16528. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The Role of Short-Chain Fatty Acids in Intestinal Barrier Function, Inflammation, Oxidative Stress, and Colonic Carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef]
- Vinolo, M.A.R.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of Inflammation by Short Chain Fatty Acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef]
- Yukino-Iwashita, M.; Nagatomo, Y.; Kawai, A.; Taruoka, A.; Yumita, Y.; Kagami, K.; Yasuda, R.; Toya, T.; Ikegami, Y.; Masaki, N.; et al. Short-Chain Fatty Acids in Gut–Heart Axis: Their Role in the Pathology of Heart Failure. J. Pers. Med. 2022, 12, 1805. [Google Scholar] [CrossRef]
- Wang, A.; Li, Z.; Sun, Z.; Zhang, D.; Ma, X. Gut-Derived Short-Chain Fatty Acids Bridge Cardiac and Systemic Metabolism and Immunity in Heart Failure. J. Nutr. Biochem. 2023, 120, 109370. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal Microbiota Metabolism of L-Carnitine, a Nutrient in Red Meat, Promotes Atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- González-Gómez, Á.; Cantone, M.; García-Muñoz, A.M.; Victoria-Montesinos, D.; Lucas-Abellán, C.; Serrano-Martínez, A.; Muñoz-Morillas, A.M.; Morillas-Ruiz, J.M. Effect of Polyphenol-Rich Interventions on Gut Microbiota and Inflammatory or Oxidative Stress Markers in Adults Who Are Overweight or Obese: A Systematic Review and Meta-Analysis. Nutrients 2025, 17, 2468. [Google Scholar] [CrossRef]
- Masenga, S.K.; Kirabo, A. Salt and Gut Microbiota in Heart Failure. Curr. Hypertens. Rep. 2023, 25, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.F.; Aden, L.A.; Barbaro, N.R.; Van Beusecum, J.P.; Xiao, L.; Simons, A.J.; Warden, C.; Pasic, L.; Himmel, L.E.; Washington, M.K.; et al. High Dietary Salt–Induced DC Activation Underlies Microbial Dysbiosis-Associated Hypertension. JCI Insight 2019, 5, e126241. [Google Scholar] [CrossRef]
- Cronin, P.; Joyce, S.A.; O’Toole, P.W.; O’Connor, E.M. Dietary Fibre Modulates the Gut Microbiota. Nutrients 2021, 13, 1655. [Google Scholar] [CrossRef] [PubMed]
- Leal-Witt, M.J.; Llobet, M.; Samino, S.; Castellano, P.; Cuadras, D.; Jimenez-Chillaron, J.C.; Yanes, O.; Ramon-Krauel, M.; Lerin, C. Lifestyle Intervention Decreases Urine Trimethylamine N-Oxide Levels in Prepubertal Children with Obesity. Obesity 2018, 26, 1603–1610. [Google Scholar] [CrossRef]
- Tuttolomondo, A.; Simonetta, I.; Daidone, M.; Mogavero, A.; Ortello, A.; Pinto, A. Metabolic and Vascular Effect of the Mediterranean Diet. Int. J. Mol. Sci. 2019, 20, 4716. [Google Scholar] [CrossRef] [PubMed]
- Crimarco, A.; Springfield, S.; Petlura, C.; Streaty, T.; Cunanan, K.; Lee, J.; Fielding-Singh, P.; Carter, M.M.; Topf, M.A.; Wastyk, H.C.; et al. A Randomized Crossover Trial on the Effect of Plant-Based Compared with Animal-Based Meat on Trimethylamine-N-Oxide and Cardiovascular Disease Risk Factors in Generally Healthy Adults: Study with Appetizing Plantfood—Meat Eating Alternative Trial (SWAP-MEAT). Am. J. Clin. Nutr. 2020, 112, 1188–1199. [Google Scholar]
- Argyridou, S.; Davies, M.J.; Biddle, G.J.H.; Bernieh, D.; Suzuki, T.; Dawkins, N.P.; Rowlands, A.V.; Khunti, K.; Smith, A.C.; Yates, T. Evaluation of an 8-Week Vegan Diet on Plasma Trimethylamine-N-Oxide and Postchallenge Glucose in Adults with Dysglycemia or Obesity. J. Nutr. 2021, 151, 1844–1853. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.T.; Ettinger, G.; Huang, C.X.; Burton, J.P.; Haist, J.V.; Rajapurohitam, V.; Sidaway, J.E.; Martin, G.; Gloor, G.B.; Swann, J.R.; et al. Probiotic Administration Attenuates Myocardial Hypertrophy and Heart Failure after Myocardial Infarction in the Rat. Circ. Heart Fail. 2014, 7, 491–499. [Google Scholar] [CrossRef]
- Awoyemi, A.; Mayerhofer, C.; Felix, A.S.; Hov, J.R.; Moscavitch, S.D.; Lappegård, K.T.; Hovland, A.; Halvorsen, S.; Halvorsen, B.; Gregersen, I.; et al. Rifaximin or Saccharomyces Boulardii in Heart Failure with Reduced Ejection Fraction: Results from the Randomized GutHeart Trial. EBioMedicine 2021, 70, 103511. [Google Scholar] [CrossRef]
- Taslim, N.A.; Yusuf, M.; Ambari, A.M.; Del Rosario Puling, I.M.; Ibrahim, F.Z.; Hardinsyah, H.; Kurniawan, R.; Gunawan, W.B.; Mayulu, N.; Joseph, V.F.F.; et al. Anti-Inflammatory, Antioxidant, Metabolic and Gut Microbiota Modulation Activities of Probiotic in Cardiac Remodeling Condition: Evidence from Systematic Study and Meta-Analysis of Randomized Controlled Trials. Probiotics Antimicrob. Proteins 2023, 15, 1049–1061. [Google Scholar] [CrossRef]
- Yan, Q.; Zhai, W.; Yang, C.; Li, Z.; Mao, L.; Zhao, M.; Wu, X. The Relationship among Physical Activity, Intestinal Flora, and Cardiovascular Disease. Cardiovasc. Ther. 2021, 2021, 3364418. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, H.; Tanisawa, K.; Sun, X.; Kubo, T.; Hoshino, Y.; Hosokawa, M.; Takeyama, H.; Higuchi, M. Effects of Short-Term Endurance Exercise on Gut Microbiota in Elderly Men. Physiol. Rep. 2018, 6, e13935. [Google Scholar] [CrossRef]
- Jia, Q.; Li, H.; Zhou, H.; Zhang, X.; Zhang, A.; Xie, Y.; Li, Y.; Lv, S.; Zhang, J. Role and Effective Therapeutic Target of Gut Microbiota in Heart Failure. Cardiovasc. Ther. 2019, 2019, 5164298. [Google Scholar] [CrossRef]
- Simadibrata, D.M.; Auliani, S.; Widyastuti, P.A.; Wijaya, A.D.; Amin, H.Z.; Muliawan, H.S.; Siswanto, B.B.; Simadibrata, M. The Gut Microbiota Profile in Heart Failure Patients: A Systematic Review. J. Gastrointestin Liver Dis. 2023, 32, 393–401. [Google Scholar] [CrossRef] [PubMed]


| Author | Year | Country | Journal | Case/Control | n | Male, n (%) | Age, Years | BMI, kg/m2 | HT, n (%) | T2DM, n (%) | eGFR, mL/min/1.73m2 | CRP, mg/L | LVEF, % | BNP, ng/L | NTproBNP, ng/L | Sequencing Method | NOS-xs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kamo et al. [27] | 2017 | Japan | PLoS One | HF < 60 y (HFpEF 0%) | 12 | 11 (92) | 47.4 ± 2.8 | 22.9 ± 1.2 | 1 (8) | 4 (33) | 54.7 ± 3.1 | NR | 20.0 ± 2.2 ** | 1060.6 ± 238.8 | NR | 16S rRNA (V1-V2) | 6 |
| HF > 60 y (HFpEF 40%) | 10 | 7 (70) | 73.8 ± 2.8 * | 24.9 ± 1.7 | 6 (60) | 3 (30) | 40.4 ± 7.4 | NR | 43.1 ± 5.8 | 697.7 ± 176.0 | NR | ||||||
| HC | 12 | 9 (75) | 41.4 ± 2.0 | 23.2 ± 0.6 | 0 (0) | 0 (0) | NR | NR | NR | NR | NR | ||||||
| Luedde et al. [32] | 2017 | Germany | ESC Heart Fail | HFrEF | 20 | 11 (55) | 65 ± 3.2 | 29.7 ± 1.4 | 14 (70) | 7 (35) | NR | 11.1 ± 2.1 * | 22.3 ± 2.9 | NR | 6564,5 ± 1187,23 * | 16S rRNA (V1-V2) | 7 |
| HC | 20 | 11 (55) | 65 ± 3.1 | 29.1 ± 1.3 | 8 (40) | 3 (15) | NR | 4.2 ± 1.1 | NR | NR | 109,2 ± 45,91 | ||||||
| Cui et al. [21] | 2018 | China | Sci Rep | HFrEF | 53 | 44 (83) | 58.1 ± 13.3 | 24.4 ± 4.5 | 30 (57) * | 15 (28) * | NR | 3.4 (2.3–8.4) * | 29.8 ± 6.6 | NR | NR | Metagenomic sequencing | 7 |
| HC | 41 | 32 (78) | 53.7 ± 5.9 | 25.2 ± 3.3 | 0 (0) | 2 (5) | NR | 2 (1–3) | NR | NR | NR | ||||||
| Katsimichas et al. [28] | 2018 | Japan | Circ J | HFrEF | 28 | 21 (75) | 51 ± 10 * | 21.7 ± 3.4 | NR | NR | 64.4 ± 25.2 * | 6.7 (3.8–35.2) * | 25 ± 9 * | 375 (145–630) * | NR | 16S rRNA (V1-V2) | 8 |
| HC | 19 | 15 (84.2) | 36 ± 6 | 21.8 ± 1.9 | NR | NR | 87.0 ± 16.9 * | 2.9 (1.9–3.8) | 62 ± 4 | 2 (2–6) | NR | ||||||
| Hayashi et al. [20] | 2019 | Japan | Circ J | Decomp HF | 22 | 14 (64) | 72 ± 18 | 25.8 ± 7.1 † | 21 (95) | 8 (36) | 48.8 ± 19.4 | NR | 42 ± 17 * | 445 (328–763) *,† | NR | 16S rRNA (V3-V4) | 7 |
| Comp HF | 23.6 ± 5.9 | 49.0 ± 22.5 | NR | 284 (152–378) * | NR | ||||||||||||
| HC | 11 | 6 (55) | 72 ± 7 | 24.4 ± 3.1 | 9 (82) | 5 (45) | 54.0 ± 11.7 | NR | 63 ± 4 | 53 (23–109) | NR | ||||||
| Mayerhofer et al. [33] | 2020 | Norway | ESC Heart Fail | HFrEF | 84 | 34 (40.5) | 59 (39–74) * | 27.9 (26.8–29.1) | 25 (29.8) * | 18 (21.4) * | 68.9 (64.3–73.5) | 3.3 (2.3–4.4) | 28.2 ± 7.3 | NR | 2664.1 (1726.2–3602.0) | 16S rRNA (V3-V4) | 7 |
| HC | 266 | 107 (40.2) | 46 (30–61) | 26.4 (25.9–26.9) | 11 (4.1) | 2 (0.8) | NR | NR | NR | NR | NR | ||||||
| Beale et al. [30] | 2021 | Australia | J Am Heart Assoc | HFpEF | 26 | 6 (23) ‡ | 68 ± 7.5 * | 32.8 ± 5.8 * | 18 (69) * | 4 (15) * | NR | NR | 60.5 ± 5.1 | NR | NR | 16S rRNA (V4-V5) | 8 |
| Metropolitan HC | 39 | 22 (56) | 58.3 ± 7.9 | 25.1 ± 2.9 | 15 (38) | 0 (0) | NR | NR | NR | NR | NR | ||||||
| Regional HC | 28 | 9 (32) | 61 ± 6 | 25.3 ± 2.5 | 5 (18) | 0 (0) | NR | NR | NR | NR | NR | ||||||
| Wang et al. [22] | 2021 | China | Mediators Inflamm | HF | 25 | 14 (56) | 65 ± 3.2 | 29.7 ± 1.4 | NR | NR | NR | 11.1 ± 2.1 * | NR | NR | 6564.5 ± 1187.2 * | 16S rDNA (V3-V4) | 5 |
| HC | 25 | 13 (52) | 65 ± 3.1 | 29.1 ± 1.3 | NR | NR | NR | 4.2 ± 1.1 | NR | NR | 109.2 ± 45.9 | ||||||
| Huang et al. [23] | 2022 | China | Front Cardiovasc Med | HFpEF | 30 | 19 (63.3) | 71.2 ± 9.4 | 23.8 ± 3 | 25 (83.3) | NR | NR | NR | NR | NR | NR | 16S rRNA (V4-V5) | 5 |
| HC | 30 | 17 (56.7) | 67 ± 7.4 | 23.9 ± 3 | NR | NR | NR | Nr | NR | NR | NR | ||||||
| Sun et al. [24] | 2022 | China | Front Microbiol | HF | 29 | 24 (82.8) * | 60.7 ± 11.7 | 24.0 ± 3.5 | 14 (48) | 10 (34) | NR | NR | 33.8 ± 9.1* | NR | 4745.7 (1130–16,755) * | 16S rRNA (V3-V4) | 5 |
| HC | 30 | 10 (33.3) | 60 ± 9.6 | 24.9 ± 3.1 | 11 (37) | 5 (16.7) | NR | NR | 63.2 ± 4.7 | NR | 124 (25–258) | ||||||
| Zhang et al. [25] | 2023 | China | Front Cardiovasc Med | HF NYHA III | 29 | 26 (44.8) * | 77 (73.5–83.5) | NR | 19 (65.5) | NR | NR | NR | 63 (42.5–66.5) * | 1014.7 (802.6–1321) * | NR | 16S rRNA (V3-V4) | 5 |
| HF NYHA IV | 29 | 79 (69.5–86.5) | NR | 17 (58.6) | NR | NR | NR | 43 (38.5–50) * | 2789.2 (2256.8–3781.6) * | NR | |||||||
| HC | 22 | 13 (59) | 76 (73.8–80) | NR | 13 (59) | NR | NR | NR | 67 (62–71.3) | 42.5 (15.5–102.7) | NR | ||||||
| Peng et al. [26] | 2023 | China | Front Cell Infect Microbiol | No sarcopenia HF | 33 | 24 (72.7) | 71.8 ± 7.9 | 24.2 ± 2.8 | NR | NR | NR | NR | 57 (39.5–61.5) * | NR | 1084 (372–3200) * | 16S rRNA (V3-V4) | 5 |
| Sarcopenia HF | 29 | 13 (44.8) | 75.1 ± 8.2 | 20.7 ± 3.8 * | NR | NR | NR | NR | 55 (38–60) * | NR | 1424 (514–4830) * | ||||||
| HC | 15 | 8 (53.3) | 67.7 ± 9.8 | 23.5 ± 3.1 | NR | NR | NR | NR | 63 (60–65) | NR | 73.6 (27.1–120) | ||||||
| Ahmad et al. [31] | 2023 | Australia | Am J Physiol Heart Circ Physiol | HFrEF | 73 | 61 (83.5) * | 59.8 ± 12.4 | 30.71 ± 6.1 * | 31 (42.5) | 20 (27.4) | NR | 35.9 ± 46.3 | 29.5 ± 9.4 | 765.2 ± 1051.1 | 2983.7 ± 5245.5 | 16S rRNA (V3-V4) | 8 |
| HC | 59 | 11 (18.6) | 56.0 ± 9.2 | 26.4 ± 3.9 | NR | NR | NR | NR | NR | NR | NR | ||||||
| Modrego et al. [34] | 2023 | Spain | Int J Mol Sci | de novo HF | 18 | 7 (38.9) | 67.6 ± 4.1 | NR | NR | 7 (38.9) | NR | 7.5 (3.7–22.2) § | 36.2 ± 4.3 § | NR | 7081 ± 3544 § | 16S rRNA | 7 |
| 12-months follow-up | 68.3 ± 4.3 | NR | NR | NR | 2.9 (2.7–5.3) | 56.7 ± 3.5 | NR | 358 ± 69 |
| Alpha Diversity | Beta Diversity | |||
|---|---|---|---|---|
| Overall Diversity | Richness | Evenness | ||
| HFpEF + HFrEF | ||||
| Kamo et al. (2017) [27] | (=) | (=) | NR | Significant differences between groups |
| Hayashi et al. (2019) [29] | (=) | NR | NR | NR |
| Wang et al. (2021) [22] | ↓ | ↓ | ↓ | Significant differences between groups |
| Sun et al. (2022) [24] | ↓ | ↓ | ↓ | Significant differences between groups |
| Zhang et al. (2023) [25] | ↓ | ↓ | NR | Significant differences between groups |
| Peng et al. (2023) [26] | ↓ | ↓ | ↓ | Significant differences between groups |
| Modrego et al. (2023) [34] | (=) | NR | (=) | No differences between baseline vs. 12-months follow-up |
| HFpEF | ||||
| Beale et al. (2021) [30] | ↓ | ↓ | NR | Significant differences between groups |
| Huang et al. (2022) [23] | ↓ | ↓ | (=) | Significant differences between groups |
| HFrEF | ||||
| Luedde et al. (2017) [32] | ↓ | (=) | NR | Significant differences between groups |
| Cui et al. (2018) [21] | NR | NR | NR | Significant differences between groups |
| Katsimichas et al. (2018) [28] | (=) | (=) | (=) | Significant differences between groups |
| Mayerhofer et al. (2020) [33] | NR | ↓ | NR | Significant differences between groups |
| Ahmad et al. (2023) [31] | ↓ | NR | NR | NR |
| Phylum | Class | Family | Genus | Species |
|---|---|---|---|---|
| Bacillota (Firmicutes) = [27], ↓ [24,25,26,33] | Clostridia ↓ [26,31] | Clostridiaceae | Clostridium ↓ [27] | |
| SMB53 ↓ [28] | ||||
| Ruminococcaceae ↑ [22], ↓ [24,30,31] | Ruminococcus = [27], ↑ [21,22], ↓ [30] | Ruminococcus gnavus ↑ [21] | ||
| Faecalibacterium = [27], ↓ [21,24,26,32,33] | Faecalibacterium prausnitzii ↓ [21] | |||
| Oscillibacter ↓ [21] | Oscillibacter sp. ↓ [21] | |||
| Butyricicoccus ↓ [23] | ||||
| Ruminiclostridium ↓ [23] | ||||
| Uncl. Ruminococcaceae ↓ [32] | ||||
| Lachnospiraceae ↓ [24,25,31,33] | Lachnospira ↓ [23,31] | |||
| Blautia = [27], ↓ [26,31,33] | ||||
| Anaerostipes = [27], ↓ [33] | ||||
| Dorea ↓ [27] | ||||
| Hungatella ↑ [33] | ||||
| Fusicatenibacter ↓ [33] | ||||
| Pseudobutyrivibrio ↓ [33] | ||||
| Coprococcus ↓ [33] | ||||
| Agathobacter ↓ [25] | ||||
| Caldicellulosiruptoraceae | Caldicellulosiruptor ↓ [30] | |||
| Eubacteriaceae | Eubacterium ↓ [27,33] | Eubacterium rectale ↓ [27] | ||
| Peptostreptococcaceae ↓ [26] | Romboutsia ↑ [22] | |||
| Negativicutes | Veillonellaceae | Veillonella ↑ [21,28] | Veillonella sp. ↑ [21] | |
| Megamonas ↑ [31] **, ↓ [25,29] | ||||
| Megasphaera ↑ [30] | ||||
| Mitsuokella ↓ [30] | ||||
| Dialister ↓ [24] | ||||
| Acidaminococcaceae | Acidaminococcus ↑ [34] * | |||
| Succiniclasticum ↑ [33] | ||||
| Erysipelotrichia | Erysipelotrichaceae ↓ [32] | Uncl. Erysipelotrichaceae ↓ [32] | ||
| L7A_E11 ↓ [30] | ||||
| Bacilli | Enterococcaceae ↑ [24] | Enterococcus ↑ [23,24] | ||
| Leuconostocaceae ↑ [26] | ||||
| Streptococcaceae | Streptococcus ↑ [21,22,25,27,28,30] | Streptococcus sp. ↑ [21] | ||
| Lactobacillaceae | Lactobacillus ↑ [21,22,23,25,27] | |||
| Lacticaseibacillus ↑ [31] § | ||||
| Ammoniphilaceae | Ammoniphilus ↑ [30] | |||
| RF39 ↓ [34] * | ||||
| Mollicutes | Acholeplasmataceae | Acholeplasma ↑ [30] | ||
| Bacteroidota (Bacteroidetes) = [25,27], ↑ [33], ↓ [24,26] | Bacteroidia | Prevotellaceae ↓ [26] | Prevotella = [27], ↑ [31] **, [33], ↓ [26] | |
| Barnesiellaceae ↑ [31] ** | ||||
| Bacteroidaceae | Bacteroides = [27], ↑ [30] | |||
| Tannerellaceae | Parabacteroides = [27] | |||
| Rikenellaceae | Alistipes ↓ [21] | |||
| Actinomycetota (Actinobacteria) = [27], ↑ [24,26,29] | Coriobacteriia | Coriobacteriaceae ↓ [32] | Collinsella = [27], ↓ [32] | |
| Atopobiaceae | Atopobium ↑ [22] | |||
| Libanicoccus ↑ [31] ** | ||||
| Eggerthellaceae | Slackia ↑ [26] | |||
| Actinomycetes | Nocardiaceae ↑ [26] | |||
| Pseudonocardiaceae ↑ [26] | ||||
| Bifidobacteriaceae | Bifidobacterium = [27], ↑ [25], [29] †, [34] *, ↓ [33] | |||
| Pseudomonadota (Proteobacteria) = [27], ↑ [24,26], ↓ [34] * | Alphaproteobacteria ↑ [26] | Sphingosinicellaceae | Sphingosinicella ↓ [34] * | |
| Sphingomonadaceae | Sphingomonas ↓ [34] * | |||
| Bradyrhizobiaceae | Bradyrhizobium ↓ [34] * | |||
| Betaproteobacteria | Sutterellaceae | Sutterella ↑ [30], ↓ [23] | Sutterella wadsworthensis ↓ [21] | |
| Gammaproteobacteria | Enterobacteriaceae ↑ [25] | Escherichia-Shigella = [27], ↑ [22,24,25], [29] ‡, [32] | ||
| Klebsiella = [27], ↑ [22,24,25] | ||||
| Pasteurellaceae | Haemophilus ↑ [22] | |||
| Erwiniaceae | Erwinia ↑ [30] | |||
| Moraxellaceae | Acinetobacter ↑ [21] | |||
| Pectobacteriaceae | Pectobacterium ↓ [34] * | |||
| Synergistota ↑ [27] | ||||
| Verrucomicrobiota | Verrucomicrobiae | Akkermansiaceae | Akkermansia ↑ [30] |
| Phylum | Class | Family | Genus | ||||
|---|---|---|---|---|---|---|---|
| Bacillota | ↓ 29% | Clostridia | ↓ 14% | Lachnospiraceae | ↓ 30% | Lachnospira | ↓ 15% |
| Blautia | ↓ 23% | ||||||
| Ruminococcaceae | ↓ 21% | ||||||
| Eubacteriaceae | Eubacterium | ↓ 15% | |||||
| Oscillospiraceae | Faecalibacterium | ↓ 38% | |||||
| Bacilli | Streptococcaceae | Streptococcus | ↑ 46% | ||||
| Lactobacillaceae | Lactobacillus | ↑ 38% | |||||
| Enterococcaceae | Enterococcus | ↑ 15% | |||||
| Pseudomonadota | ↑ 14% | Gammaproteobacteria | Enterobacteriaceae | Escherichia-Shigella | ↑ 38% | ||
| Klebsiella | ↑ 23% | ||||||
| Alphaproteobacteria | ↑ 7% | ||||||
| Actinomycetota | ↑ 21% | ||||||
| Synergistota | ↑ 7% |
| Author | Country | Antibiotics | Probiotics | Proton Pump Inhibitors | Diuretics | Diet |
|---|---|---|---|---|---|---|
| Kamo et al., 2017 [27] | Japan | Excluded if taken 2 months before | Excluded if taken 2 months before | Collected No analysis | Not collected | Not collected |
| Luedde et al., 2017 [32] | Germany | Excluded if taken 3 months before | Excluded if taken 3 months before | Not collected | Collected Multivariate analysis | All HF patients and HC consumed a mixed European diet |
| Cui et al., 2018 [21] | China | Excluded if taken 1 month before | Excluded if taken 1 month before | Collected Multivariate analysis | Collected as part of the study population characteristics | Not collected |
| Katsimichas et al., 2018 [28] | Japan | Excluded if taken 1 month before | Excluded if taken 1 month before | Collected Multivariate analysis | Not collected | Analysis of diet differences between HF and HC groups |
| Hayashi et al., 2019 [20] | Japan | Excluded if taken 1 month before | Not collected | Collected Analysis of differences in genera between users vs. non-users | Collected as part of the study population characteristics | Not collected |
| Mayerhofer et al., 2020 [33] | Norway | Excluded if taken 3 months before | Not collected | Not collected | Collected as part of the study population characteristics | Collected in HF validation group Correlation analysis between diet, bacteria and metabolites |
| Beale et al., 2021 [30] | Australia | Excluded if taken 3 months before | Excluded if taken 3 months before | Not collected | Collected as part of the study population characteristics | Multivariable Analysis between HF patients and HC |
| Wang et al., 2021 [22] | China | Not collected | Not collected | Not collected | Not collected | Not collected |
| Huang et al., 2022 [23] | China | Excluded if taken 3 months before | Excluded if taken 3 months before | Not collected | Not collected | Not collected |
| Sun et al., 2022 [24] | China | Excluded if taken 1 month before | Excluded if taken 1 month before | Not collected | Not collected | Not collected |
| Zhang et al., 2023 [25] | China | Excluded if taken 3 months before | Excluded if taken 3 months before | Not collected | Collected as part of the study population characteristics | Not collected |
| Peng et al., 2023 [26] | China | Excluded if taken 1 month before | Excluded if taken 1 month before | Not collected | Collected as part of the study population characteristics | Not collected |
| Ahmad et al., 2023 [31] | Australia | Excluded if taken 3 months before | Excluded if taken 3 months before | Not collected | Collected as part of the study population characteristics | Not collected |
| Modrego et al., 2023 [34] | Spain | Excluded if taken 2 months before | Excluded if taken 2 months before | Not collected | Collected as part of the study population characteristics | Analysis of diet differences between admission and 12 months follow-up |
| Increase | Decrease |
|---|---|
| Para-Tolyl octanoate [21] | Niacin [21] |
| Homocitrulline [22] | Cinnamic acid [21] |
| Sphingosine 1− phosphate [21] | Orotic acid [21] |
| Ethylsalicylate [22] | Riboflavin [22] |
| Acetate [31] | Citramalate [22] |
| Phenylacetylglutamine [25] | Biocytin [22] |
| Soluble CD14 [31] | Indoxyl sulfate [29] |
| Trimethylamine N-oxide [29,31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Báez-Ferrer, N.; Lemus-Martín, A.; Castro-Hernández, M.B.; Avanzas, P.; Martínez-González, S.; Lecuona-Fernández, M.; Domínguez-Rodríguez, A. Gut Microbiota Alterations in Heart Failure Patients: Insights from a Systematic Review. J. Clin. Med. 2025, 14, 8110. https://doi.org/10.3390/jcm14228110
Báez-Ferrer N, Lemus-Martín A, Castro-Hernández MB, Avanzas P, Martínez-González S, Lecuona-Fernández M, Domínguez-Rodríguez A. Gut Microbiota Alterations in Heart Failure Patients: Insights from a Systematic Review. Journal of Clinical Medicine. 2025; 14(22):8110. https://doi.org/10.3390/jcm14228110
Chicago/Turabian StyleBáez-Ferrer, Néstor, Alejandro Lemus-Martín, María Beatriz Castro-Hernández, Pablo Avanzas, Susana Martínez-González, María Lecuona-Fernández, and Alberto Domínguez-Rodríguez. 2025. "Gut Microbiota Alterations in Heart Failure Patients: Insights from a Systematic Review" Journal of Clinical Medicine 14, no. 22: 8110. https://doi.org/10.3390/jcm14228110
APA StyleBáez-Ferrer, N., Lemus-Martín, A., Castro-Hernández, M. B., Avanzas, P., Martínez-González, S., Lecuona-Fernández, M., & Domínguez-Rodríguez, A. (2025). Gut Microbiota Alterations in Heart Failure Patients: Insights from a Systematic Review. Journal of Clinical Medicine, 14(22), 8110. https://doi.org/10.3390/jcm14228110









