General Anesthesia or Spinal Anesthesia and Serum Endocan Release After Surgery: A Prospective Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Data Collection
2.3. Statistical Analysis
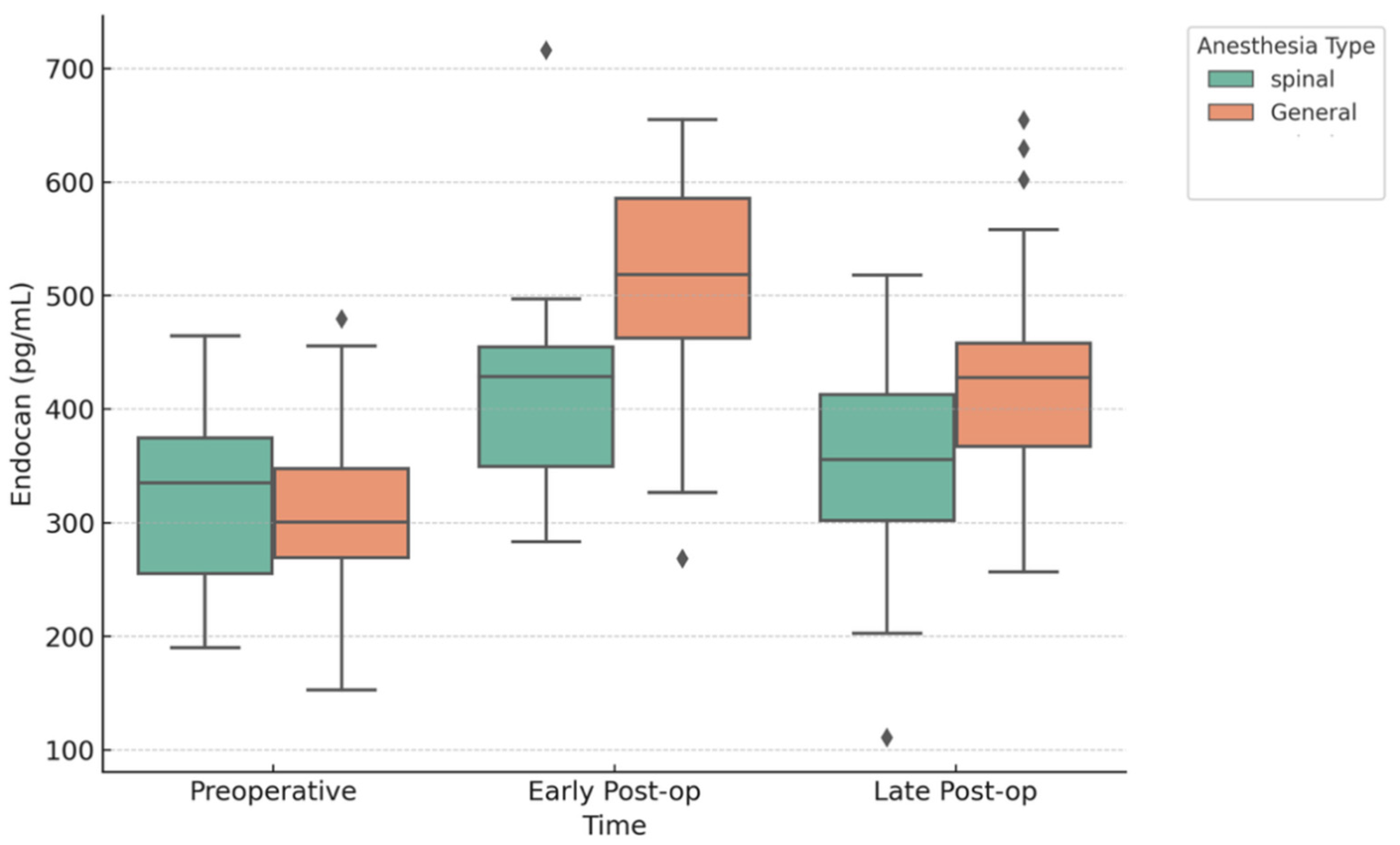
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Letchuman, V.; Agarwal, N.; Mummaneni, V.P.; Wang, M.Y.; Shabani, S.; Patel, A.; Mummaneni, P.V. Awake spinal surgery: Simplifying the learning curve with a patient selection algorithm. Neurosurg. Focus 2021, 51, E2. [Google Scholar] [CrossRef]
- De Biase, G.; Gruenbaum, S.E.; West, J.L.; Chen, S.; Bojaxhi, E.; Kryzanski, J.; Abode-Iyamah, K. Spinal versus general anesthesia for minimally invasive transforaminal lumbar interbody fusion: Implications on operating room time, pain, and ambulation. Neurosurg. Focus 2021, 51, E3. [Google Scholar] [CrossRef]
- Wang, M.Y.; Grossman, J. Endoscopic minimally invasive transforaminal interbody fusion without general anesthesia: Initial clinical experience with 1-year follow-up. Neurosurg. Focus 2016, 40, E13. [Google Scholar] [CrossRef]
- De Biase, G.; Gruenbaum, S.E.; Quiñones-Hinojosa, A.; Abode-Iyamah, K.O. Spine Surgery Under Spinal vs. General Anesthesia: Prospective Analysis of Quality of Life, Fatigue, and Cognition. Neurosurgery 2022, 90, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Rajjoub, R.; Ghaith, A.K.; El-Hajj, V.G.; Rios-Zermano, J.; De Biase, G.; Atallah, E.; Abode-Iyamah, K. Comparative outcomes of awake spine surgery under spinal versus general anesthesia: A comprehensive systematic review and meta-analysis. Eur. Spine J. 2024, 33, 985–1000. [Google Scholar] [CrossRef] [PubMed]
- Taborsky, A.; Dexter, F.; Novak, A.; Espy, J.L.; Sondekoppam, R.V. The impact of spinal versus general anesthesia on the variability of surgical times: A systematic review and meta-analysis. L’impact de la rachianesthésie par rapport à l’anesthésie générale sur la variabilité des temps chirurgicaux: Une revue systématique et méta-analyse. Can. J. Anaesth. 2025, 72, 91–105. [Google Scholar] [CrossRef]
- Sarrazin, S.; Adam, E.; Lyon, M.; Depontieu, F.; Motte, V.; Landolfi, C.; Delehedde, M. Endocan or endothelial cell-specific molecule-1 (ESM1): A potential novel endothelial cell marker and a new target for cancer therapy. Biochim. Biophys. Acta (BBA) Rev. Cancer 2006, 1765, 25–37. [Google Scholar] [CrossRef]
- Balta, S.; Mikhailidis, D.P.; Demirkol, S.; Ozturk, C.; Celik, T.; Iyisoy, A. Endocan: A novel inflammatory indicator in cardiovascular disease? Atherosclerosis 2015, 243, 339–343. [Google Scholar] [CrossRef]
- Bidlan, M.; Kumar, M.; Bhargava, A.; Goel, V.; Kumar, V. Endocan, a Potential Biomarker of Endothelial Dysfunction: A Brief Overview. Angiology 2025. [Google Scholar] [CrossRef]
- Kucukbas, G.N.; Sanhal, C.Y.; Uygur, D. Plasma Endocan Levels in Early and Late-Onset Preeclampsia. Fetal Pediatr. Pathol. 2021, 40, 214–221. [Google Scholar] [CrossRef]
- Boavista, B.H.L.; Leme, S.P.; Ferreira, C.F.; Pelosi, P.; Rieken, M.R.P. Immunomodulatory effects of anesthetic agents in perioperative medicine. Minerva Anestesiol. 2020, 86, 181–195. [Google Scholar] [CrossRef]
- White, C.W.; Avery, E.; Müller, A.; Li, Y.; Le, H.; Thliveris, J.; Freed, D.H. Avoidance of profound hypothermia during initial reperfusion improves the functional recovery of hearts donated after circulatory death: Hypothermia inhibits DCD heart resuscitation. Am. J. Transplant. 2016, 16, 773–782. [Google Scholar] [CrossRef]
- Méndez-Carmona, N.; Wyss, R.K.; Arnold, M.; Segiser, A.; Kalbermatter, N.; Joachimbauer, A.; Longnus, S.L. Effects of graft preservation conditions on coronary endothelium and cardiac functional recovery in a rat model of donation after circulatory death. J. Heart Lung Transpl. 2021, 40, 1396–1407. [Google Scholar] [CrossRef]
- Zwissler, B. Anästhesieverfahren-Auswirkungen auf die postoperative Phase [Anesthesia procedures–postoperative effects]. Anaesthesist 1997, 46 (Suppl 2), S99–S108. [Google Scholar] [CrossRef]
- Murray, P.A.; Fehr, D.M.; Chen, B.B.; Rock, P.; Esther, J.W.; Desai, P.M.; Nyhan, D.P. Differential effects of general anesthesia on cGMP-mediated pulmonary vasodilation. J. Appl. Physiol. 1992, 73, 721–727. [Google Scholar] [CrossRef]
- Honca, M.; Purtuloglu, T.; Akgul, E.O.; Oztosun, M.; Honca, T.; Sizlan, A.; Yaman, H. Effects of general and spinal anesthetic techniques on endothelial adhesion molecules in cesarean section. Korean J. Anesthesiol. 2014, 66, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Vosoughian, M.; Dahi, M.; Dabir, S.; Moshari, M.; Tabashi, S.; Mosavi, Z. Effects of General Anesthesia Versus Spinal Anesthesia on Serum Cytokine Release After Cesarean Section: A Randomized Clinical Trial. Anesth. Pain Med. 2021, 11, e111272. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, L.M.; Bijkerk, V.; van Eijk, L.T.; Joosten, L.A.; Keijzer, C.; Visser, J.; Warlé, M.C. The effect of general versus spinal anesthesia on perioperative innate immune function in patients undergoing total hip arthroplasty. BMC Anesthesiol. 2025, 25, 10. [Google Scholar] [CrossRef] [PubMed]
- Djuric, M.; Nenadic, I.; Radisavljevic, N.; Todorovic, D.; Stojanovic, M.; Dimic, N.; Bobos, M.; Bojic, S.; Stevanovic, P.; Savic, P.; et al. The Influence of Anesthetics on the Functions of the Endothelium and Oxidative Stress: A Critical Review. Biomedicines 2025, 13, 2357. [Google Scholar] [CrossRef]
- Bouglé, A.; Allain, P.A.; Favard, S.; Ait Hamou, N.; Carillion, A.; Leprince, P.; Amour, J. Postoperative serum levels of Endocan are associated with the duration of norepinephrine support after coronary artery bypass surgery. Anaesth. Crit. Care Pain Med. 2018, 37, 565–570. [Google Scholar] [CrossRef]
- Dogdus, M.; Yenercag, M.; Ozyasar, M.; Yilmaz, A.; Can, L.H.; Kultursay, H. Serum Endocan Levels Predict Angiographic No-Reflow Phenomenon in Patients With ST-Segment Elevation Myocardial Infarction Undergoing Primary Coronary Intervention. Angiology 2021, 72, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.F.; Zhao, Z.W.; Luo, Y.K.; Dong, X.F.; Yan, Y.M. Elevated endocan concentration is associated with coronary slow flow. Scand. J. Clin. Lab. Investig. 2016, 76, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Scherpereel, A.; Depontieu, F.; Grigoriu, B.; Cavestri, B.; Tsicopoulos, A.; Gentina, T.; Lassalle, P. Endocan, a new endothelial marker in human sepsis. Crit. Care Med. 2006, 34, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, M.E.; Shah, C.V.; Scherpereel, A.; Lanken, P.N.; Lassalle, P.; Bellamy, S.L.; Christie, J.D. Lower serum endocan levels are associated with the development of acute lung injury after major trauma. J. Crit. Care 2012, 27, 522.e11–522.e17. [Google Scholar] [CrossRef]
- Stoppelkamp, S.; Veseli, K.; Stang, K.; Schlensak, C.; Wendel, H.P.; Walker, T. Identification of Predictive Early Biomarkers for Sterile-SIRS after Cardiovascular Surgery. PLoS ONE 2015, 10, e0135527. [Google Scholar] [CrossRef]
- Madhivathanan, P.R.; Fletcher, N.; Gaze, D.; Thomson, R.; Chandrasekaran, V.; Al-Subaie, N.; Sharma, V. Perioperative kinetics of endocan in patients undergoing cardiac surgery with and without cardiopulmonary bypass. Cytokine 2016, 83, 8–12. [Google Scholar] [CrossRef]
- Perrotti, A.; Chenevier-Gobeaux, C.; Ecarnot, F.; Bardonnet, K.; Barrucand, B.; Flicoteaux, G.; Chocron, S. Is Endocan a Diagnostic Marker for Pneumonia After Cardiac Surgery? The ENDOLUNG Study. Ann. Thorac. Surg. 2018, 105, 535–541. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, L.; Yu, X.H.; Hu, M.; Zhang, Y.K.; Liu, X.; Ouyang, X. Endocan: A Key Player of Cardiovascular Disease. Front. Cardiovasc. Med. 2022, 8, 798699. [Google Scholar] [CrossRef]
| Surgical Procedure | GA n (%) | SA n (%) | Total n (%) |
|---|---|---|---|
| Arthroscopy | 1 (2.4%) | 4 (10.5%) | 5 (6.2%) |
| Knee prosthesis | 1 (2.4%) | 5 (13.2%) | 6 (7.5%) |
| Inguinal hernia | 2 (4.8%) | 2 (5.3%) | 4 (5.0%) |
| Hip prosthesis | 0 (0.0%) | 1 (2.6%) | 1 (1.2%) |
| Cystectomy | 1 (2.4%) | 0 (0.0%) | 1 (1.2%) |
| Lumbar disc herniation | 4 (9.5%) | 0 (0.0%) | 4 (5.0%) |
| Liposuction | 9 (21.4%) | 0 (0.0%) | 9 (11.2%) |
| Breast prosthesis | 1 (2.4%) | 0 (0.0%) | 1 (1.2%) |
| Meniscus surgery | 0 (0.0%) | 2 (5.3%) | 2 (2.5%) |
| Osteomyelitis | 0 (0.0%) | 1 (2.6%) | 1 (1.2%) |
| Ovarian cystectomy | 1 (2.4%) | 0 (0.0%) | 1 (1.2%) |
| Pilonidal sinus | 0 (0.0%) | 3 (7.9%) | 3 (3.8%) |
| Rhinoplasty | 9 (21.4%) | 0 (0.0%) | 9 (11.2%) |
| Cystocele repair | 1 (2.4%) | 2 (5.3%) | 3 (3.8%) |
| Stapedectomy | 1 (2.4%) | 0 (0.0%) | 1 (1.2%) |
| TUR (transurethral) | 0 (0.0%) | 2 (5.3%) | 2 (2.5%) |
| Umbilical hernia | 2 (4.8%) | 0 (0.0%) | 2 (2.5%) |
| Ureterorenoscopy (URS) | 1 (2.4%) | 1 (2.6%) | 2 (2.5%) |
| Varicocele | 2 (4.8%) | 8 (21.1%) | 10 (12.5%) |
| Varicose vein surgery | 1 (2.4%) | 5 (13.2%) | 6 (7.5%) |
| Facelift | 5 (11.9%) | 0 (0.0%) | 5 (6.2%) |
| ACL reconstruction | 0 (0.0%) | 2 (5.3%) | 2 (2.5%) |
| Total | 42 (100%) | 38 (100%) | 80 (100%) |
| Variable | GA (Mean ± SD) | SA (Mean ± SD) | p-Value |
|---|---|---|---|
| (n = 42) | (n = 38) | ||
| Duration of surgery (min) | 205.60 ± 114.31 | 99.34 ± 38.40 | 5.578 × 10−7 |
| BMI | 43.97 ± 5.75 | 45.32 ± 4.56 | 0.249 |
| Glucose (mg/dL) | 95.50 ± 16.84 | 102.24 ± 22.66 | 0.133 |
| Urea (mg/dL) | 15.60 ± 3.84 | 17.36 ± 5.01 | 0.081 |
| Creatinine (mg/dL) | 0.76 ± 0.14 | 0.82 ± 0.16 | 0.073 |
| AST (U/L) | 22.60 ± 11.79 | 24.13 ± 11.76 | 0.562 |
| ALT (U/L) | 24.24 ± 8.72 | 23.71 ± 7.08 | 0.769 |
| Sodium (Na, mmol/L) | 138.83 ± 2.49 | 138.63 ± 2.55 | 0.721 |
| Potassium (K, mmol/L) | 4.02 ± 0.25 | 4.03 ± 0.21 | 0.783 |
| Hemoglobin (Hb, g/dL) | 13.32 ± 1.54 | 14.43 ± 1.99 | 0.006 |
| White Blood Cells (WBC, ×103/µL) | 7.48 ± 1.99 | 7.94 ± 2.11 | 0.316 |
| Platelets (PLT, ×103/µL) | 262.50 ± 60.65 | 249.32 ± 54.03 | 0.310 |
| Neutrophils (%) | 54.82 ± 9.17 | 55.53 ± 9.28 | 0.732 |
| Lymphocytes (%) | 39.99 ± 8.47 | 33.01 ± 8.69 | 0.278 |
| INR | 0.97 ± 0.06 | 1.19 ± 1.49 | 0.336 |
| Pulse rate (beats/min) | 76.93 ± 7.81 | 80.26 ± 9.05 | 0.081 |
| Oxygen saturation (SpO2, %) | 98.81 ± 0.71 | 98.45 ± 0.72 | 0.026 |
| Systolic BP (mmHg) | 121.48 ± 9.89 | 127.47 ± 8.82 | 0.006 |
| Diastolic BP (mmHg) | 71.69 ± 8.81 | 74.76 ± 9.37 | 0.135 |
| Preoperative Endocan | 304.50 ± 80.67 | 320.74 ± 72.50 | 0.349 |
| Post-op Early Endocan | 511.50 ± 88.73 | 415.18 ± 79.53 | 2.403 × 10−6 |
| Post-op Late Endocan | 427.50 ± 87.87 | 352.58 ± 84.67 | 2.209 × 10−4 |
| Comparison | Mean Difference | SE | df | t | Cohen’s d | p |
|---|---|---|---|---|---|---|
| Preoperative vs. Post-op Early | −207.00 | 19.97 | 41 | −10.37 | −2.41 | 1.521 × 10−12 |
| Preoperative vs. Post-op Late | −123.00 | 20.89 | 41 | −5.89 | −1.43 | 1.874 × 10−6 |
| Post-op Early vs. Late | 84.00 | 21.03 | 41 | 3.99 | 0.98 | 7.890 × 10−4 |
| Comparison | Mean Difference | SE | df | t | Cohen’s d | p |
|---|---|---|---|---|---|---|
| Preoperative vs. Post-op Early | −94.45 | 15.51 | 37 | −6.09 | −1.20 | 1.425 × 10−6 |
| Preoperative vs. Post-op Late | −31.84 | 17.55 | 37 | −1.81 | −0.40 | 0.233 |
| Post-op Early vs. Late | 62.61 | 19.53 | 37 | 3.21 | 0.79 | 0.008 |
| Dependent Variable | Predictor | β (Unstandardized) | SE | β (Standardized) | t | p | Adjusted R2 |
|---|---|---|---|---|---|---|---|
| Post-operative early Endocan | (Intercept) | 579.469 | 67.609 | — | 8.571 | <0.001 | 0.232 |
| Pre-operative endocan | −0.035 | 0.128 | −0.027 | −0.270 | 0.788 | ||
| Anesthesia Type | −98.879 | 22.361 | −0.513 | −4.422 | <0.001 | ||
| Surgery Duration | −0.025 | 0.112 | −0.026 | −0.220 | 0.826 | ||
| Age | 1.081 | 0.740 | 0.145 | 1.460 | 0.148 | ||
| Post-operative late Endocan | (Intercept) | 569.196 | 69.161 | — | 8.230 | <0.001 | 0.140 |
| Pre-operative endocan | −0.142 | 0.131 | −0.116 | −1.083 | 0.282 | ||
| Anesthesia Type | −72.637 | 22.875 | −0.390 | −3.175 | 0.002 | ||
| Surgery Duration | −0.003 | 0.115 | −0.003 | −0.023 | 0.981 | ||
| Age | −0.587 | 0.757 | −0.082 | −0.775 | 0.441 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunduz, E.; Durmus, S.; Misirlioglu, N.F.; Cucu, O.; Dumur, S.; Dundar, B.; Uzun, H. General Anesthesia or Spinal Anesthesia and Serum Endocan Release After Surgery: A Prospective Observational Study. J. Clin. Med. 2025, 14, 8076. https://doi.org/10.3390/jcm14228076
Gunduz E, Durmus S, Misirlioglu NF, Cucu O, Dumur S, Dundar B, Uzun H. General Anesthesia or Spinal Anesthesia and Serum Endocan Release After Surgery: A Prospective Observational Study. Journal of Clinical Medicine. 2025; 14(22):8076. https://doi.org/10.3390/jcm14228076
Chicago/Turabian StyleGunduz, Ergun, Sinem Durmus, Naile Fevziye Misirlioglu, Oguzhan Cucu, Seyma Dumur, Bagnu Dundar, and Hafize Uzun. 2025. "General Anesthesia or Spinal Anesthesia and Serum Endocan Release After Surgery: A Prospective Observational Study" Journal of Clinical Medicine 14, no. 22: 8076. https://doi.org/10.3390/jcm14228076
APA StyleGunduz, E., Durmus, S., Misirlioglu, N. F., Cucu, O., Dumur, S., Dundar, B., & Uzun, H. (2025). General Anesthesia or Spinal Anesthesia and Serum Endocan Release After Surgery: A Prospective Observational Study. Journal of Clinical Medicine, 14(22), 8076. https://doi.org/10.3390/jcm14228076






