Multimodal Fusion of Chest X-Rays and Blood Biomarkers for Automated Silicosis Staging
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
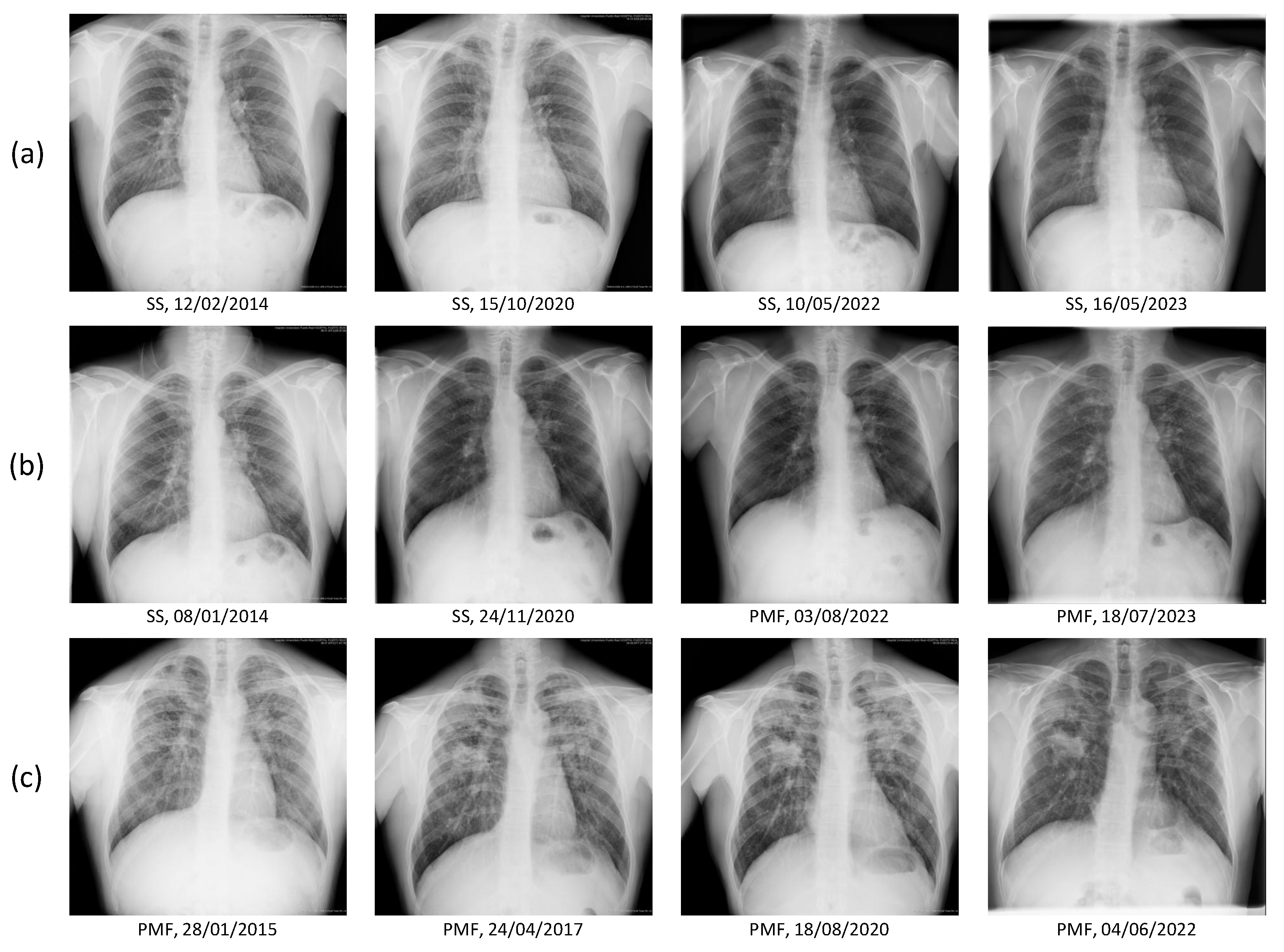
2.2. Image and Clinical Data
2.3. Demographics and Respiratory Data
2.4. Biochemical and Hematological Blood Markers
2.5. Dataset
2.6. Ethics
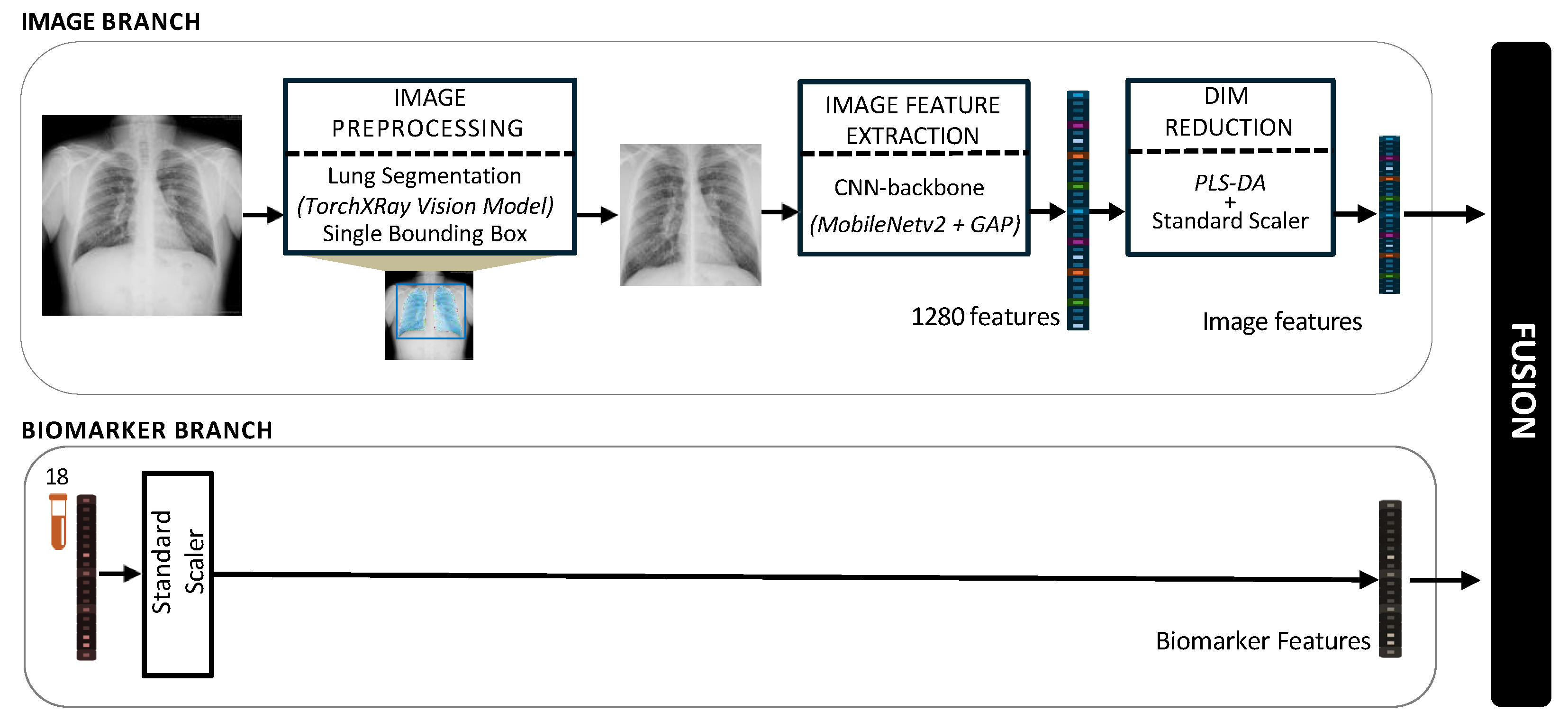
2.7. Multimodal Fusion Staging Methods
2.7.1. Image Preprocessing
2.7.2. Image Feature Extraction and Dimensionality Reduction
- Initial Feature Scaling: A standard scaler was fitted to the 1280-dimensional training features. Then, this scaler was used to transform both the training and test sets.
- Latent Space Transformation: A PLS-DA model was fitted on the scaled training features and labels (SS vs. PMF). The optimal number of latent components was determined to be 15 via a separate nested cross-validation, as this provided the best balance between explained variance and model stability. Then, this fitted PLS-DA model was used as a transformer, projecting both the training and test sets into a 15-dimensional latent space.
- Final Latent Scaling: A second standard scaler was fitted, this time only on the resulting 15-dimensional PLS scores from the training set. This final step normalized the new latent features and was then applied to the 15-dimensional test set scores.
2.7.3. Biomarkers Processing
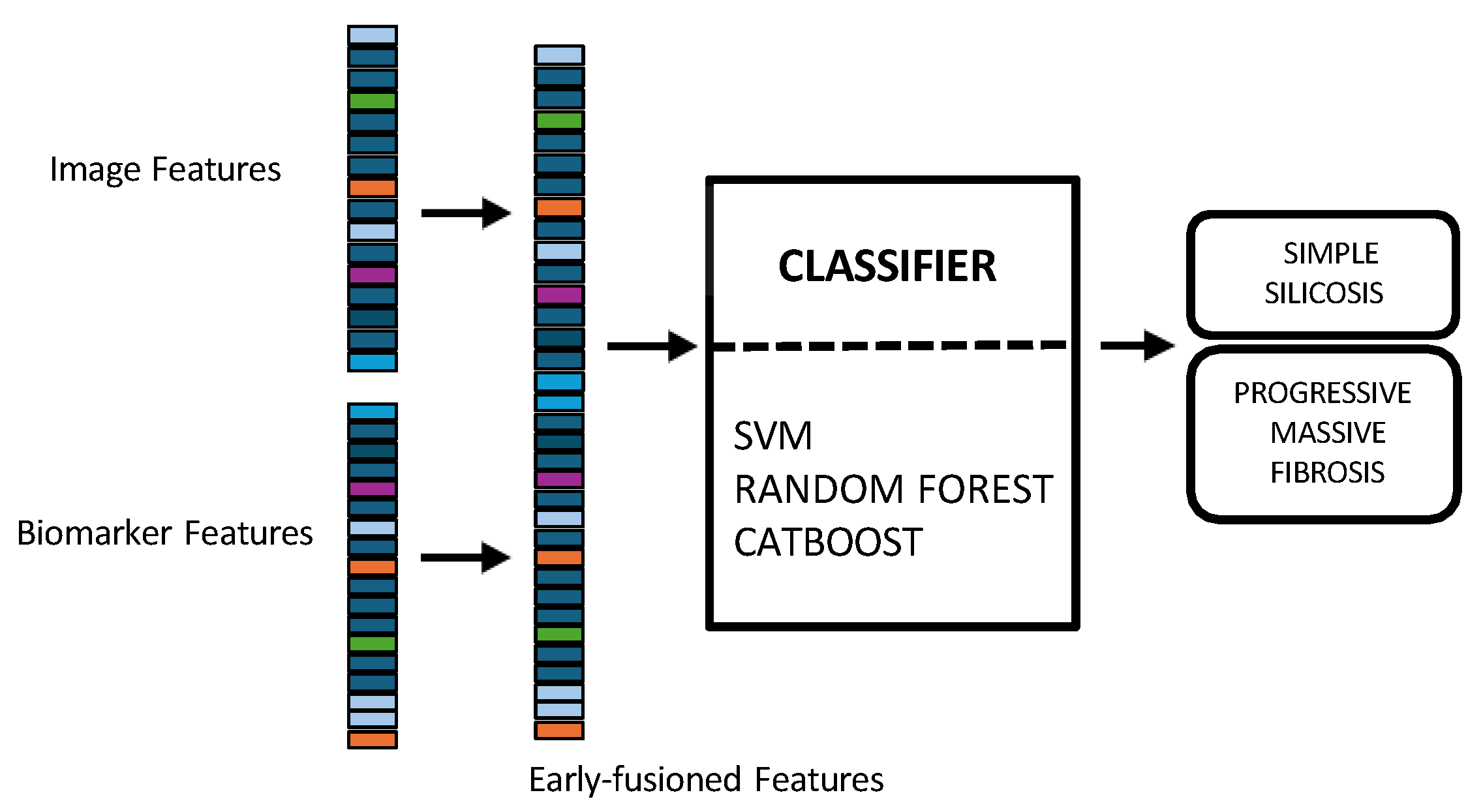
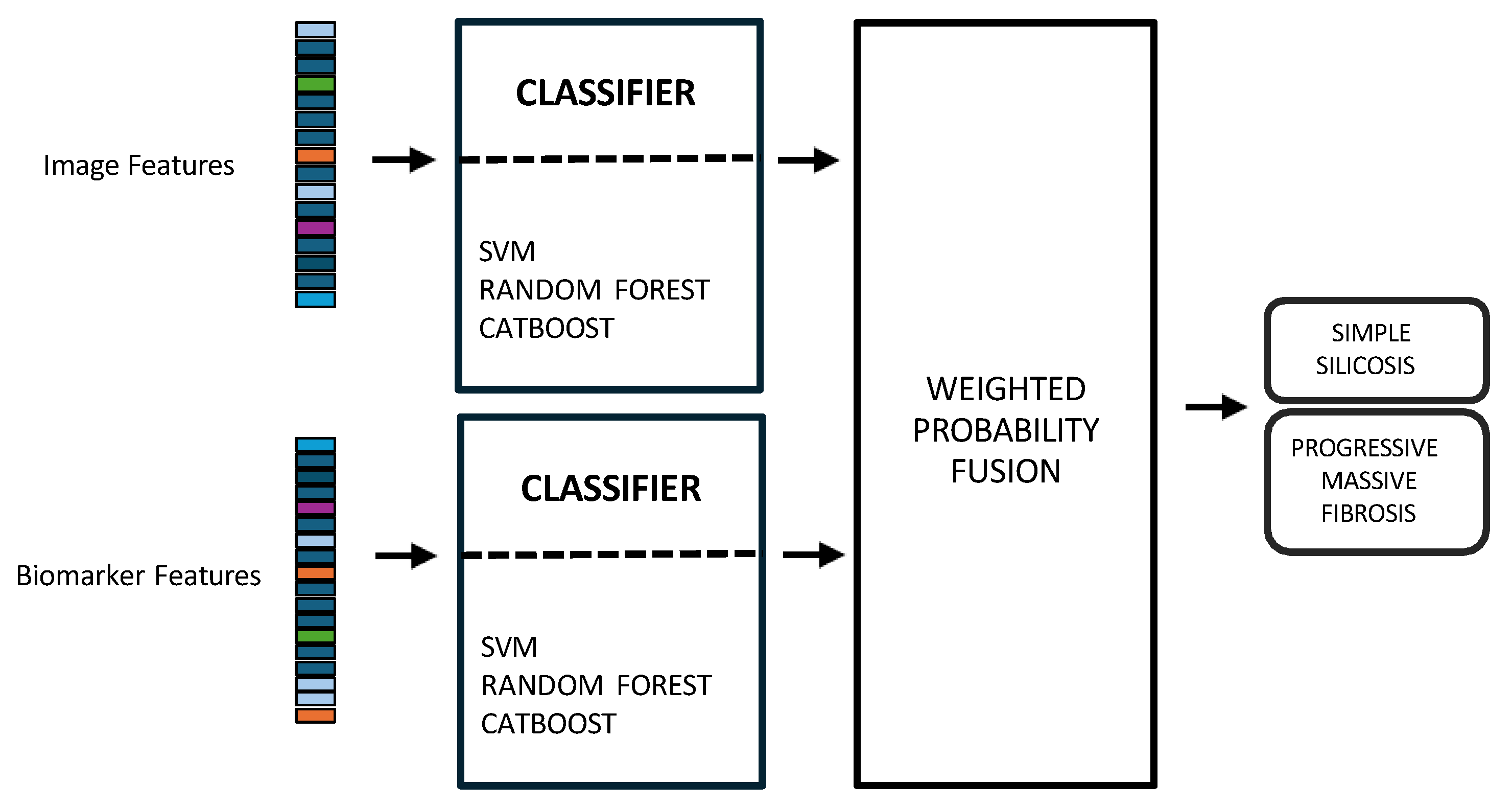
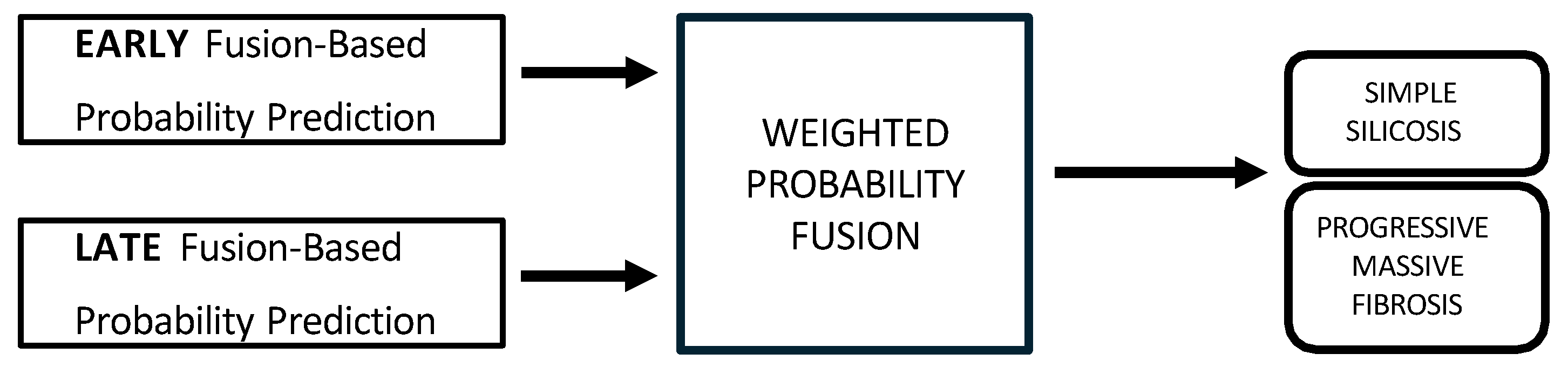
2.7.4. Multimodal Data Integration
- In early fusion, features of both modalities are combined before classification, as depicted in Figure 3. This approach has the theoretical advantage of allowing the model to learn cross-modal relationships from low-level features. Nonetheless, this early fusion is prone to overfitting when samples are limited [40] and may struggle to detect connections between the modalities if these connections only become clear at more abstract levels, as marginal representations are not specifically learned [41,42,43]. In this study, the image feature vector, derived from PLS-DA scores, and the biomarker vector were concatenated. This process resulted in a 33-dimensional vector representing each patient at a specific time point. This vector was used as input to train and evaluate three shallow ML models, selected for their different approaches to handling feature spaces: SVM [44], RF [45], and CatBoost [46].Model training and hyperparameter optimization were conducted using a nested, group-aware cross-validation framework to ensure robust, patient-level separation.
- Late fusion combined modality-specific model outputs into a final decision, offering better robustness to missing data, easier interpretability, and simpler integration into clinical workflows. Nevertheless, this strategy may lose some fine cross-modal dependencies and might sacrifice detailed inter-modality relationships [40,42]. In this work, two separate classifiers were trained in parallel: the first model used only the image-based feature vectors, while the second used the standardized biomarker vectors, as illustrated in Figure 4.Hyperparameter tuning for both models was performed independently using a nested, group-aware cross-validation procedure. During inference, each modality-specific model generates a probability score for PMF. These two scores were then fused into a single prediction using an adaptive weighting scheme, where the weights were determined by the area under the receiver operating characteristic (ROC) curve (AUC) achieved by each model on the internal validation folds. A final binary classification was made by applying a 0.5 threshold to this weighted-average probability.
- Hybrid fusion was designed to integrate the output of both the early and late fusion frameworks (Figure 5). Independent classifiers were trained for each modality, and then their probability predictions were combined.
2.8. Unimodal Approaches
2.9. Performance Metrics and Validation Scheme
2.10. Statistical Analysis
3. Results
3.1. Study Group
3.2. Ablation Study: Justification of Image Segmentation Method
3.3. Unimodal Models Performance
3.4. Multimodal Models Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, B.; Liu, X.; Peng, C.; Meng, X.; Jia, Q. Silicosis: From pathogenesis to therapeutics. Front. Pharmacol. 2025, 16, 1516200. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.C.; Yu, I.T.S.; Chen, W. Silicosis. Lancet 2012, 379, 2008–2018. [Google Scholar] [CrossRef]
- Hoy, R.F.; Jeebhay, M.F.; Cavalin, C.; Chen, W.; Cohen, R.A.; Fireman, E.; Go, L.H.; León-Jiménez, A.; Menéndez-Navarro, A.; Ribeiro, M.; et al. Current global perspectives on silicosis—Convergence of old and newly emergent hazards. Respirology 2022, 27, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Leso, V.; Fontana, L.; Romano, R.; Gervetti, P.; Iavicoli, I. Artificial stone associated silicosis: A systematic review. Int. J. Environ. Res. Public Health 2019, 16, 568. [Google Scholar] [CrossRef]
- León-Jiménez, A.; Mánuel, J.M.; García-Rojo, M.; Pintado-Herrera, M.G.; López-López, J.A.; Hidalgo-Molina, A.; García, R.; Muriel-Cueto, P.; Maira-González, N.; Del Castillo-Otero, D.; et al. Compositional and structural analysis of engineered stones and inorganic particles in silicotic nodules of exposed workers. Part. Fibre Toxicol. 2021, 18, 41. [Google Scholar] [CrossRef]
- Hoy, R.F.; Glass, D.C.; Dimitriadis, C.; Hansen, J.; Hore-Lacy, F.; Sim, M.R. Identification of early-stage silicosis through health screening of stone benchtop industry workers in Victoria, Australia. Occup. Environ. Med. 2021, 78, 296–302. [Google Scholar] [CrossRef]
- León-Jiménez, A.; Hidalgo-Molina, A.; Conde-Sánchez, M.Á.; Pérez-Alonso, A.; Morales-Morales, J.M.; García-Gámez, E.M.; Córdoba-Doña, J.A. Artificial stone silicosis: Rapid progression following exposure cessation. Chest 2020, 158, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Bai, Y.; Liang, C.; Liu, Y.; Zhou, J.; Guo, J.; Cai, X.; Hu, X.; Fang, Y.; Ding, X.; et al. Ingenol ameliorates silicosis via targeting the PTGS2/PI3K/AKT signaling axis: Implications for therapeutic intervention. Cell. Signal. 2025, 131, 111780. [Google Scholar] [CrossRef]
- Li, T.; Yang, X.; Xu, H.; Liu, H. Early identification, accurate diagnosis, and treatment of silicosis. Can. Respir. J. 2022, 2022, 3769134. [Google Scholar] [CrossRef]
- International Labour Organization (ILO). Guidelines for the Use of the ILO International Classification of Radiographs of Pneumoconioses (Rev Version 2022); Technical Report; International Labour Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Sun, W.; Wu, D.; Luo, Y.; Liu, L.; Zhang, H.; Wu, S.; Zhang, Y.; Wang, C.; Zheng, H.; Shen, J.; et al. A fully deep learning paradigm for pneumoconiosis staging on chest radiographs. IEEE J. Biomed. Health Inform. 2022, 26, 5154–5164. [Google Scholar] [CrossRef]
- Yi, J.; Tapia, K.; Robinson, J.W.; Gandomkar, Z.; Mo’ayyad, E.S.; Brennan, P.C.; Sommerfeld, N.; Taba, S.T. Radiologists’ performance in diagnosing silicosis on high-resolution computed tomography (HRCT) scans: An online platform. In Proceedings of the Medical Imaging 2024: Image Perception, Observer Performance, and Technology Assessment, San Diego, CA, USA, 18–22 February 2024; SPIE: Bellingham, WA, USA, 2024; Volume 12929, pp. 32–38. [Google Scholar]
- Jiménez-Gómez, G.; Campos-Caro, A.; García-Núñez, A.; Gallardo-García, A.; Molina-Hidalgo, A.; León-Jiménez, A. Analysis of immune cell subsets in peripheral blood from patients with engineered stone silica-induced lung inflammation. Int. J. Mol. Sci. 2024, 25, 5722. [Google Scholar] [CrossRef]
- García-Núñez, A.; Jiménez-Gómez, G.; Hidalgo-Molina, A.; Córdoba-Doña, J.A.; León-Jiménez, A.; Campos-Caro, A. Inflammatory indices obtained from routine blood tests show an inflammatory state associated with disease progression in engineered stone silicosis patients. Sci. Rep. 2022, 12, 8211. [Google Scholar] [CrossRef]
- Sanchez-Morillo, D.; León-Jiménez, A.; Guerrero-Chanivet, M.; Jiménez-Gómez, G.; Hidalgo-Molina, A.; Campos-Caro, A. Integrating routine blood biomarkers and artificial intelligence for supporting diagnosis of silicosis in engineered stone workers. Bioeng. Transl. Med. 2024, 9, e10694. [Google Scholar] [CrossRef]
- Sanchez-Morillo, D.; Martín-Carrillo, A.; Priego-Torres, B.; Sopo-Lambea, I.; Jiménez-Gómez, G.; León-Jiménez, A.; Campos-Caro, A. Cytokine profiles as predictive biomarkers of disease severity and progression in engineered stone silicosis: A machine learning approach. Diagnostics 2025, 15, 2413. [Google Scholar] [CrossRef]
- Obuchowicz, R.; Strzelecki, M.; Piórkowski, A. Clinical applications of artificial intelligence in medical imaging and image processing-A review. Cancers 2024, 16, 1870. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Yang, J.; Teng, F. Role of artificial intelligence in medical image analysis: A review of current trends and future directions. J. Med. Biol. Eng. 2024, 44, 231–243. [Google Scholar] [CrossRef]
- Sun, G.K.; Xiang, Y.H.; Wang, L.; Xiang, P.P.; Wang, Z.X.; Zhang, J.; Wu, L. Development of a multi-laboratory integrated predictive model for silicosis utilizing machine learning: A retrospective case-control study. Front. Public Health 2025, 12, 1450439. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.K.; Harjule, P.; Agarwal, B.; Kumar, R. Silicosis detection using extended transfer learning model. In Communications in Computer and Information Science; Springer Nature: Cham, Switzerland, 2024; pp. 111–126. [Google Scholar]
- Zhang, Y.; Zheng, B.; Zeng, F.; Cheng, X.; Wu, T.; Peng, Y.; Zhang, Y.; Xie, Y.; Yi, W.; Chen, W.; et al. Potential of digital chest radiography-based deep learning in screening and diagnosing pneumoconiosis: An observational study. Medicine 2024, 103, e38478. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Fang, Y.; Xie, Y.; Zheng, H.; Li, X.; Wu, D.; Zhang, T. Deep learning pneumoconiosis staging and diagnosis system based on multi-stage joint approach. BMC Med. Imaging 2024, 24, 165. [Google Scholar] [CrossRef]
- Li, X.; Xu, M.; Yan, Z.; Xia, F.; Li, S.; Zhang, Y.; Xing, Z.; Guan, L. Deep convolutional network-based chest radiographs screening model for pneumoconiosis. Front. Med. 2024, 11, 1290729. [Google Scholar] [CrossRef] [PubMed]
- Devnath, L.; Luo, S.; Summons, P.; Wang, D.; Shaukat, K.; Hameed, I.A.; Alrayes, F.S. Deep ensemble learning for the automatic detection of pneumoconiosis in coal worker’s chest X-ray radiography. J. Clin. Med. 2022, 11, 5342. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Jin, N.; Qiu, C.; Ba, K.; Wang, X.; Zhang, H.; Zhao, Q.; Huang, B. Balanced convolutional neural networks for pneumoconiosis detection. Int. J. Environ. Res. Public Health 2021, 18, 9091. [Google Scholar] [CrossRef]
- Zhang, L.; Rong, R.; Li, Q.; Yang, D.M.; Yao, B.; Luo, D.; Zhang, X.; Zhu, X.; Luo, J.; Liu, Y.; et al. A deep learning-based model for screening and staging pneumoconiosis. Sci. Rep. 2021, 11, 2201. [Google Scholar] [CrossRef]
- Yang, F.; Tang, Z.R.; Chen, J.; Tang, M.; Wang, S.; Qi, W.; Yao, C.; Yu, Y.; Guo, Y.; Yu, Z. Pneumoconiosis computer aided diagnosis system based on X-rays and deep learning. BMC Med. Imaging 2021, 21, 189. [Google Scholar] [CrossRef]
- Priego-Torres, B.; Sanchez-Morillo, D.; Khalili, E.; Conde-Sánchez, M.Á.; García-Gámez, A.; León-Jiménez, A. Automated engineered-stone silicosis screening and staging using Deep Learning with X-rays. Comput. Biol. Med. 2025, 191, 110153. [Google Scholar] [CrossRef]
- Krones, F.; Marikkar, U.; Parsons, G.; Szmul, A.; Mahdi, A. Review of multimodal machine learning approaches in healthcare. Inf. Fusion 2025, 114, 102690. [Google Scholar] [CrossRef]
- Kline, A.; Wang, H.; Li, Y.; Dennis, S.; Hutch, M.; Xu, Z.; Wang, F.; Cheng, F.; Luo, Y. Multimodal machine learning in precision health: A scoping review. NPJ Digit. Med. 2022, 5, 171. [Google Scholar] [CrossRef] [PubMed]
- Hardy-Werbin, M.; Maiques, J.M.; Busto, M.; Cirera, I.; Aguirre, A.; Garcia-Gisbert, N.; Zuccarino, F.; Carbullanca, S.; Del Carpio, L.A.; Ramal, D.; et al. MultiCOVID: A multi modal deep learning approach for COVID-19 diagnosis. Sci. Rep. 2023, 13, 18761. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, J.; Huang, X.; Ling, S.H.; Su, S.W. DeepMMSA: A novel multimodal deep learning method for non-small cell lung cancer survival analysis. In Proceedings of the 2021 IEEE International Conference on Systems, Man, and Cybernetics (SMC), Melbourne, Australia, 17–20 October 2021; pp. 1468–1472. [Google Scholar]
- Suganuma, N.; Kusaka, Y.; Hering, K.G.; Vehmas, T.; Kraus, T.; Arakawa, H.; Parker, J.E.; Kivisaari, L.; Letourneux, M.; Gevenois, P.A.; et al. Reliability of the proposed international classification of high-resolution computed tomography for occupational and environmental respiratory diseases. J. Occup. Health 2009, 51, 210–222. [Google Scholar] [CrossRef]
- Rahman, T.; Khandakar, A.; Kadir, M.A.; Islam, K.R.; Islam, K.F.; Mazhar, R.; Hamid, T.; Islam, M.T.; Kashem, S.; Mahbub, Z.B.; et al. Reliable tuberculosis detection using chest X-ray with deep learning, segmentation and visualization. IEEE Access 2020, 8, 191586–191601. [Google Scholar] [CrossRef]
- Loveless, I.; Liu, M.; Rosenman, K.; Wang, L.; Alessio, A. Characterizing inherent image ordinality to improve multiclass classification. In Proceedings of the Medical Imaging 2025: Computer-Aided Diagnosis, San Diego, CA, USA, 18–22 February 2024; Astley, S.M., Wismüller, A., Eds.; SPIE: Bellingham, WA, USA, 2025; p. 2. [Google Scholar]
- Liu, M.; Loveless, I.; Huang, Z.; Rosenman, K.; Wang, L.; Alessio, A. Deep learning methods for multi-class pneumoconioses grading of chest radiographs. In Proceedings of the Medical Imaging 2024: Computer-Aided Diagnosis, San Diego, CA, USA, 18–22 February 2024; Astley, S.M., Chen, W., Eds.; SPIE: Bellingham, WA, USA, 2024. [Google Scholar]
- Cohen, J.P.; Viviano, J.D.; Bertin, P.; Morrison, P.; Torabian, P.; Guarrera, M.; Lungren, M.P.; Chaudhari, A.; Brooks, R.; Hashir, M.; et al. TorchXRayVision: A library of chest X-ray datasets and models. In Proceedings of the International Conference on Medical Imaging with Deep Learning, Zurich, Switzerland, 6–8 July 2022; PMLR: New York, NY, USA, 2022; pp. 231–249. [Google Scholar]
- Sandler, M.; Howard, A.; Zhu, M.; Zhmoginov, A.; Chen, L.C. MobileNetV2: Inverted residuals and linear bottlenecks. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 18–22 June 2018; pp. 4510–4520. [Google Scholar]
- Brereton, R.G.; Lloyd, G.R. Partial least squares discriminant analysis: Taking the magic away. J. Chemom. 2014, 28, 213–225. [Google Scholar] [CrossRef]
- Huang, S.C.; Pareek, A.; Seyyedi, S.; Banerjee, I.; Lungren, M.P. Fusion of medical imaging and electronic health records using deep learning: A systematic review and implementation guidelines. NPJ Digit. Med. 2020, 3, 136. [Google Scholar] [CrossRef]
- Ramachandram, D.; Taylor, G.W. Deep multimodal learning: A survey on recent advances and trends. IEEE Signal Process. Mag. 2017, 34, 96–108. [Google Scholar] [CrossRef]
- Guarrasi, V.; Aksu, F.; Caruso, C.M.; Di Feola, F.; Rofena, A.; Ruffini, F.; Soda, P. A systematic review of intermediate fusion in multimodal deep learning for biomedical applications. Image Vis. Comput. 2025, 158, 105509. [Google Scholar] [CrossRef]
- Stahlschmidt, S.R.; Ulfenborg, B.; Synnergren, J. Multimodal deep learning for biomedical data fusion: A review. Brief. Bioinform. 2022, 23, bbab569. [Google Scholar] [CrossRef]
- Pisner, D.A.; Schnyer, D.M. Support vector machine. In Machine Learning; Elsevier: Amsterdam, The Netherlands, 2020; pp. 101–121. [Google Scholar]
- Parmar, A.; Katariya, R.; Patel, V. A review on random forest: An ensemble classifier. In International Conference on Intelligent Data Communication Technologies and Internet of Things (ICICI) 2018; Springer International Publishing: Cham, Switzerland, 2019; pp. 758–763. [Google Scholar]
- Prokhorenkova, L.; Gusev, G.; Vorobev, A.; Dorogush, A.V.; Gulin, A. CatBoost: Unbiased boosting with categorical features. In Proceedings of the Advances in Neural Information Processing Systems; Curran Associates Inc.: Red Hook, NY, USA, 2018; Volume 31. [Google Scholar]
- Devnath, L.; Summons, P.; Luo, S.; Wang, D.; Shaukat, K.; Hameed, I.A.; Aljuaid, H. Computer-aided diagnosis of coal workers’ pneumoconiosis in chest X-ray radiographs using machine learning: A systematic literature review. Int. J. Environ. Res. Public Health 2022, 19, 6439. [Google Scholar] [CrossRef]
- Zhang, Y. Computer-aided diagnosis for pneumoconiosis staging based on multi-scale feature mapping. Int. J. Comput. Intell. Syst. 2021, 14, 191. [Google Scholar] [CrossRef]
- Wang, X.; Yu, J.; Zhu, Q.; Li, S.; Zhao, Z.; Yang, B.; Pu, J. Potential of deep learning in assessing pneumoconiosis depicted on digital chest radiography. Occup. Environ. Med. 2020, 77, 597–602. [Google Scholar] [CrossRef]
- Hoy, R.F.; Hansen, J.; Glass, D.C.; Dimitriadis, C.; Hore-Lacy, F.; Sim, M.R. Serum angiotensin converting enzyme elevation in association with artificial stone silicosis. Respir. Med. 2021, 177, 106289. [Google Scholar] [CrossRef]
- Anwar, S.M.; Parida, A.; Atito, S.; Awais, M.; Nino, G.; Kittler, J.; Linguraru, M.G. SS-CXR: Self-supervised pretraining using chest X-rays towards A domain specific foundation model. In Proceedings of the 2024 IEEE International Conference on Image Processing (ICIP), Abu Dhabi, United Arab Emirates, 27–30 October 2024; IEEE: Piscataway, NJ, USA, 2024; pp. 2975–2981. [Google Scholar]
- Pandey, S.; Saha, P.; Sharan, G.; Sandosh, S. Enhancing chest X-ray analysis using encoder-decoder with GRU for report generation. In Proceedings of the 2024 IEEE Fourth International Conference on Advances in Electrical, Computing, Communication and Sustainable Technologies (ICAECT), Bhilai, India, 11–12 January 2024; IEEE: Piscataway, NJ, USA, 2024; pp. 1–8. [Google Scholar]
- Yu, Q.; Ma, Q.; Da, L.; Li, J.; Wang, M.; Xu, A.; Li, Z.; Li, W.; Alzheimer’s Disease Neuroimaging Initiative. A transformer-based unified multimodal framework for Alzheimer’s disease assessment. Comput. Biol. Med. 2024, 180, 108979. [Google Scholar] [CrossRef]
- Fang, M.; Xu, B. Transformer-based multi-modal learning for breast cancer screening: Merging imaging and genetic data. J. Radiat. Res. Appl. Sci. 2025, 18, 101586. [Google Scholar] [CrossRef]



| Parameter | SS | PMF |
|---|---|---|
| FEV1 (mL) | 3271.77 ± 660.01 | 2972.25 ± 736.83 |
| FEV1 (%) | 87.38 ± 13.97 | 78.35 ± 17.94 |
| FVC (mL) | 4256.43 ± 777.93 | 4099.46 ± 834.28 |
| FVC (%) | 90.93 ± 15.54 | 86.59 ± 15.90 |
| FEV1/FVC | 0.78 ± 0.05 | 0.72 ± 0.09 |
| DLCO (mmol/min/kPa) | 9.29 ± 2.16 | 8.79 ± 1.59 |
| DLCO (%) | 91.88 ± 21.55 | 85.48 ± 15.75 |
| Segmentation Method | Classifier | Accuracy (%) | F1-Score (%) | AUC |
|---|---|---|---|---|
| Lung Bounding Box | CatBoost | 69.18 [60.96–77.40] | 64.59 [58.16–71.02] | 0.77 [0.68–0.86] |
| RF | 69.77 [60.82–78.72] | 64.77 [56.40–73.14] | 0.77 [0.70–0.84] | |
| SVM | 69.10 [59.02–79.18] | 62.84 [52.98– 72.70] | 0.74 [0.65–0.83] | |
| Anatomical Rib-Segmentation | CatBoost | 75.84 [72.08–79.60] | 70.04 [63.65–76.43] | 0.83 [0.79–0.87] |
| RF | 72.01 [65.58–78.44] | 65.72 [56.71–74.73] | 0.81 [0.77–0.85] | |
| SVM | 74.83 [67.75–81.91] | 69.10 [61.59–76.61] | 0.83 [0.79–0.87] |
| Strategy | Classifier | Accuracy (%) | Precision (%) | Recall (%) | F1-Score (%) | AUC |
|---|---|---|---|---|---|---|
| Image Based Model | CatBoost | 75.84 [72.08–79.60] | 80.04 [62.64–97.44] | 65.12 [51.97–78.27] | 70.04 [63.65–76.43] | 0.83 [0.79–0.87] |
| RF | 72.01 [65.58–78.44] | 74.40 [58.08–90.72] | 62.48 [45.37–79.59] | 65.72 [56.71–74.73] | 0.81 [0.77–0.85] | |
| SVM | 74.83 [67.75–81.91] | 77.51 [65.42–89.60] | 64.45 [49.74–79.16] | 69.10 [61.59–76.61] | 0.83 [0.80–0.88] | |
| Biomarker Based Model | CatBoost | 62.32 [55.06–69.58] | 61.81 [40.17–83.45] | 57.60 [44.83–70.37] | 57.19 [47.77–66.61] | 0.69 [0.62–0.76] |
| RF | 62.66 [55.53–69.79] | 61.39 [40.78–82.00] | 58.07 [48.55–67.59] | 57.76 [48.19–67.33] | 0.70 [0.64–0.76] | |
| SVM | 59.75 [52.18–67.32] | 60.85 [37.04–84.66] | 46.08 [40.84–51.32] | 50.46 [43.53–57.39] | 0.65 [0.56–0.74] |
| Strategy | Classifier | Accuracy (%) | Precision (%) | Recall (%) | F1-Score (%) | AUC |
|---|---|---|---|---|---|---|
| Early Fusion | CatBoost | 75.51 [69.72–81.30] | 80.67 [62.54–98.80] | 64.14 [50.30–77.98] | 69.42 [61.59–77.25] | 0.83 [0.79–0.87] |
| RF | 71.68 [64.51–78.85] | 75.58 [58.42–92.74] | 62.67 [47.21–78.13] | 66.11 [62.26–69.96] | 0.82 [0.78–0.86] | |
| SVM | 72.63 [67.61–77.65] | 74.62 [61.01–88.23] | 63.38 [51.82–74.94] | 67.13 [64.41–69.85] | 0.82 [0.77–0.87] | |
| Hybrid Fusion | CatBoost | 74.37 [69.40–79.34] | 79.12 [60.12–98.12] | 62.59 [49.53–75.65] | 67.96 [60.57–75.35] | 0.85 [0.80–0.90] |
| RF | 71.72 [65.54–77.90] | 75.97 [57.19–94.75] | 61.55 [48.08–75.02] | 65.53 [59.98–71.08] | 0.84 [0.80–0.88] | |
| SVM | 73.14 [66.47–79.81] | 77.18 [63.82–90.54] | 61.66 [46.71–76.61] | 66.77 [61.12–72.42] | 0.82 [0.77–0.87] | |
| Late Fusion | CatBoost | 74.63 [68.96–80.30] | 80.34 [62.93–97.75] | 62.38 [48.23–76.53] | 68.17 [61.85–74.49] | 0.85 [0.79–0.91] |
| RF | 71.80 [64.81–78.79] | 76.74 [57.78–95.70] | 60.51 [47.04–73.98] | 65.34 [58.98–71.70] | 0.83 [0.79–0.87] | |
| SVM | 73.62 [66.06–81.18] | 79.52 [65.16–93.88] | 59.55 [44.49–74.61] | 66.37 [58.98–73.76] | 0.82 [0.78–0.86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Priego-Torres, B.; Sopo-Lambea, I.; Khalili, E.; Martín-Carrillo, A.; Campos-Caro, A.; León-Jiménez, A.; Sanchez-Morillo, D. Multimodal Fusion of Chest X-Rays and Blood Biomarkers for Automated Silicosis Staging. J. Clin. Med. 2025, 14, 8074. https://doi.org/10.3390/jcm14228074
Priego-Torres B, Sopo-Lambea I, Khalili E, Martín-Carrillo A, Campos-Caro A, León-Jiménez A, Sanchez-Morillo D. Multimodal Fusion of Chest X-Rays and Blood Biomarkers for Automated Silicosis Staging. Journal of Clinical Medicine. 2025; 14(22):8074. https://doi.org/10.3390/jcm14228074
Chicago/Turabian StylePriego-Torres, Blanca, Iris Sopo-Lambea, Ebrahim Khalili, Ana Martín-Carrillo, Antonio Campos-Caro, Antonio León-Jiménez, and Daniel Sanchez-Morillo. 2025. "Multimodal Fusion of Chest X-Rays and Blood Biomarkers for Automated Silicosis Staging" Journal of Clinical Medicine 14, no. 22: 8074. https://doi.org/10.3390/jcm14228074
APA StylePriego-Torres, B., Sopo-Lambea, I., Khalili, E., Martín-Carrillo, A., Campos-Caro, A., León-Jiménez, A., & Sanchez-Morillo, D. (2025). Multimodal Fusion of Chest X-Rays and Blood Biomarkers for Automated Silicosis Staging. Journal of Clinical Medicine, 14(22), 8074. https://doi.org/10.3390/jcm14228074








