1. Introduction
Aortic stenosis (AS) is the most common valvular heart disease in developed countries, with moderate AS (aortic valve area (AVA) 1.0–1.5 cm
2, mean gradient 20–40 mmHg), affecting a significant proportion of elderly patients (estimated prevalence 2–4% in those >65 years) [
1,
2]. AS is a complex disease affecting the aortic valve, myocardium, coronary microcirculation, and the entire cardiovascular system. Subclinical cardiac dysfunction—manifesting as impaired LV, left atrial (LA), and right ventricular (RV) mechanics—often precedes overt symptoms or ejection fraction (EF) decline, highlighting the need for sensitive tools to detect early impairments and stratify risk [
3,
4,
5,
6,
7]. Additionally, the assessment of flow, specifically stroke volume indexed to body surface area (stroke volume index (SVi)), is crucial: a low SVi value (<35 mL/m
2) may indicate a “paradoxical low-flow” form of AS, even in moderate stenosis [
8]. Moderate AS is a condition often understudied compared to severe AS.
Cardiopulmonary exercise testing (CPET) is a cornerstone for assessing functional capacity in heart failure (HF) and valvular diseases, providing objective metrics beyond resting echocardiography. In moderate AS, stratifying patients into low and good functional groups may identify those at higher risk, guiding clinical management [
9]. Peak oxygen consumption (VO
2) is the most recognized functional parameter [
10,
11]. However, peak VO
2 is effort-dependent and influenced by non-cardiac factors such as age, skeletal muscle deconditioning and obesity, limiting its specificity in moderate AS with preserved EF. Absolute VO
2 peak can be misleading in older adults unconditioned who naturally have lower values. In this study, we selected % predicted VO
2 with a threshold of ≥83% to define preserved functional capacity in elderly patients with moderate aortic stenosis (AS) and preserved ejection fraction (EF ≥ 50%), which is in line with literature suggestions [
9,
12]. The ventilation to carbon dioxide production slope (VE/VCO
2 slope) measures ventilatory efficiency, capturing HF-specific pathophysiology like ventilation-perfusion mismatch, elevated pulmonary pressures, and chemoreflex hypersensitivity. VE/VCO
2 slope is effort-independent, submaximal, and has demonstrated superior prognostic value over peak VO
2 in HF with preserved EF (HFpEF). Value of VE/VCO
2 slope ≥ 34 has high specificity in severe outcomes, but ≥30 is more sensitive for detecting early inefficiency [
12,
13,
14,
15]. In our study, we defined ventilatory inefficiency as a VE/VCO
2 slope ≥ 30.
Echocardiography, particularly advanced techniques like global longitudinal strain (GLS) and myocardial work (MW), offers insights into subclinical LV dysfunction, which may precede overt heart failure in AS [
16,
17,
18]. Echocardiographic strain analysis using two-dimensional speckle-tracking echocardiography (STE) provides insights into subclinical myocardial deformation across the left atrium (LA), left ventricle (LV), and right ventricle (RV). LAstrain, is a sensitive tool for assessing LA function across its three physiological phases: reservoir (peak atrial longitudinal strain (PALS)), conduit (LA conduit strain (LAScd)), and contractile (LA contractile strain (LA Scr)). Each phase reflects a distinct aspect of LA mechanics. Parameters such as LV global longitudinal strain (GLS), LA reservoir strain (PALS), and RV free wall strain (RV FWS) detect early dysfunction in AS, correlating with symptoms, fibrosis, and prognosis [
19,
20,
21,
22]. In the literature and clinical practice, two abbreviations are commonly encountered for Left atrial reservoir strain: LASr and PALS (peak atrial longitudinal strain). The abbreviation “PALS” is frequently utilised in clinical practice. In moderate AS with preserved EF, integrating CPET-derived functional classifications with strain parameters may enhance risk stratification, as reduced functional capacity (low % predicted VO
2 or poor VE/VCO
2) is linked to worse cardiac mechanics [
23].
Despite these advances, few studies have compared strain parameters in moderate AS patients stratified by both % predicted VO
2 and VE/VCO
2 slope. Leveraging CPET phenotyping in HF cohorts, where low functional is associated with progressive echocardiographic worsening, this approach may identify high-risk subgroups in moderate AS [
14].
Parameters like LV EF and GLS evaluate LV systolic performance in AS but do not account for afterload, which varies with AS severity and peripheral vascular resistance, nor do they reflect LV myocardial work or oxygen demand. Noninvasive LV myocardial work (MW), a novel method using pressure-strain loops that integrate GLS with afterload estimates (from blood pressure and transvalvular gradients), provides insights into LV energetics, including global work index (GWI; total work during the cardiac cycle), global constructive work (GCW; work contributing to systolic contraction and diastolic relaxation), global wasted work (GWW; inefficient work due to dyssynchrony), and global work efficiency (GWE; percentage of useful work). Low GLS values indicate impaired LV mechanics, while myocardial work indices quantify LV efficiency, providing prognostic value beyond ejection fraction [
24]. In severe AS, MW indices like GWI and GCW have shown independent associations with heart failure symptoms and may outperform GLS in reflecting myocardial remodelling [
25].
Therefore, the objectives of this study were: (1) to divide patients with moderate AS and preserved EF into groups based on low versus preserved % predicted VO2 and good versus poor ventilatory efficiency (VE/VCO2 slope); (2) to compare LA, LV, and RV strain parameters across these groups; and (3) to identify which echocardiographic parameters are best predictors for reduced functional capacity. By integrating CPET and STE, this study aims to refine prognostic phenotyping in moderate AS, facilitating risk stratification.
2. Methodology
A total of 116 patients were screened from the outpatient clinic of the Cardiology Department at the University Clinical Center of Serbia between July 2024 and June 2025. Participants presented with either no symptoms or equivocal complaints, including mild dyspnea, worsening dyspnea among those with chronic obstructive pulmonary disease (COPD), nonspecific chest pain, or atypical angina and dizziness unrelated to exertion.
All patients fulfilled predefined inclusion criteria for moderate aortic stenosis (AS), as per the European Association of Cardiovascular Imaging (EACVI) guidelines: aortic valve area (AVA) between 1.0 and 1.5 cm2, mean transvalvular pressure gradient of 20–40 mmHg, peak aortic jet velocity of 3.0–4.0 m/s, and preserved left ventricular ejection fraction (LVEF ≥ 50%) confirmed by two-dimensional echocardiography.
Exclusion criteria encompassed LVEF < 50%, resting heart rate > 90 beats per minute, significant concomitant valvular pathology (≥moderate aortic regurgitation, ≥moderate mitral stenosis or regurgitation), moderate-to-severe mitral annular calcification, unstable coronary artery disease, known significant epicardial coronary artery disease, other cardiac disorders (e.g., constrictive pericarditis, pulmonary thromboembolism, primary pulmonary hypertension, or high-output heart failure), and major noncardiac conditions impairing functional capacity.
Of the screened cohort, five patients were excluded due to orthopedic limitations (knee or hip pain) preventing cardiopulmonary exercise testing (CPET). An additional four were excluded post-CPET or multidetector computed tomography (MDCT) due to newly identified severe coronary artery disease. This yielded 107 patients eligible for predicted peak oxygen uptake (VO
2) assessment. However, five more were excluded from ventilatory efficiency (VE/VCO
2) analysis owing to erratic breathing patterns, resulting in a final analytical cohort of 102 patients for VE/VCO
2 analysis. All included patients exhibited normal-flow physiology, with stroke volume index (SVi) ≥ 35 mL/m
2; no instances of low-flow, low-gradient, or paradoxical low-flow AS were observed (
Figure 1).
2.1. Echocardiography
All selected patients underwent a standard comprehensive echocardiographic study using a Vivid E95 imaging device (GE Healthcare, Chicago, IL, USA) and stored on a workstation for off-line analysis (EchoPAC, GE Healthcare), including M-mode, 2D echocardiogram, Doppler, tissue Doppler, and multiple transducer positions to record aortic valve jet velocity. Aortic valve area (AVA) was calculated using the continuity equation as per EACVI guidelines. The following parameters were measured offline: left ventricular (LV) wall thickness, volumes, and ejection fraction (EF). E-wave velocity, A-wave velocity, and septal and lateral e’ velocities were measured from mitral valve inflow and mitral annulus tissue Doppler. Tricuspid lateral annulus S’ were measured from tissue Doppler. Right ventricular systolic pressure (RVSP) was derived as the sum of the tricuspid regurgitation jet peak velocity and the estimated mean right atrial pressure based on the diameter and collapsibility of the inferior vena cava. Mitral regurgitation and tricuspid regurgitation were defined as significant when graded as moderate or severe according to current recommendations. LV stroke volume (SV) was derived from the left ventricular outflow tract (LVOT). The biplane Simpson’s method was used to measure LV and left atrial (LA) volumes, indexed to body surface area (BSA).
Offline 2D speckle tracking strain analysis was performed to measure left atrial (LA) and left ventricular (LV) longitudinal strain on the same image and cardiac cycle to eliminate beat-to-beat variability. Analyzed image frame rates were ≥60 frames/s. LA cardiac cycles were defined as: (i) reservoir phase: from ventricular end-diastole until mitral valve opening; (ii) conduit phase: from mitral valve opening through diastasis until the onset of atrial contraction; and (iii) contractile phase: from the onset of atrial contraction until the end of ventricular diastole. Endocardial border tracing was performed automatically, and segments with persistently inadequate tracking after manual adjustment were excluded. LV myocardial systolic function and LA phasic function were studied on apical views. The following myocardial and chamber function values were recorded: LV global longitudinal peak systolic strain (GLS), LA reservoir (PALS), LA contractile and conduit strains (LA Sct and LA Scd, respectively), and LA biplane EF. The time to peak strain (PSD) for both chambers was also recorded. Strain analysis was feasible in all patients included. All echocardiographic and myocardial work analyses were performed by a single experienced echocardiographer (>15 years’ experience), blinded to clinical and CPET data, to minimize variability.
Myocardial work indices were derived using proprietary software (EchoPAC version 204) that integrates LV GLS with blood pressure recordings to construct pressure–strain loops over cardiac cycles. LV GLS was measured from the apical four-, two-, and three- or five-chamber views by tracing the endocardial border at an end-systolic frame, after which the software automatically defined a region of interest, manually corrected to include the entire myocardial thickness when needed. After GLS measurement, the timing of aortic and mitral valve opening and closure, and LV systolic blood pressure were entered into the software. LV systolic pressure was estimated by the sum of the mean aortic transvalvular gradient and non-invasively measured systolic pressure to correct for afterload, as previously described and validated. The software provided four indices of global myocardial work: (i) Left ventricular global work index (LV GWI), calculated as the area within the pressure–strain loop from mitral valve closure to opening; (ii) Left ventricular global constructive work (LV GCW), defined as shortening during systole and lengthening during relaxation; (iii) Left ventricular global wasted work (LV GWW), determined as lengthening during systole and shortening during relaxation; and (iv) Left ventricular global work efficiency (LV GWE), calculated by dividing LV GCW by the sum of LV GCW and LV GWW. All readings were performed by one experienced echocardiographer.
For RV strain measurement, RV-focused apical four-chamber views were acquired in the left lateral position, ensuring no RV foreshortening. Frame rates were set at 50–90 fps, optimized for endocardial definition, with 3–5 cardiac cycles recorded, ECG-gated, and timed to pulmonary valve closure. In the end-diastolic frame, points were placed at the basal septum, basal RV free wall, and apex. The endocardial border was traced semi-automatically, covering the RV free wall and septum, with ROI width adjusted to myocardial thickness. Tracking quality was verified, and poor segments were adjusted or excluded. Three segments (basal, mid, apical for RV free wall) were analyzed with RV free wall strain (FWS) as the average. TAPSE was automatically derived from the longitudinal displacement of the basal RV free wall segment during systole using the speckle-tracking software (EchoPAC, GE Healthcare, version 204).
2.2. CPET
The cardiopulmonary exercise test (CPET) was performed using a Schiller CS-200 cycle ergometer (Schiller, CARDIOVIT CS-200 Excellence, Germany) and the R15 ramp protocol, with continuous breath-by-breath analysis of expired gases collected through a well-sealed facemask. CPET data were acquired on a breath-by-breath basis and averaged over 8–10-s intervals to reduce short-term variability. All tests underwent visual quality control, and patients with erratic breathing patterns or poor signal stability were excluded from the analysis.
Throughout the CPET, a 12-lead ECG was continuously recorded, and ventilatory gas exchange variables were measured in real-time, including oxygen uptake (VO2) at the first anaerobic threshold (AT), identified using the V-slope method and peak oxygen consumption (peak VO2), representing the subject’s maximal aerobic capacity. Additional ventilatory parameters, such as minute ventilation (VE) and breathing reserve, were monitored to evaluate ventilatory efficiency and potential respiratory limitations. Blood pressure and heart rate were measured at baseline, at the end of each workload stage, and during the recovery phase. No participant exhibited electrocardiographic or clinical signs suggestive of myocardial ischemia during or after testing. Tests were terminated upon symptom onset, abnormal hemodynamics, or achievement of RER > 1.05, per safety guidelines for older cardiac patients. All participants completed graded cycle ergometer exercise testing until they reached the point of volitional exhaustion. Submaximal effort was expected in some participants due to the advanced age of the study cohort, the presence of comorbidities and factors such as reduced motivation or lack of familiarity with the CPET procedure. Peak oxygen uptake was determined as the maximum value recorded in the final 30 s of the exercise test, reported as absolute VO2 peak (mL/min), adjusted VO2 peak (mL/kg/min), or % predicted VO2 (% of predicted value based on age, sex, and weight). Exercise ventilation efficiency was addressed by the VE increase for a given VCO2 slope and calculated.
Patients were classified according to functional capacity and ventilatory efficiency. Functional capacity was defined by % predicted peak VO
2, with values ≥ 83% indicating preserved capacity and <83% indicating reduced capacity [
12]. Ventilatory efficiency was defined by VE/VCO
2 slope, with values < 30 considered good and ≥30 considered poor [
26,
27].
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the University Clinical Center of Serbia and the Faculty of Medicine, University of Belgrade (protocol code 25/XI-4, 20 November 2024).
3. Statistics
Data are expressed as mean ± standard deviation (SD) or percentages unless otherwise specified. Group differences were evaluated using the Student t test for normally distributed continuous variables, Mann-Whitney U tests for non–normally distributed continuous variables, and the chi-square or Fisher exact tests for categorical variables. Pearson or Spearman correlation coefficients were used to examine the relationship between continuous variables. To identify independent predictors of a positive exercise test, logistic multivariate analysis was performed, with significant variables included in the statistical model. A p-value < 0.05 was considered statistically significant. Associations between CPET and echocardiographic parameters were determined using logistic regression analysis. Only variables that were significantly correlated with the outcome (% predicted VO2 < 83% or VE/VCO2 slope ≥ 30%) in univariate analysis were considered for entry into the logistic regression model using the Forward LR method, which retained only the most significant predictors based on likelihood ratio tests (p < 0.05 for entry, p < 0.10 for retention). Data were analyzed using SPSS version 23.
To identify independent predictors of reduced exercise capacity (% predicted VO2 < 83%) or poor ventilatory efficiency (VE/VCO2 slope ≥ 30%), logistic regression models were constructed. Candidate covariates were selected based on clinical relevance (e.g., age, sex, BMI, β-blocker therapy, AF, COPD, CKD, SBP) and univariate significance (p < 0.10). The primary model used forward stepwise selection (likelihood ratio method; p < 0.05 for entry, p < 0.10 for retention), adhering to the events-per-variable (EPV) rule (≤1 predictor per 10 events; max 5 predictors for ~50 events).
Collinearity was assessed via variance inflation factors (VIF; cutoff > 3 for exclusion) and correlation matrices (Pearson/Spearman |r| > 0.7, with exclusion/replacement of highly correlated variables). Model fit was evaluated using the Hosmer-Lemeshow test (p > 0.05) and ROC curve analysis (AUC with 95% CIs). A sensitivity analysis employed the Enter method, forcing inclusion of key covariates, with internal validation via bootstrap resampling (1000 iterations) for optimism-corrected coefficients and 95% CIs.
To address multiple testing across echocardiographic and myocardial work parameters, Benjamini-Hochberg false discovery rate (FDR) correction was applied (q < 0.05 for significance).
4. Results
4.1. Patient Characteristics
The study included 107 patients with moderate aortic stenosis (AS) and preserved ejection fraction (EF ≥ 50%), recruited between April 2024 and June 2025. Patients were stratified by functional capacity (% predicted peak VO2 < 83%, n = 42; ≥83%, n = 65) and ventilatory efficiency (VE/VCO2 slope ≥ 30, n = 41; <30, n = 61). Five patients were excluded from VE/VCO2 analysis due to irregular breathing patterns affecting measurement reliability, leaving 102 patients for this comparison.
In the % predicted VO
2 groups, those with reduced functional capacity (<83%) were more often male (77% vs. 53%,
p = 0.014), while age, body mass index (BMI), blood pressure, comorbidities, and medical therapy were similar (all
p > 0.05,
Table 1). There was no significant difference in β-blocker therapy between groups. All included patients had acceptable spirometry, and no patient met criteria for moderate or severe obstructive impairment prior to CPET.Echocardiographic and CPET Findings.
Patients with reduced functional capacity (% predicted VO
2 < 83%) demonstrated larger LV end-systolic volumes (43.6 ± 13.4 vs. 38.5 ± 11.3 mL,
p = 0.035), smaller indexed aortic valve area (0.61 ± 0.09 vs. 0.67 ± 0.14 cm
2/m
2,
p = 0.025), and lower stroke volume index (47.4 ± 8.4 vs. 51.7 ± 9.4 mL/m
2,
p = 0.020) compared with those with preserved capacity. LV end-diastolic volume (
p = 0.055) and global work index (
p = 0.068) showed trends toward significance. Other strain parameters, including GLS, LA strain, and RV free wall strain, did not differ significantly between groups (
Table 2).
In the VE/VCO
2 groups, patients with a slope ≥ 30 were older (73.9 ± 6.5 vs. 70.2 ± 7.4 years,
p = 0.011), but no other baseline characteristics differed significantly (
Table 3).
By contrast, ventilatory inefficiency (VE/VCO
2 slope ≥ 30) was associated with more pronounced myocardial dysfunction. These patients had worse LV GLS (−15.8 ± 3.1% vs. −17.6 ± 2.6%,
p = 0.002), lower TAPSE (16.1 ± 4.4 vs. 18.8 ± 4.8 mm,
p = 0.005), reduced LA reservoir strain (21.6 ± 8.0% vs. 25.6 ± 8.6%,
p = 0.019), and impaired LA conduit strain (−8.4 ± 5.1% vs. −12.7 ± 5.3%,
p < 0.001). RV free wall strain was also reduced (−19.5 ± 5.6% vs. −22.0 ± 5.8%,
p = 0.029). Myocardial work indices were significantly altered, with lower GWI and GCW, higher GWW, and reduced GWE (all
p < 0.05). Additionally, patients with VE/VCO
2 ≥30 had smaller AVA (1.16 ± 0.17 vs. 1.26 ± 0.18 cm
2,
p = 0.010) and higher mean pressure gradients (26.2 ± 5.8 vs. 24.0 ± 4.9 mmHg,
p = 0.043) (
Table 4).
Because multiple echocardiographic and myocardial work parameters were tested, we applied false discovery rate (FDR) correction to minimize the risk of type I error. The main associations remained significant after correction, supporting the robustness of the findings and reducing the likelihood that they are driven by multiple comparisons.
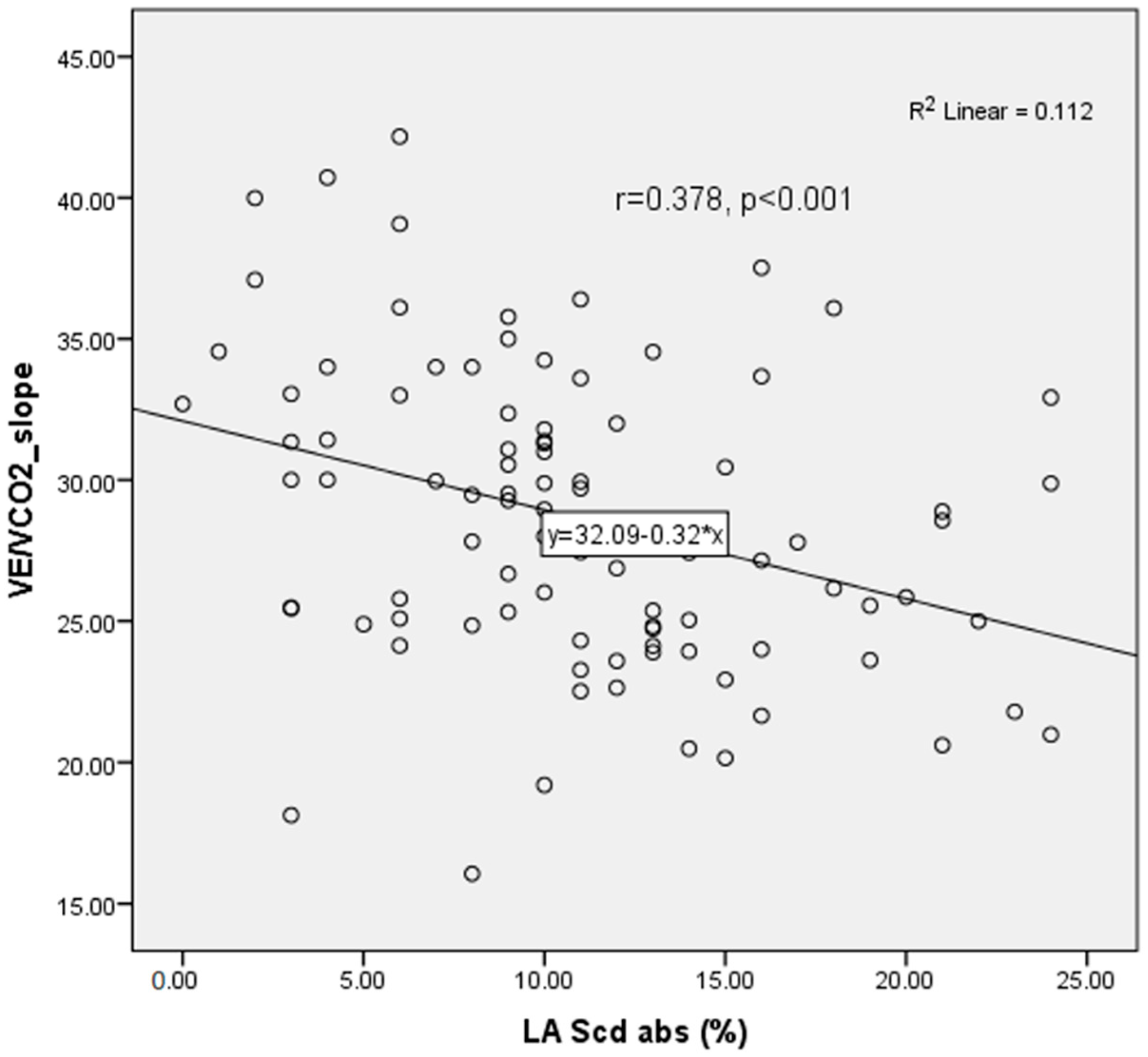
LAScd showed a significant inverse correlation with the VE/VCO
2 slope (r = 0.378,
p < 0.001), consistent with impaired left atrial–pulmonary coupling in patients with reduced ventilatory efficiency (
Figure 2).
Receiver operating characteristic (ROC) analysis was performed to assess the ability of LAScd to identify patients with ventilatory inefficiency (VE/VCO
2 ≥ 30). The area under the curve (AUC) was 0.723 (95% CI 0.623–0.823,
p < 0.001), indicating fair discrimination (
Supplementary Materials Figure S1).
To evaluate threshold robustness, the analysis was repeated using a VE/VCO2 slope ≥ 34 to define ventilatory inefficiency. In this model, only TAPSE remained independently associated with the outcome (B = −1.76, SE = 0.62, p = 0.005, OR = 0.17 (95% CI not computed by SPSS for bootstrap model)), while the direction of associations for other variables remained consistent but no longer reached statistical significance. These results suggest that right ventricular function retains prognostic relevance even when a stricter criterion for ventilatory inefficiency is applied.
4.2. Univariate and Multivariate Analyses
In univariate analysis, % predicted VO2 correlated positively with age, AVAi, stroke volume index, and absolute value of RV free wall strain. These findings indicate that forward flow reserve, rather than subclinical LV dysfunction, is the principal determinant of aerobic capacity in moderate AS. Although SVi and AVAi both showed moderate correlations with exercise performance, neither variable remained an independent predictor in multivariable modeling.
In contrast, VE/VCO
2 slope showed stronger and more consistent associations with indices of myocardial mechanics. Age and systolic blood pressure correlated modestly with ventilatory efficiency, but echocardiographic parameters demonstrated the most robust relationships. GLS, GWI, GCW, LA Scd, and TAPSE were all significantly associated with VE/VCO
2 slope, highlighting the integrated contribution of LV, LA, and RV mechanics to ventilatory control. Among these, LA Scd emerged as the only independent predictor in multivariable analysis (
Table 5). This underscores the pivotal role of atrial conduit function in determining ventilatory efficiency, likely through its impact on LV filling pressures, pulmonary vascular load, and RV–pulmonary coupling.
Internal validation with 1000 bootstrap samples showed overall model stability and low coefficient bias. The optimism-corrected regression coefficients (B (95% CI)) were: age 0.047 (−0.043 to 0.178), GLS 0.361 (−0.114 to 1.026), TAPSE −0.421 (−1.903 to 0.961), LAScd 0.131 (0.025 to 0.309), GWI −0.001 (−0.006 to 0.005), GCW 0.003 (−0.002 to 0.008], and systolic blood pressure (SBP) −0.045 (−0.120 to −0.004). Among these, LAScd (
p = 0.019) and SBP (
p = 0.083) remained significant or near-significant after correction, confirming limited overfitting and good internal consistency of the final model (
Supplementary Materials Table S1).
In the sensitivity multivariable model incorporating prespecified clinical covariates (age, sex, BMI, β-blocker therapy, AF, COPD, CKD, and SBP) irrespective of univariable significance, associations aligned closely with the main analysis. Following 1000 bootstrap resamples, optimism-corrected coefficients (B [95% CI]) included: age 0.043 (−0.071–0.212), GLS 0.269 (−0.359–1.336), TAPSE −0.769 (−3.042–0.995), LAScd 0.160 (0.054–0.521), GWI −0.002 (−0.011–0.008), GCW 0.003 (−0.003–0.012), SBP−0.052 (−0.186–0.000), BMI 0.046 (−0.116–0.257), and COPD −20.661 (−23.281–−17.539). Among these, LAScd (p = 0.024) and COPD (p = 0.003) remained independently associated with VE/VCO2 slope ≥ 30, affirming model robustness against confounders and minimal overfitting post-validation. The markedly large negative coefficient for COPD (B = −20.661, p = 0.003), although statistically significant, is attributable to its rarity, coded dichotomously as yes/no for diagnosis, and quasi-complete separation—stemming from the pre-CPET spirometry exclusion of moderate-to-severe cases, which yielded few or no instances of ventilatory inefficiency among affected individuals and thus unstable parameter estimates. Nonetheless, this underscores the primary model’s resilience to confounding and minimal overfitting after validation, with COPD’s association warranting cautious interpretation.
5. Discussion
5.1. Main Findings
In this prospective cohort, 40% of patients with moderate AS and preserved EF exhibited reduced exercise capacity, and 40% demonstrated ventilatory inefficiency. Severity of AS and comorbidity burden were less influential than underlying myocardial dysfunction. Strain-derived indices—including LV GLS, LA reservoir and conduit strain, and RV free wall strain—were strongly associated with ventilatory inefficiency but not with exercise capacity. By contrast, exercise capacity was linked to forward flow measures (stroke volume index, AVAi). Notably, LA conduit strain emerged as the strongest independent predictor of ventilatory inefficiency.
5.2. Underlying Mechanisms and Pathophysiology
Moderate AS is increasingly recognized as a heterogeneous disease, with variable patterns of LV remodeling and atrial–ventricular interaction that explain differences in clinical outcomes [
28,
29].
Accurate risk stratification is critical, as many patients with preserved EF harbour subclinical myocardial damage not evident on resting echocardiography. Functional testing offers an integrated assessment of cardiovascular reserve that complements structural imaging.
Our data show that impaired forward flow (SVi, AVAi) primarily determines exercise capacity, whereas ventilatory inefficiency reflects a more global cardiopulmonary burden. The strong link between LA conduit strain and ventilatory inefficiency highlights the pathophysiological role of atrial function in LV filling and pulmonary vascular load. Impaired LA conduit function elevates filling pressures, increases pulmonary resistance, and predisposes to RV dysfunction, all of which worsen ventilatory efficiency. This aligns with prior studies demonstrating that LA strain, particularly the conduit component, is a sensitive marker of diastolic dysfunction and pulmonary hypertension, and correlates with reduced exercise tolerance in both HF and valvular disease [
30,
31].
5.3. Clinical Implications
Risk stratification in moderate AS remains a pressing challenge. Conventional echocardiography often underestimates disease burden in patients with preserved EF, while functional assessment via CPET provides objective, integrated measures of reserve. In our study, VE/VCO
2 slope ≥ 30 was a more robust marker of subclinical dysfunction than % predicted VO
2, echoing prior evidence from HF and AS cohorts [
30]. Resting echocardiography often fails to detect these abnormalities, whereas functional assessment provides an objective and integrated measure of cardiovascular reserve.
Gherbesi et al. reviewed the physical basis and clinical applications of speckle-tracking echocardiography, emphasizing its role in assessing left ventricular function [
32].
Our findings align with recent research highlighting the importance of left atrial (LA) function in aortic stenosis (AS). Specifically, Tan et al. demonstrated the prognostic value of LA strain in AS using a competing risk analysis, further solidifying the role of LA strain as a valuable marker for risk stratification [
33].
Beyond LA metrics, right heart strain also offers robust prognostic value: a meta-analysis establishes a substantial association between RV FWS and adverse outcomes (e.g., HF hospitalisation, mortality) in the AS population, independent of LV function and AS severity. This incremental utility enhances multiparametric models for early decompensation detection [
34]. Clinically, STE integration could refine early TAVR: in equivocal moderate AS, LA/RV/RA strain abnormalities flag high-risk phenotypes for timely intervention, mitigating ischemia and optimizing recovery [
35]. Serial STE/CPET at 6–12 months could detect early all-chamber dysfunction and guide interventions in moderate AS.
Importantly, ongoing trials are investigating whether earlier intervention benefits selected patients with moderate AS. TAVR-UNLOAD reported improved quality of life without mortality benefit, while PROGRESS and EXPAND TAVR II are evaluating early TAVR in high-risk phenotypes [
36,
37].
Our findings suggest that patients with impaired ventilatory efficiency and abnormal LA conduit strain may represent one such high-risk subgroup warranting closer surveillance and possibly earlier intervention [
31,
38].
5.4. The Role of LA Conduit Strain
Among strain parameters, LA conduit strain was the only independent predictor of ventilatory inefficiency. This reflects its integrative role in preload reserve, LV filling, and pulmonary vascular coupling. Beyond mechanics, impaired LA conduit strain serves as a sensitive marker of cardiomyopathy severity, capturing subclinical dysfunction not visible with traditional measures. Prior studies in AS and cardiomyopathy support its prognostic value, with LAScd consistently associated with adverse outcomes, atrial fibrosis, and impaired remodelling [
33,
39,
40,
41].
In ROC analysis, LAScd demonstrated fair discriminatory power for identifying ventilatory inefficiency. This supports its physiological relevance as a marker of impaired atrial–pulmonary coupling in moderate AS. Although discrimination was moderate, this finding reinforces the concept that LA conduit function reflects early hemodynamic adaptation in this population. External validation in larger cohorts is needed to confirm these results and establish clinical cut-off values. Calibration plots and decision-curve analyses were not performed; clinical utility requires further testing.
6. Limitations
This study has several limitations. First, the sample size discrepancy between % predicted VO2 groups (n = 107) and VE/VCO2 groups (n = 102, due to the exclusion of five patients with irregular breathing) suggests potential data inconsistencies, which may affect generalizability. Second, the modest sample size may limit statistical power for detecting differences, such as GLS in the % predicted VO2 groups. Third, the forward likelihood ratio (LR) method in multivariate analysis may have excluded relevant predictors due to its data-driven approach, and a larger sample could enable more robust modeling. Fifth, the study did not assess long-term outcomes (e.g., mortality, heart failure hospitalization), limiting prognostic conclusions. Finally, the single-center design and exclusion of patients with significant comorbidities may reduce generalizability.
Reproducibility of advanced imaging (e.g., GLS, LAScd, MW) relies on single-observer analysis here, without ICC reporting. While blinding reduced bias, unassessed intra-/inter-observer variability introduces potential measurement error, warranting multi-observer validation in future studies.
We acknowledge that ventilatory and chronotropic factors may influence exercise capacity and ventilatory efficiency. Although β-blocker therapy was comparable between groups, spirometry was performed in all patients with exclusion of relevant pulmonary impairment, and patients with erratic breathing patterns were excluded from CPET analysis, residual confounding from heart-rate reserve or subtle ventilatory limitation cannot be fully excluded.
The inclusion of clinically relevant confounders in the sensitivity model confirmed that the observed associations were not driven by differences in baseline therapy or comorbidities. Nevertheless, although the model was internally validated by bootstrap resampling, external validation in an independent population was not possible. Thus, while the results appear stable, residual confounding and limited generalizability cannot be completely excluded.
The absence of explicit low-flow stratification (all SVi ≥35 mL/m2) limits applicability to low-flow cases.
7. Conclusions
This study demonstrates that ventilatory inefficiency is more closely associated with subclinical LV, LA, and RV dysfunction than with exercise capacity in patients with moderate aortic stenosis and preserved ejection fraction. LA conduit strain (LAScd) emerged as the strongest independent predictor, underscoring its potential mechanistic role in atrial–pulmonary coupling and its value as a sensitive marker of early hemodynamic impairment. These findings are exploratory and hypothesis-generating; they suggest that combined CPET and strain analysis may improve physiological characterisation and identify patients who could benefit from closer follow-up. However, the absence of longitudinal or outcome data precludes conclusions regarding prognosis or treatment effects.
Supplementary Materials
The following supporting information can be downloaded at:
https://www.mdpi.com/article/10.3390/jcm14228065/s1, Figure S1: ROC curve regarding diagnostic accuracy of LA conduit strain (LAScd) in predicting patients with ventilatory inefficiency (VE/VCO
2 ≥30); Figure S2: Scatter plot of GLS % and VE/VCO
2 Slope in the study population. Spearman correlation r = 0.264,
p = 0.007; Figure S3: Scatter plot of GWI and VE/VCO
2 Slope in the study population. Spearman correlation of GWI and VE/VCO
2 Slope r = −3.08,
p = 0.001; Table S1: Bootstrap Linear Regression Analysis of Echocardiographic and Clinical Variables.
Author Contributions
Conceptualization, O.P., DZ. and S.V.; methodology, O.P., D.Z., S.V. and D.T.-Z.; validation, D.T.-Z. and S.V.; formal analysis, O.P.; investigation, O.P., I.N., O.N.-A., A.P., R.M., S.S., I.P., I.J., A.U., M.T. and M.O.; resources—A.P., S.S., M.T., A.U., I.J. and J.V.; writing—original draft preparation, O.P.; writing—review and editing, O.P., D.Z., S.V., D.T.-Z. and G.S.; visualization, O.P.; supervision, D.T.-Z. and G.S.; project administration, D.T.-Z. and G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was part of project No. 451-03-66/2024-03/200110 by the Ministry of Science, Technological Development, and Innovation of the Republic of Serbia. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the University Clinical Center of Serbia and the Faculty of Medicine, University of Belgrade (protocol code 25/XI-4, 20 November 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Osnabrugge, R.L.; Mylotte, D.; Head, S.J.; Van Mieghem, N.M.; Nkomo, V.T.; LeReun, C.M.; Bogers, A.J.; Piazza, N.; Kappetein, A.P. Aortic stenosis in the elderly: Disease prevalence and number of candidates for transcatheter aortic valve replacement: A meta-analysis and modeling study. J. Am. Coll. Cardiol. 2013, 62, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 43, 561–632. [Google Scholar] [CrossRef]
- Strange, G.; Stewart, S.; Celermajer, D.; Prior, D.; Scalia, G.M.; Marwick, T.; Ilton, M.; Joseph, M.; Codde, J.; Playford, D. Poor Long-Term Survival in Patients with Moderate Aortic Stenosis. J. Am. Coll. Cardiol. 2019, 74, 1851–1863. [Google Scholar] [CrossRef]
- Zhu, D.; Ito, S.; Miranda, W.R.; Nkomo, V.T.; Pislaru, S.V.; Villarraga, H.R.; Pellikka, P.A.; Crusan, D.J.; Oh, J.K. Left Ventricular Global Longitudinal Strain Is Associated with Long-Term Outcomes in Moderate Aortic Stenosis. Circ. Cardiovasc. Imaging 2020, 13, e009958. [Google Scholar] [CrossRef] [PubMed]
- McConkey, H.Z.R.; Marber, M.; Chiribiri, A.; Pibarot, P.; Redwood, S.R.; Prendergast, B.D. Coronary Microcirculation in Aortic Stenosis. Circ. Cardiovasc. Interv. 2019, 12, e007547. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.; Petraglia, L.; Campana, P.; Gerundo, G.; Caruso, A.; Grimaldi, M.G.; Russo, V.; Attena, E.; Leosco, D.; Parisi, V. The role of inflammation and metabolic risk factors in the pathogenesis of calcific aortic valve stenosis. Aging Clin. Exp. Res. 2021, 33, 1765–1770. [Google Scholar] [CrossRef]
- Singh, A.; Greenwood, J.P.; Berry, C.; Dawson, D.K.; Hogrefe, K.; Kelly, D.J.; Dhakshinamurthy, V.; Lang, C.C.; Khoo, J.P.; Sprigings, D.; et al. Comparison of exercise testing and CMR measured myocardial perfusion reserve for predicting outcome in asymptomatic aortic stenosis: The PRognostic Importance of MIcrovascular Dysfunction in Aortic Stenosis (PRIMID AS) Study. Eur. Heart J. 2017, 38, 1222–1229. [Google Scholar] [CrossRef]
- Clavel, M.-A.; Magne, J.; Pibarot, P. Low-gradient aortic stenosis. Eur. Heart J. 2016, 37, 2645–2657. [Google Scholar] [CrossRef]
- Le, V.D.; Jensen, G.V.; Kjøller-Hansen, L. Prognostic Usefulness of Cardiopulmonary Exercise Testing for Managing Patients with Severe Aortic Stenosis. Am. J. Cardiol. 2017, 120, 844–849. [Google Scholar] [CrossRef][Green Version]
- Juarez, M.; Castillo-Rodriguez, C.; Soliman, D.; Del Rio-Pertuz, G.; Nugent, K. Cardiopulmonary Exercise Testing in Heart Failure. J. Cardiovasc. Dev. Dis. 2024, 11, 70. [Google Scholar] [CrossRef]
- Domínguez-Rodríguez, A. The cardiopulmonary exercise testing in asymptomatic severe aortic stenosis: Not to be forgotten in 2021 ESC Guidelines of valvular heart disease. Eur. J. Cardiothorac. Surg. 2022, 62, ezac119. [Google Scholar] [CrossRef] [PubMed]
- American Thoracic Society; American College of Chest Physicians. ATS/ACCP Statement on Cardiopulmonary Exercise Testing. Am. J. Respir. Crit. Care Med. 2003, 167, 211–277. [Google Scholar] [CrossRef]
- Guazzi, M.; Adams, V.; Conraads, V.; Halle, M.; Mezzani, A.; Vanhees, L.; Arena, R.; Fletcher, G.F.; Forman, D.E.; Kitzman, D.W.; et al. Clinical Recommendations for Cardiopulmonary Exercise Testing Data Assessment in Specific Patient Populations. Circulation 2012, 126, 2261–2274. [Google Scholar] [CrossRef]
- Guazzi, M.; Borlaug, B.; Metra, M.; Losito, M.; Bandera, F.; Alfonzetti, E.; Boveri, S.; Sugimoto, T. Revisiting and Implementing the Weber and Ventilatory Functional Classifications in Heart Failure by Cardiopulmonary Imaging Phenotyping. J. Am. Heart Assoc. 2021, 10, e018822. [Google Scholar] [CrossRef]
- Campodonico, J.; Agostoni, P. One-size-fits-all peak VO2, a dream or a nightmare. Int. J. Cardiol. 2018, 263, 94–95. [Google Scholar] [CrossRef]
- Abawi, D.; Rinaldi, T.; Faragli, A.; Pieske, B.; Morris, D.A.; Kelle, S.; Tschöpe, C.; Zito, C.; Alogna, A. The non-invasive assessment of myocardial work by pressure-strain analysis: Clinical applications. Heart Fail. Rev. 2022, 27, 1261–1279. [Google Scholar] [CrossRef]
- Ribic, D.; Remme, E.W.; Smiseth, O.A.; Massey, R.J.; Eek, C.H.; Kvitting, J.P.; Gullestad, L.; Broch, K.; Russell, K. Non-invasive myocardial work in aortic stenosis: Validation and improvement in left ventricular pressure estimation. Eur. Heart J. Cardiovasc. Imaging 2024, 25, 201–212. [Google Scholar] [CrossRef]
- Moya, A.; Buytaert, D.; Penicka, M.; Bartunek, J.; Vanderheyden, M. State-of-the-Art: Noninvasive Assessment of Left Ventricular Function Through Myocardial Work. J. Am. Soc. Echocardiogr. 2023, 36, 1027–1042. [Google Scholar] [CrossRef]
- Vollema, E.M.; Sugimoto, T.; Shen, M.; Tastet, L.; Ng, A.C.T.; Abou, R.; Marsan, N.A.; Mertens, B.; Dulgheru, R.; Lancellotti, P.; et al. Association of Left Ventricular Global Longitudinal Strain with Asymptomatic Severe Aortic Stenosis: Natural Course and Prognostic Value. JAMA Cardiol. 2018, 3, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Marques-Alves, P.; Marinho, A.V.; Teixeira, R.; Baptista, R.; Castro, G.; Martins, R.; Gonçalves, L. Going beyond classic echo in aortic stenosis: Left atrial mechanics, a new marker of severity. BMC Cardiovasc. Disord. 2019, 19, 215. [Google Scholar] [CrossRef] [PubMed]
- Winkler, N.E.; Anwer, S.; Reeve, K.A.; Michel, J.M.; Kasel, A.M.; Tanner, F.C. Right vs. left ventricular longitudinal strain for mortality prediction after transcatheter aortic valve implantation. Front. Cardiovasc. Med. 2023, 10, 1252872. [Google Scholar] [CrossRef]
- Stassen, J.; Pio, S.M.; Ewe, S.H.; Singh, G.K.; Hirasawa, K.; Butcher, S.C.; Cohen, D.J.; Généreux, P.; Leon, M.B.; Marsan, N.A.; et al. Left Ventricular Global Longitudinal Strain in Patients with Moderate Aortic Stenosis. J. Am. Soc. Echocardiogr. 2022, 35, 791–800.e4. [Google Scholar] [CrossRef] [PubMed]
- Hamada, A.; Utsunomiya, H.; Tohgi, K.; Takeuchi, M.; Hyodo, Y.; Mogami, A.; Tsuchiya, A.; Takahari, K.; Ueda, Y.; Nakano, Y. Prevalence, Mechanisms, and Prognostic Impact of Effort Intolerance in Patients with Asymptomatic/Minimally Symptomatic Aortic Stenosis. J. Am. Heart Assoc. 2025, 14, e041414. [Google Scholar] [CrossRef]
- Banovic, M.; Mileva, N.; Moya, A.; Paolisso, P.; Beles, M.; Boskovic, N.; Jovanovic, M.; Nedeljkovic, I.; Radunovic, A.; Radjenovic, M.; et al. Myocardial Work Predicts Outcome in Asymptomatic Severe Aortic Stenosis. JACC Cardiovasc. Imaging 2023, 16, 708–710. [Google Scholar] [CrossRef]
- Fortuni, F.; Butcher, S.C.; van der Kley, F.; Lustosa, R.P.; Karalis, I.; de Weger, A.; Priori, S.G.; van der Bijl, P.; Bax, J.J.; Delgado, V.; et al. Left Ventricular Myocardial Work in Patients with Severe Aortic Stenosis. J. Am. Soc. Echocardiogr. 2021, 34, 257–266. [Google Scholar] [CrossRef]
- Naeije, R.; Faoro, V. The great breathlessness of cardiopulmonary diseases. Eur. Respir. J. 2018, 51, 1702517. [Google Scholar] [CrossRef] [PubMed]
- Arena, R.; Myers, J.; Abella, J.; Peberdy, M.A.; Bensimhon, D.; Chase, P.; Guazzi, M. The Ventilatory Classification System Effectively Predicts Hospitalization in Patients with Heart Failure. J. Cardiopulm. Rehabil. Prev. 2008, 28, 195–198. [Google Scholar] [CrossRef]
- Stassen, J.; Ewe, S.H.; Hirasawa, K.; Butcher, S.C.; Singh, G.K.; Amanullah, M.R.; Sin, K.Y.K.; Ding, Z.P.; Pio, S.M.; Chew, N.W.S.; et al. Left ventricular remodelling patterns in patients with moderate aortic stenosis. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 1326–1335. [Google Scholar] [CrossRef] [PubMed]
- Treibel Thomas, A.; Badiani, S.; Lloyd, G.; Moon James, C. Multimodality Imaging Markers of Adverse Myocardial Remodeling in Aortic Stenosis. JACC Cardiovasc. Imaging 2019, 12 Pt 1, 1532–1548. [Google Scholar] [CrossRef]
- Fulop, P.; Valocik, G.; Barbierik Vachalcova, M.; Zenuch, P.; Filipova, L. Aortic stenosis and right ventricular dysfunction. Int. J. Cardiovasc. Imaging 2024, 40, 299–305. [Google Scholar] [CrossRef]
- Le, T.T.; Huang, W.; Singh, G.K.; Toh, D.F.; Ewe, S.H.; Tang, H.C.; Loo, G.; Bryant, J.A.; Ang, B.; Tay, E.L.; et al. Echocardiographic Global Longitudinal Strain Is Associated with Myocardial Fibrosis and Predicts Outcomes in Aortic Stenosis. Front Cardiovasc. Med. 2021, 8, 750016. [Google Scholar] [CrossRef]
- Gherbesi, E.; Gianstefani, S.; Angeli, F.; Ryabenko, K.; Bergamaschi, L.; Armillotta, M.; Guerra, E.; Tuttolomondo, D.; Gaibazzi, N.; Squeri, A.; et al. Myocardial strain of the left ventricle by speckle tracking echocardiography: From physics to clinical practice. Echocardiography 2024, 41, e15753. [Google Scholar] [CrossRef]
- Tan, E.S.J.; Jin, X.; Oon, Y.Y.; Chan, S.P.; Gong, L.; Lunaria, J.B.; Liew, O.-W.; Chong, J.P.-C.; Tay, E.L.W.; Soo, W.M.; et al. Prognostic Value of Left Atrial Strain in Aortic Stenosis: A Competing Risk Analysis. J. Am. Soc. Echocardiogr. 2023, 36, 29–37.e5. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Nabeshima, Y.; Kitano, T.; Parasca, C.A.; Calin, A.; Popescu, B.A.; Takeuchi, M. Prognostic value of right ventricular free-wall longitudinal strain in aortic stenosis: A systematic review and meta-analysis. J. Cardiol. 2024, 84, 80–85. [Google Scholar] [CrossRef]
- Grevious, S.N.; Fernandes, M.F.; Annor, A.K.; Ibrahim, M.; Saint Croix, G.R.; de Marchena, E.; Cohen, M.G.; Alfonso, C.E. Prognostic Assessment of Right Ventricular Systolic Dysfunction on Post-Transcatheter Aortic Valve Replacement Short-Term Outcomes: Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2020, 9, e014463. [Google Scholar] [CrossRef] [PubMed]
- Van Mieghem, N.M.; Elmariah, S.; Spitzer, E.; Pibarot, P.; Nazif, T.M.; Bax, J.J.; Hahn, R.T.; Popma, A.; Ben-Yehuda, O.; Kallel, F.; et al. Transcatheter Aortic Valve Replacement in Patients with Systolic Heart Failure and Moderate Aortic Stenosis: TAVR UNLOAD. J. Am. Coll. Cardiol. 2025, 85, 878–890. [Google Scholar] [CrossRef]
- Théron, A.; Ternacle, J.; Pibarot, P. Moderate aortic stenosis: The next frontier of transcatheter aortic valve implantation? Arch. Cardiovasc. Dis. 2023, 116, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Arena, R.; Myers, J.; Aslam, S.S.; Varughese, E.B.; Peberdy, M.A. Peak VO2 and VE/VCO2 slope in patients with heart failure: A prognostic comparison. Am. Heart J. 2004, 147, 354–360. [Google Scholar] [CrossRef]
- White James, A. Left Atrial Strain in Dilated Cardiomyopathy. JACC Cardiovasc. Imaging 2022, 15, 1027–1029. [Google Scholar] [CrossRef]
- Fang, Q.; Kan, A.; Li, S.; Yu, Y.; Dai, J.; Song, Y.; Wang, X.; Xiao, X.; Xu, L.; Gong, L. Predictive value of left atrial strain for left ventricular reverse remodeling in dilated cardiomyopathy. Int. J. Cardiol. 2025, 423, 133020. [Google Scholar] [CrossRef]
- Raafs, A.G.; Vos, J.L.; Henkens, M.T.H.M.; Slurink, B.O.; Verdonschot, J.A.J.; Bossers, D.; Roes, K.; Gerretsen, S.; Knackstedt, C.; Hazebroek, M.R.; et al. Left Atrial Strain Has Superior Prognostic Value to Ventricular Function and Delayed-Enhancement in Dilated Cardiomyopathy. JACC Cardiovasc. Imaging 2022, 15, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).