Primary Intramedullary Spinal Melanocytomas: Case Report and Review of Clinical Features, Diagnosis, and Management
Abstract
1. Introduction
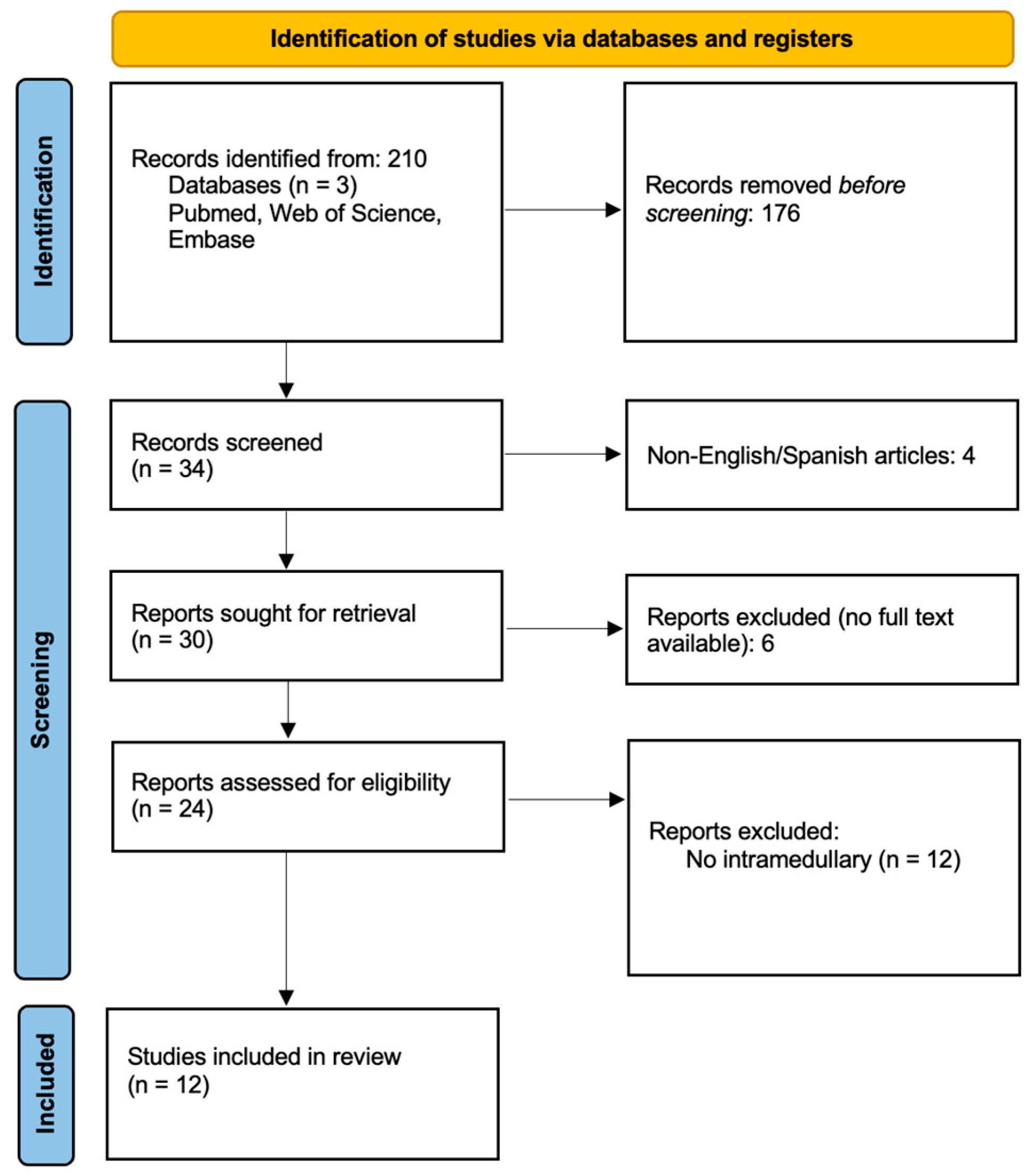
2. Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
2.3. Literature Search Strategy
2.4. Study Selection
2.5. Data Extraction and Analysis
3. Results
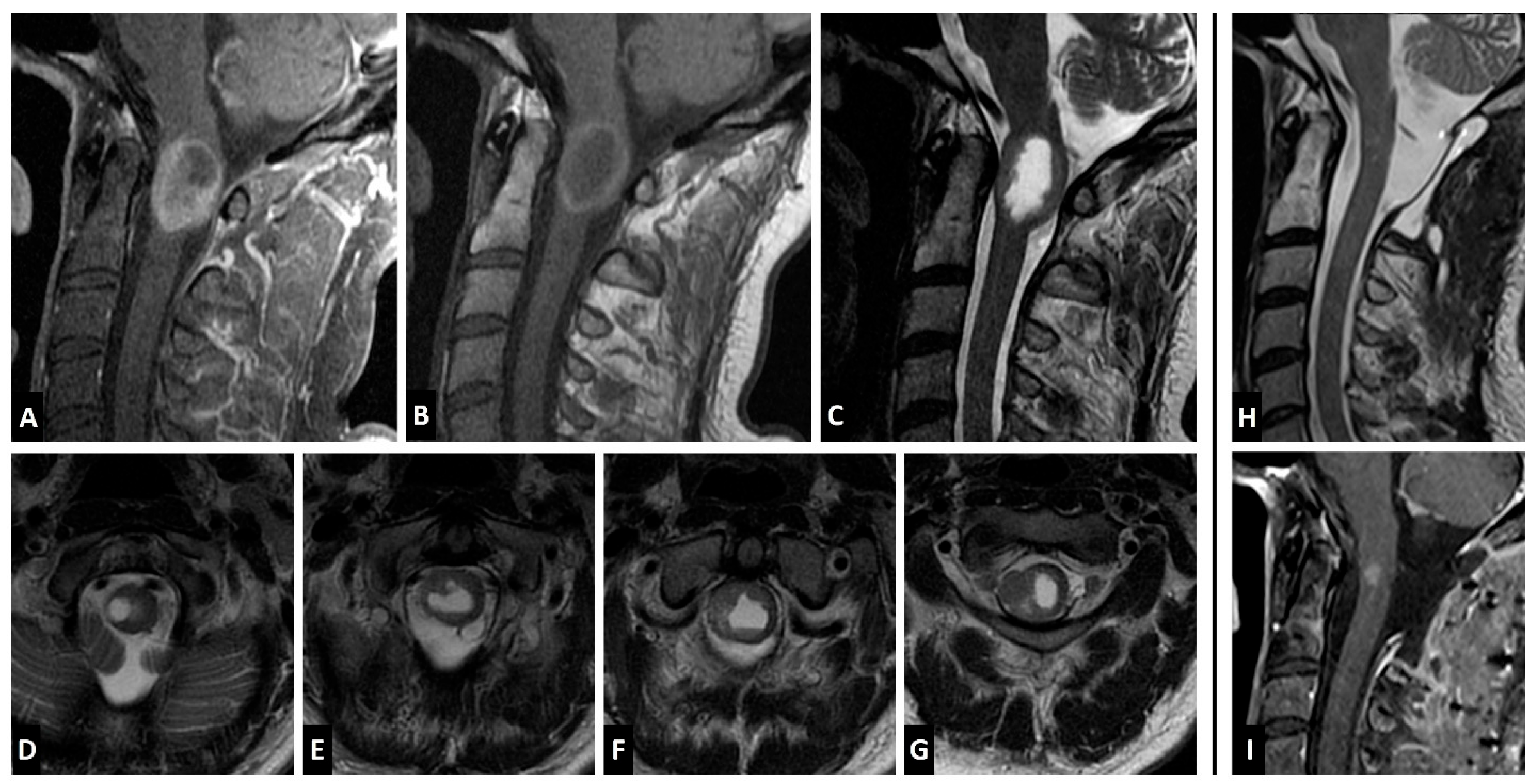
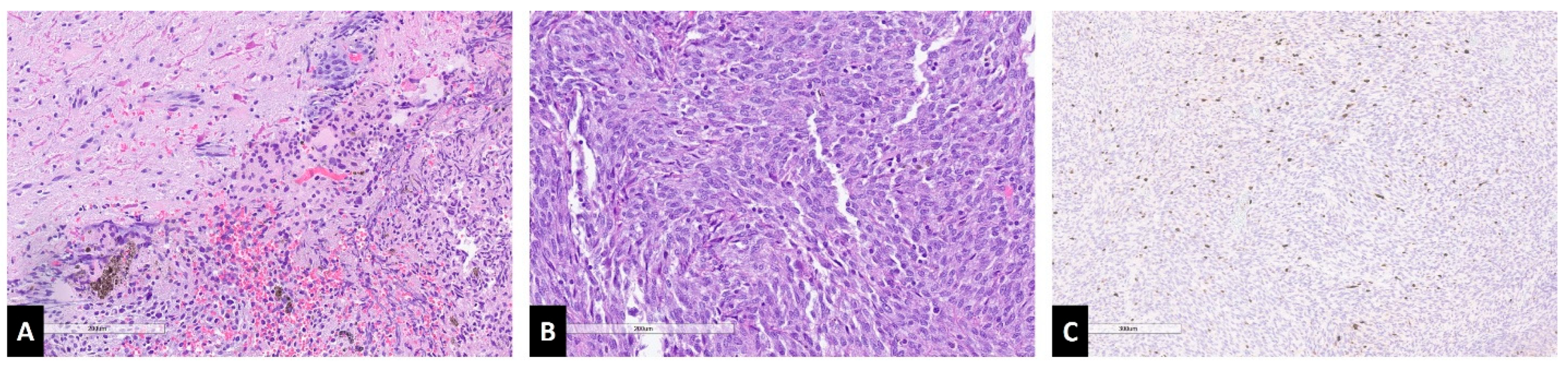
Case Report
4. Discussion
4.1. Epidemiology
4.2. Histopathology and Genetic Markers
4.3. Imaging Characteristics
4.4. Clinical Presentation
4.5. Treatment and Outcomes
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perrini, P.; Caniglia, M.; Pieroni, M.; Castagna, M.; Parenti, G.F. Malignant Transformation of Intramedullary Melanocytoma: Case Report. Neurosurgery 2010, 67, E867–E869, Discussion E869. [Google Scholar] [CrossRef]
- Pellerino, A.; Verdijk, R.M.; Nichelli, L.; Andratschke, N.H.; Idbaih, A.; Goldbrunner, R. Primary Meningeal Melanocytic Tumors of the Central Nervous System: A Review from the Ultra-Rare Brain Tumors Task Force of the European Network for Rare Cancers (EURACAN). Cancers 2024, 16, 2508. [Google Scholar] [CrossRef]
- Küsters-Vandevelde, H.V.N.; Klaasen, A.; Küsters, B.; Groenen, P.J.T.A.; van Engen-van Grunsven, I.A.C.H.; van Dijk, M.R.C.F.; Reifenberger, G.; Wesseling, P.; Blokx, W.A.M. Activating Mutations of the GNAQ Gene: A Frequent Event in Primary Melanocytic Neoplasms of the Central Nervous System. Acta Neuropathol. 2010, 119, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Foit, N.A.; Neidert, M.C.; Woernle, C.M.; Rushing, E.J.; Krayenbühl, N. Bifocal Extra- and Intradural Melanocytoma of the Spine: Case Report and Literature Review. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2013, 22 (Suppl. S3), S521–S525. [Google Scholar] [CrossRef] [PubMed]
- Karikari, I.O.; Powers, C.J.; Bagley, C.A.; Cummings, T.J.; Radhakrishnan, S.; Friedman, A.H. Primary Intramedullary Melanocytoma of the Spinal Cord: Case Report. Neurosurgery 2009, 64, E777–E778, Discussion E778. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shao, X.; Zou, Y. Primary Intramedullary Melanocytoma in the Thoracic Cord: A Case Report and Literature Review. Transl. Cancer Res. 2022, 11, 928–934. [Google Scholar] [CrossRef]
- Kim, K.; Choi, J.; Cha, Y.J.; Kim, K.H.; Cho, Y.E. Primary Intramedullary Meningeal Melanocytoma in Cervical Spine: A Case Report and Literature Review. Nerve 2021, 7, 1–6. [Google Scholar] [CrossRef]
- Dubey, A.; Kataria, R.; Sardana, V. Intramedullary Melanocytoma of the Cervicothoracic Cord: Case Report and Review of Literature. Asian J. Neurosurg. 2022, 13, 478–481. [Google Scholar] [CrossRef]
- Wagner, F.; Berezowska, S.; Wiest, R.; Gralla, J.; Beck, J.; Verma, R.K.; Huber, A. Primary Intramedullary Melanocytoma in the Cervical Spinal Cord: Case Report and Literature Review. Radiol. Case Rep. 2015, 10, 1010. [Google Scholar] [CrossRef]
- Muthappan, M.; Muthu, T.; Hussain, Z.; Lamont, D.; Balakrishnan, V. Cervical Intramedullary Melanocytoma: A Case Report and Review of Literature. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2012, 19, 1450–1453. [Google Scholar] [CrossRef]
- Eskandari, R.; Schmidt, M.H. Intramedullary Spinal Melanocytoma. Rare Tumors 2010, 2, 64–67. [Google Scholar] [CrossRef]
- Caruso, R.; Marrocco, L.; Wierzbicki, V.; Salvati, M. Intramedullary Melanocytoma: Case Report and Review of Literature. Tumori J. 2009, 95, 389–393. [Google Scholar] [CrossRef]
- Chacko, G.; Rajshekhar, V. Thoracic Intramedullary Melanocytoma with Long-Term Follow-Up. J. Neurosurg. Spine 2008, 9, 589–592. [Google Scholar] [CrossRef]
- Horn, E.M.; Nakaji, P.; Coons, S.W.; Dickman, C.A. Surgical Treatment for Intramedullary Spinal Cord Melanocytomas. J. Neurosurg. Spine 2008, 9, 48–54. [Google Scholar] [CrossRef]
- Turhan, T.; Oner, K.; Yurtseven, T.; Akalin, T.; Ovul, I. Spinal Meningeal Melanocytoma. J. Neurosurg. Spine 2004, 100, 287–290. [Google Scholar] [CrossRef]
- Battistelli, M.; Grilli, F.; Rapisarda, A.; Di Domenico, M.; Montano, N.; Gessi, M.; Olivi, A.; Albanese, A.; Polli, F.M. Unsatisfactory Neurological Outcome in an Intramedullary Thoracic Intermediate-Grade Melanocytoma-Systematic Review and Illustrative Case. Cancers 2024, 16, 1867. [Google Scholar] [CrossRef] [PubMed]
- Brat, D.J.; Giannini, C.; Scheithauer, B.W.; Burger, P.C. Primary Melanocytic Neoplasms of the Central Nervous Systems. Am. J. Surg. Pathol. 1999, 23, 745–754. [Google Scholar] [CrossRef]
- Cornejo, K.M.; Hutchinson, L.; Cosar, E.F.; Smith, T.; Tomaszewicz, K.; Dresser, K.; Deng, A. Is It a Primary or Metastatic Melanocytic Neoplasm of the Central Nervous System?: A Molecular Based Approach. Pathol. Int. 2013, 63, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Küsters-Vandevelde, H.V.N.; Küsters, B.; van Engen-van Grunsven, A.C.H.; Groenen, P.J.T.A.; Wesseling, P.; Blokx, W.A.M. Primary Melanocytic Tumors of the Central Nervous System: A Review with Focus on Molecular Aspects. Brain Pathol. 2015, 25, 209–226. [Google Scholar] [CrossRef]
- Liubinas, S.V.; Maartens, N.; Drummond, K.J. Primary Melanocytic Neoplasms of the Central Nervous System. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2010, 17, 1227–1232. [Google Scholar] [CrossRef]
- Sahm, F.; Reuss, D.E.; Giannini, C. WHO 2016 Classification: Changes and Advancements in the Diagnosis of Miscellaneous Primary CNS Tumours. Neuropathol. Appl. Neurobiol. 2018, 44, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Vij, M.; Tungria, A.; Jaiswal, A.K.; Srivastava, A.K.; Behari, S. Primary Melanocytic Tumors of the Central Nervous System: A Neuroradiological and Clinicopathological Study of Five Cases and Brief Review of Literature. Neurol. India 2011, 59, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Dar, N.; Mantziaris, G.; Pikis, S.; Young, L.; Sheehan, J. Stereotactic Radiosurgery for Intracranial Primary Melanocytomas. World Neurosurg. 2022, 164, 160–166. [Google Scholar] [CrossRef] [PubMed]
| Study Author | Year | Age | Sex | Signs and Symptoms | Location | Imaging | Resection | RT | IHC/Molecular Markers (+) | Symptom Control | Follow-up (Months) | Recurrence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wang et al. [6] | 2022 | 56 | M | Paraparesis. Bilateral pain, temperature, and touch hypoesthesia below inguinal level. | T10–T12 | MRI: T1 hyperintense and T2 hypointense. Uncertain enhancement. | NT | No | HMB-45, MelanA Ki-67 = 5% | Worsened | 17 | No |
| Kim et al. [7] | 2021 | 78 | M | Right hemiparesis with numbness. Gait disturbance. | C3–C5 | CT: hyperdense mass with heterogeneous core; MRI: T1 hyperintense, T2 hypointense. Mild enhancement. | T | No | MelanA, S-100, CD-68. Ki-67 < 5%. | - | - | - |
| Dubey et al. [8] | 2018 | 35 | F | Cervicothoracic back pain. Spastic paraparesis. | C6–T6 | MRI: T1 hyperintense, T2 hypointense. Syrinx formation cranial and caudal. Heterogeneous enhancement. | NT | No | - | Improved | 6 | No |
| Wagner et al. [9] | 2015 | 63 | M | Right progressive hemiparesis. Bilateral positive Babinski sign. | C2–C3 | MRI: T1 hyperintense, T2 isointense. No diffusion restriction. | T | Yes | S-100, MelanA, HMB-45. | Improved | 18 | Yes |
| Muthappan et al. [10] | 2012 | 61 | F | Right spastic hemiparesis and paresthesia. Loss of fine motor skills. Right hyperreflexia with positive Hoffman and Babinski signs. | C3–C4 | MRI: T2 hyperintense. | T | No | S-100, MelanA, HMB-45. Ki-67 < 2%. | Stable | 36 | No |
| Eskandari et al. [11] | 2010 | 45 | M | Paraparesis with paresthesia and pain, worse on the right. Bowel and bladder urgency. LE hyperreflexia. | T11 | MRI: T1 hyperintense, T2 hypointense with associated syrinx. | ST | Yes | - | Stable | 36 | Yes |
| Perrini et al. [1] | 2009 | 79 | F | Paraparesis with paresthesia. Urinary sphincter dysfunction. Hyperreflexia and myelopathy. | T10–T11 | MRI: T1 hyperintense, T2 hypointense. Homogeneous enhancement. | ST | No | S-100, MelanA, HMB-45 | Improved | 30 | Yes |
| Caruso et al. [12] | 2009 | 62 | M | Progressive paraparesis, spastic gait, hyperreflexia in both legs, and clonus on the left. Bilateral positive Babinski sign. Touch and pain hypoesthesia at T11 level. | T11–T12 | MRI: T1 hyperintense, T2 hypointense. | T | No | S-100, HMB-45. Ki-67 < 2%. | Improved | 24 | No |
| Karikari et al. [5] | 2009 | 20 | M | Right LE paresis. | T12 | MRI: T2 hyperintense. Homogeneous enhancement. | T | No | S-100, HMB-45, MART-1. | - | 1.5 | No |
| 32 | F | Bilateral LE paresthesia. Patchy sensory loss on the left foot. | T10 | MRI: T1 hyperintense. Homogeneous enhancement. | T | No | S-100, MART-1. | Resolved | 3 | No | ||
| Chacko et al. [13] | 2008 | 22 | M | Spastic paraparesis, bowel and bladder dysfunction. Pain, touch, and temperature hypoesthesia below T6. Reduced lower limbs proprioception. Hyperreflexia. | T6–T11 | MRI: T2 hypointense. Homogeneous enhancement. Associated syrinx. | T | No | S-100, HMB-45, vimentin. | Improved | 96 | No |
| Horn et al. [14] | 2008 | 37 | F | Progressive paresthesia and dysesthesias of bilateral UE and LE. | C1–C3 | MRI: T1 hyperintense, T2 hypointense. Homogeneous enhancement. | T | No | MelanA | Stable | 38 | Yes |
| 37 | F | Thoracic back and left LE pain. | T9–T10 | MRI: T1 hyperintense, T2 isointense. Homogeneous enhancement. | T | No | S-100, MelanA | Improved | 16 | Yes | ||
| 48 | M | Paraparesis, bilateral LE paresthesia and urinary incontinence. Hyperreflexia with myelopathic signs. | T12 | MRI: T2 hypointense. Homogeneous enhancement. | T | No | S-100 | Improved before recurrence. | 185 | Yes | ||
| Turhan et al. [15] | 2004 | 19 | F | Lumbar pain. Paraparesis with hyperreflexia. Hypoesthesia below T10. | T8 | MRI: T1 hyperintense, T2 hypointense. Associated syrinx. Homogeneous enhancement. | T | No | S-100, HMB-45. Ki-67 < 1%. | - | 36 | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimchi, G.; Varela, S.; Zuluaga-Garcia, J.P.; Call-Orellana, F.; Ramirez Ferrer, E.; Andrade de Almeida, R.A.; Gubbiotti, M.A.; Glitza, I.C.; Bishop, A.J.; Grant, J.D.; et al. Primary Intramedullary Spinal Melanocytomas: Case Report and Review of Clinical Features, Diagnosis, and Management. J. Clin. Med. 2025, 14, 8047. https://doi.org/10.3390/jcm14228047
Kimchi G, Varela S, Zuluaga-Garcia JP, Call-Orellana F, Ramirez Ferrer E, Andrade de Almeida RA, Gubbiotti MA, Glitza IC, Bishop AJ, Grant JD, et al. Primary Intramedullary Spinal Melanocytomas: Case Report and Review of Clinical Features, Diagnosis, and Management. Journal of Clinical Medicine. 2025; 14(22):8047. https://doi.org/10.3390/jcm14228047
Chicago/Turabian StyleKimchi, Gil, Samantha Varela, Juan Pablo Zuluaga-Garcia, Francisco Call-Orellana, Esteban Ramirez Ferrer, Romulo Augusto Andrade de Almeida, Maria A. Gubbiotti, Isabella C. Glitza, Andrew J. Bishop, Jonathan D. Grant, and et al. 2025. "Primary Intramedullary Spinal Melanocytomas: Case Report and Review of Clinical Features, Diagnosis, and Management" Journal of Clinical Medicine 14, no. 22: 8047. https://doi.org/10.3390/jcm14228047
APA StyleKimchi, G., Varela, S., Zuluaga-Garcia, J. P., Call-Orellana, F., Ramirez Ferrer, E., Andrade de Almeida, R. A., Gubbiotti, M. A., Glitza, I. C., Bishop, A. J., Grant, J. D., North, R. Y., Alvarez-Breckenridge, C. A., Rhines, L. D., & Tatsui, C. E. (2025). Primary Intramedullary Spinal Melanocytomas: Case Report and Review of Clinical Features, Diagnosis, and Management. Journal of Clinical Medicine, 14(22), 8047. https://doi.org/10.3390/jcm14228047






