Contemporary and Future Perspectives on Thoracic Trauma Care: Surgical Stabilization, Multidisciplinary Approaches, and the Role of Artificial Intelligence
Abstract
1. Introduction
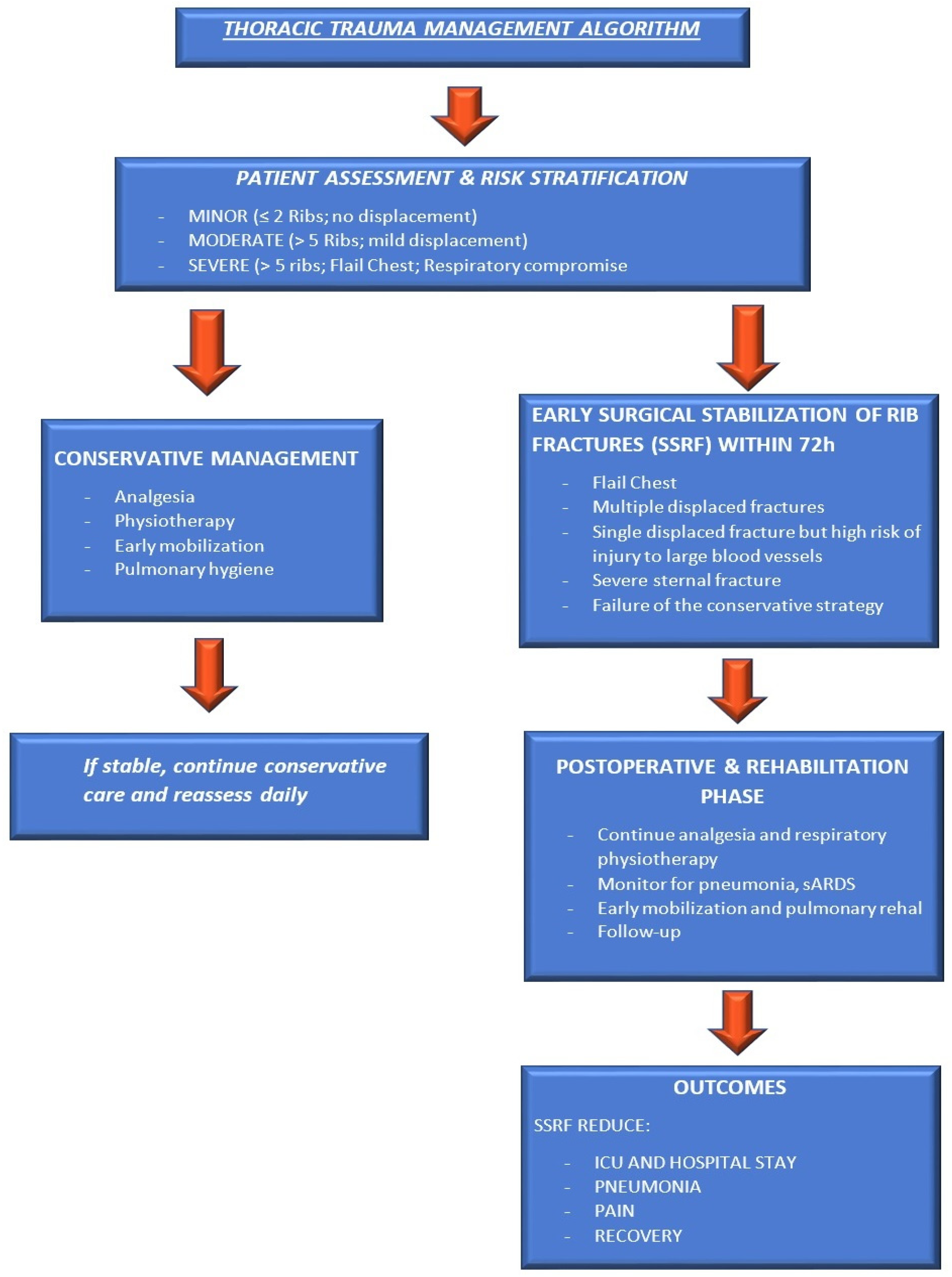
2. Current State of Management: Old and New Solutions
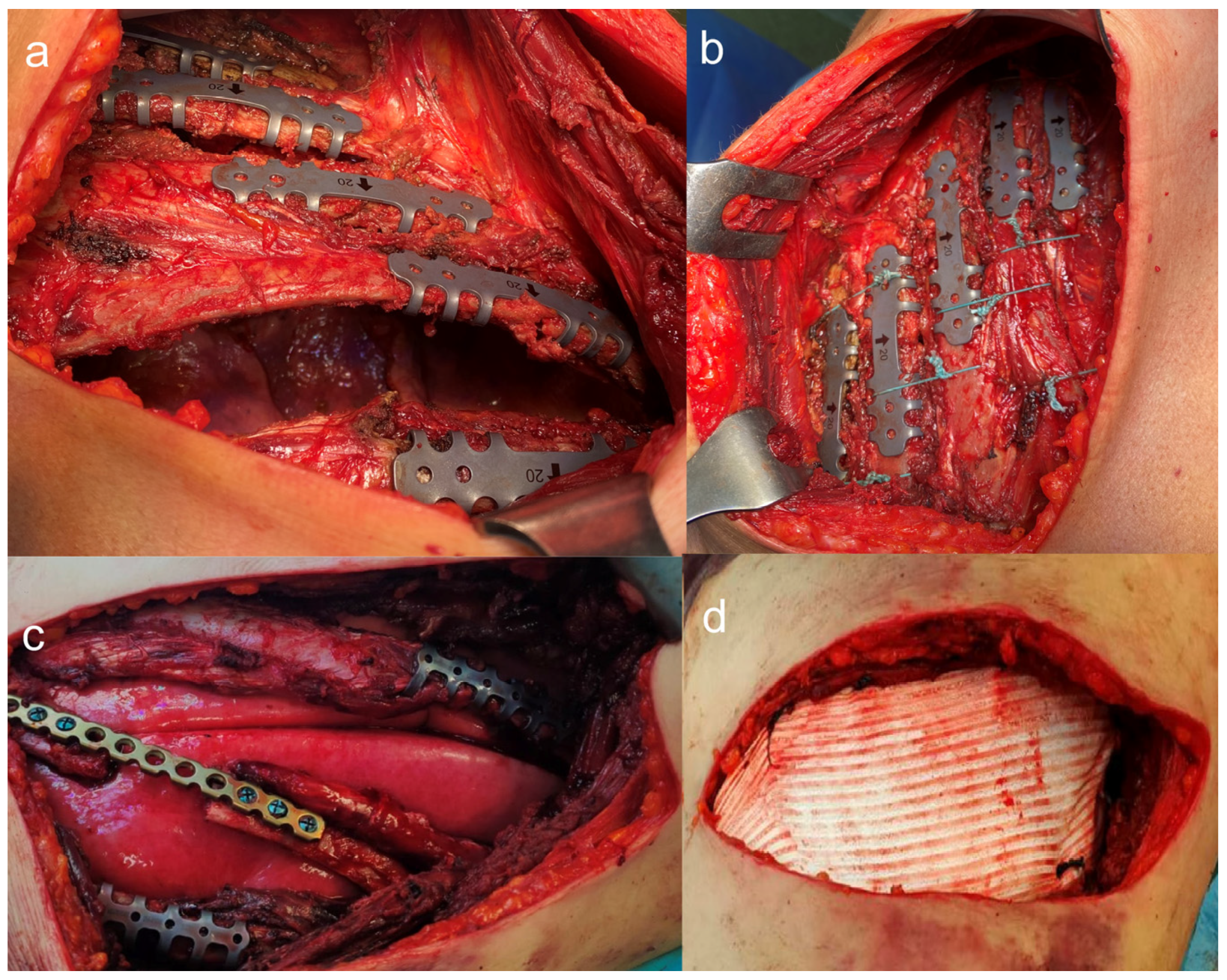
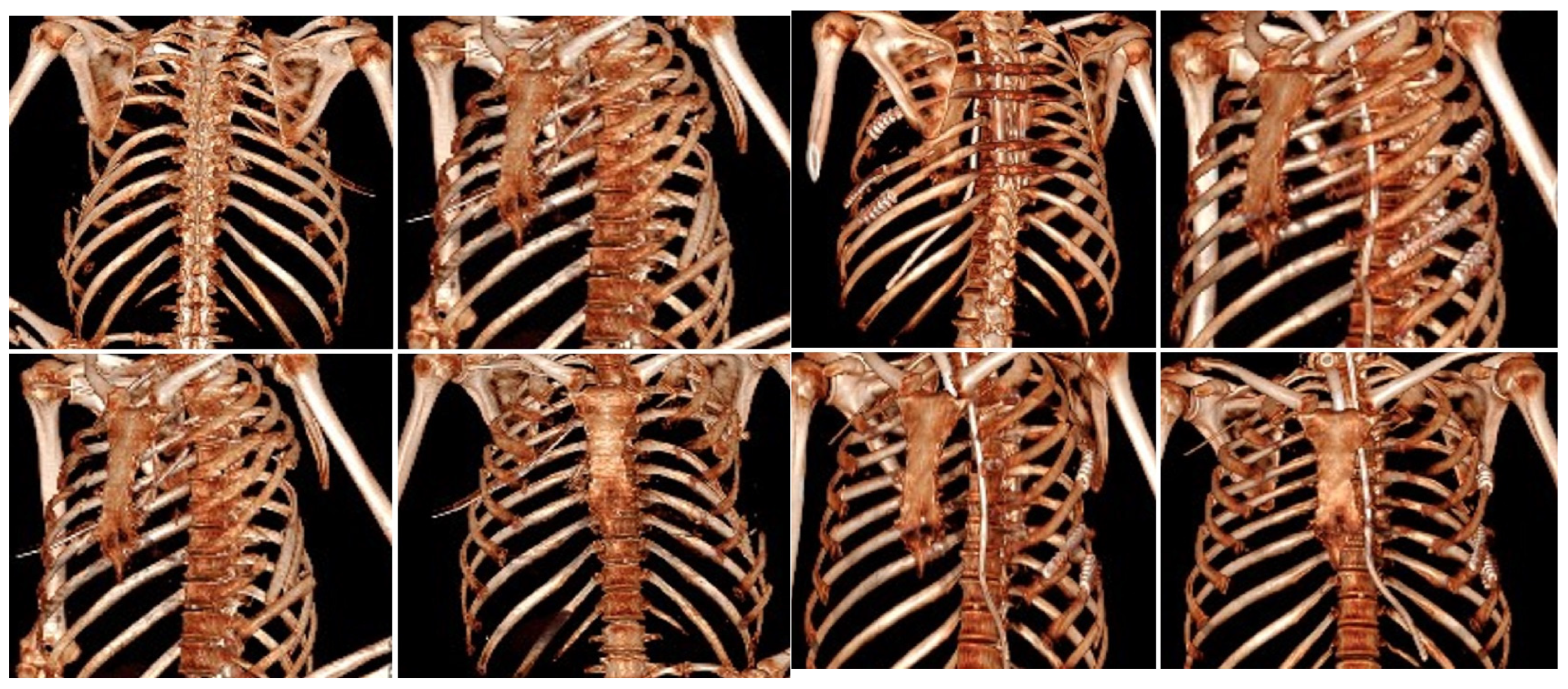
2.1. Surgical Stabilization of Rib Fractures (SSRF)
- The guidelines synthesize evidence from 287 studies and issue 39 recommendations covering: indications for flail chest, multiple displaced rib fractures, and failure of conservative management.
- Timing: Early intervention (within 72 h) leads to better outcomes.
- Techniques and implants: Various fixation devices are available, with no single method universally superior.
2.2. Integrated Diagnostic and Therapeutic Approach for Rib Fractures
2.2.1. Imaging-Based Assessment and Early Diagnostic Stratification
2.2.2. Clinical Decision-Making: Conservative Versus Surgical Stabilization
2.3. Regional Anesthesia in Trauma Chest Pain Management
3. Emerging Role of AI in Thoracic Trauma
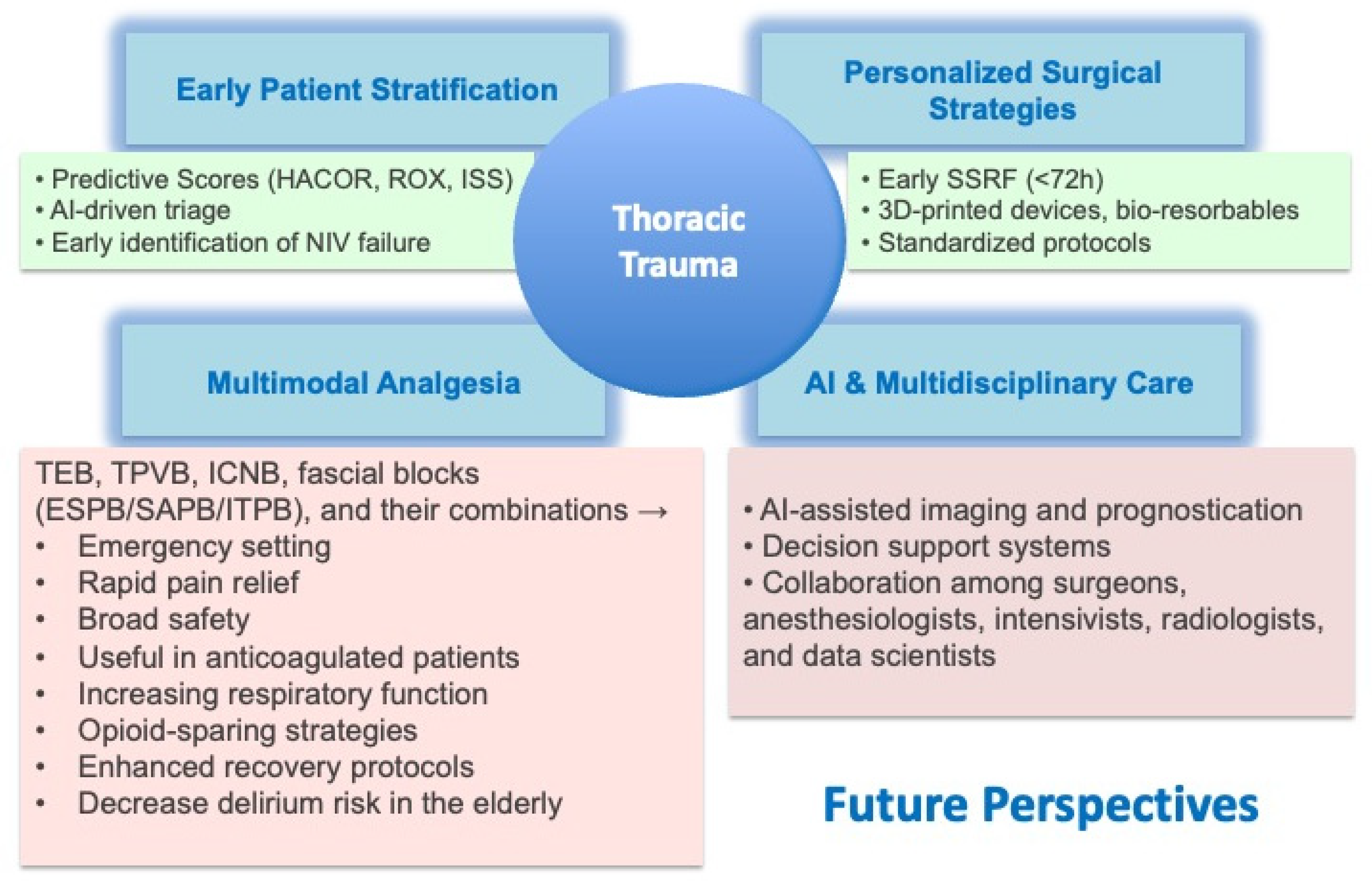
4. Perspectives
5. Conclusions
6. Key Messages
- Thoracic trauma is responsible for 25% of deaths related to injuries.
- SSRF reduces mortality, particularly in patients with flail chest and the elderly.
- Fascial plane blocks (ESPB, SAPB) offer safe and effective alternatives to neuraxial analgesia.
- AI enhances the detection of rib fractures, risk stratification, and prognostication.
- Future models should incorporate early surgery, multimodal pain management, and AI-driven multidisciplinary care.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SSRF | Surgical Stabilization of Rib Fractures |
| ESPB | Erector Spinae Plane Block |
| SAPB | Serratus Anterior Plane Block |
| TEA | Thoracic Epidural Analgesia |
| AI | Artificial Intelligence |
| NIV | Non-invasive ventilation |
| HFNC | High-Flow Nasal Cannula, |
| CPAP | Continuous positive airway pressure |
| BiPAP | stands for Bilevel Positive Airway Pressure. |
| WSES | World Society of Emergency Surgery |
| CWIS | Chest Wall Injury Society |
| VAC | Vacuum-assisted closure |
| QALYs | Quality-Adjusted Life Years |
| VATS | Video-assisted thoracoscopic surgery |
| ICNB | Intercostal Nerve Block |
| ITPB | Intertransverse Process Block |
| IPP | Interpectoral Plane block |
| PSP | Pectoserratus Plane block |
| TTMPB | Transversus Thoracic Muscle Plane Block |
| PSIP | Parascapular Sub-Iliocostalis Plane block |
| RCT | Randomized Controlled Trial |
| CAD | computer-aided design |
| CT | computerized tomography scan |
| GCS | Glasgow Coma Scale |
| PTSD | Post-traumatic stress disorder |
References
- Adal, O.; Tareke, A.A.; Bogale, E.K.; Anagaw, T.F.; Tiruneh, M.G.; Fenta, E.T.; Endeshaw, D.; Delie, A.M. Mortality of traumatic chest injury and its predictors across sub-saharan Africa: Systematic review and meta-analysis, 2024. BMC Emerg. Med. 2024, 24, 32. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, N.; Nauth, A.; Schemitsch, E.; Vicente, M.; Jenkinson, R.; Kreder, H.; McKee, M. Operative vs Nonoperative Treatment of Acute Unstable Chest Wall Injuries: A Randomized Clinical Trial. JAMA Surg. 2022, 157, 983–990. [Google Scholar] [CrossRef]
- Alanwer, K.M.; Refat, A.M.; Negm, E.M. Impact of flail chest injury on morbidity and outcome: Ten years’ experience at a tertiary care hospital in a developing country. BMC Anesthesiol. 2023, 23, 229. [Google Scholar] [CrossRef]
- Sermonesi, G.; Bertelli, R.; Pieracci, F.M.; Balogh, Z.J.; Coimbra, R.; Galante, J.M.; Hecker, A.; Weber, D.; Bauman, Z.M.; Kartiko, S.; et al. Surgical stabilization of rib fractures (SSRF): The WSES and CWIS position paper. World J. Emerg. Surg. 2024, 19, 33. [Google Scholar] [CrossRef]
- Dilday, J.; Chien, C.Y.; Lewis, M.; Emigh, B.; Benjamin, E.R.; Demetriades, D. Surgical Rib Fixation in Obese Patients with Isolated Flail Chest Improves Outcomes: A Matched Cohort Study. World J. Surg. 2022, 46, 2890–2899. [Google Scholar] [CrossRef]
- Battle, C.E.; Hutchings, H.; Evans, P.A. Risk factors that predict mortality in patients with blunt chest wall trauma: A systematic review and meta-analysis. Injury 2012, 43, 8–17. [Google Scholar] [CrossRef]
- Divisi, D.; Di Leonardo, G.; Crisci, R. Surgical management of traumatic isolated sternal fracture and manubriosternal dislocation. J. Trauma Acute Care Surg. 2013, 75, 824–829. [Google Scholar] [CrossRef]
- Divisi, D.; Crisci, R. Use of demineralized bone matrix and plate for sternal stabilization after traumatic dislocation. Gen. Thorac. Cardiovasc. Surg. 2011, 59, 52–56. [Google Scholar] [CrossRef]
- Peek, J.; Ochen, Y.; Saillant, N.; Groenwold, R.H.H.; Leenen, L.P.H.; Uribe-Leitz, T.; Houwert, R.M.; Heng, M. Traumatic rib fractures: A marker of severe injury. A nationwide study using the National Trauma Data Bank. Trauma Surg. Acute Care Open. 2020, 5, e000441. [Google Scholar] [CrossRef]
- Kane, E.D.; Jeremitsky, E.; Pieracci, F.M.; Majercik, S.; Doben, A.R. Quantifying and exploring the recent national increase in surgical stabilization of rib fractures. J. Trauma. Acute Care Surg. 2017, 83, 1047–1052. [Google Scholar] [CrossRef]
- Gamberini, L.; Moro, F.; Dallari, C.; Tartaglione, M.; Mazzoli, C.A.; Allegri, D.; Scquizzato, T.; Chiarini, V.; Coniglio, C.; Brogi, E. Regional anesthesia modalities in blunt thoracic trauma: A systematic review and Bayesian network meta-analysis. Am. J. Emerg. Med. 2025, 89, 199–208. [Google Scholar] [CrossRef]
- Hargrave, J.; Grant, M.C.; Kolarczyk, L.; Kelava, M.; Williams, T.; Brodt, J.; Neelankavil, J.P. An Expert Review of Chest Wall Fascial Plane Blocks for Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2023, 37, 279–290. [Google Scholar] [CrossRef]
- Lodhia, J.V.; Eyre, L.; Smith, M.; Toth, L.; Troxler, M.; Milton, R.S. Management of thoracic trauma. Anaesthesia 2023, 78, 225–235. [Google Scholar] [CrossRef]
- Bethlahmy, J.M.; Hanst, B.A.; Giafaglione, S.M.; Elia, J.M. Perioperative considerations for patients undergoing surgical stabilization of rib fractures: A narrative review. J. Clin. Anesth. 2023, 91, 111275. [Google Scholar] [CrossRef]
- Crisci, R.; Divisi, D. Chest wall surgical stabilization after thoracic trauma: Indications and techniques. Shanghai Chest 2017, 1, 5. [Google Scholar] [CrossRef]
- Cipulli, F.; Miori, S.; Balzani, E.; Zanella, R.; Zanon, F.; Bellani, G. Noninvasive ventilation failure in thoracic trauma: A retrospective study on predictive scores, ventilatory strategies and pain management’. J. Crit. Care 2025, 89, 155137. [Google Scholar] [CrossRef]
- Zhao, P.; Ge, Q.; Zheng, H.; Luo, J.; Song, X.; Hu, L. Clinical outcome analysis for surgical fixation versus conservative treatment on rib fractures: A systematic evaluation and meta-analysis. World J. Emerg. Surg. 2025, 20, 10. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.E.; Harvin, J.A.; Vincent, L.; Motley, K.; Wandling, M.W.; Puzio, T.J.; Moore, L.J.; Cotton, B.A.; Wade, C.E.; Kao, L.S. Randomized Controlled Trial of Surgical Rib Fixation to Nonoperative Management in Severe Chest Wall Injury. Ann. Surg. 2023, 278, 357–365. [Google Scholar] [CrossRef]
- de Moya, M.; Brasel, K.J.; Brown, C.V.; Hartwell, J.L.; Inaba, K.; Ley, E.J.; Moore, E.E.; Peck, K.A.; Rizzo, A.G.; Rosen, N.G.; et al. Evaluation and management of traumatic pneumothorax: A Western Trauma Association critical decisions algorithm. J. Trauma Acute Care Surg. 2022, 92, 103–107. [Google Scholar] [CrossRef]
- Divisi, D.; Mucilli, F.; Di Leonardo, G.; Zaccagna, G.; De Vico, A.; Camplese, P.; Angeletti, C.; Crisci, R. Plates versus struts versus an extracortical rib fixation in flail chest patients: Two-center experience. Injury 2021, 52, 235–242. [Google Scholar] [CrossRef]
- Divisi, D.; De Vico, A.; Zaccagna, G.; Marella, A.; De Sanctis, S.; Crisci, R. Reconstructive options of the chest wall after trauma: A narrative review. AME Surg. J. 2023, 3, 17. [Google Scholar] [CrossRef]
- Divisi, D.; Barone, M.; Crisci, R. Surgical Management of Flail Chest: State of Art and Future Perspectives. Curr. Surg. Rep. 2017, 5, 21. [Google Scholar] [CrossRef]
- Hu, M.; Sun, M.; Bao, C.; Luo, J.; Zhuo, L.; Guo, M. 3D-printed external fixation guide combined with video-assisted thoracoscopic surgery for the treatment of flail chest: A technical report and case series. Front. Surg. 2023, 10, 1272628. [Google Scholar] [CrossRef]
- Wong, Y.C.; Wang, L.J.; Kaewlai, R.; Wu, C.H. Watch Out for the Early Killers: Imaging Diagnosis of Thoracic Trauma. Korean J. Radiol. 2023, 24, 752–760. [Google Scholar] [CrossRef]
- Lin, Y.-Y.; Chen, Y.-J.; Hsu, C.-P.; Huang, J.-F.; Lin, Y.-C.; Kuo, L.-W.; Cheng, C.-T.; Liao, C.-H. Surgical stabilization of rib fractures improves survival in functionally dependent trauma patients. World J. Emerg. Surg. 2025, 20, 61. [Google Scholar] [CrossRef]
- Gordy, S.; Fabricant, L.; Ham, B.; Mullins, R.; Mayberry, J. The contribution of rib fractures to chronic pain and disability. Am. J. Surg. 2014, 207, 659–662. [Google Scholar] [CrossRef]
- Boyette-Davis, J.A.; Dougherty, P.M. Mechanisms of Pain in Thoracic Surgery. In Thoracic Anesthesia; McGraw-Hill Companies Inc.: Columbus, OH, USA, 2012; Chapter 6. [Google Scholar]
- Kim, M.; Moore, J.E. Chest Trauma: Current Recommendations for Rib Fractures, Pneumothorax, and Other Injuries. Curr. Anesthesiol. Rep. 2020, 10, 61–68. [Google Scholar] [CrossRef]
- Eaves, G.K.; Ware, E.E.; Touchet, D.R.; Hamilton, W.K.; Netterville, S.S.; Stevens, J.R.; Ahmadzadeh, S.; Shekoohi, S.; Kaye, A.D. Efficacy and Safety of Thoracic Epidural vs. Paravertebral Block for Analgesia in Thoracotomy: A Systematic Review of Randomized Controlled Trials. Curr. Pain Headache Rep. 2025, 29, 69. [Google Scholar] [CrossRef]
- Tanimoto, S.; Shakuo, T.; Dosei, T.; Sakamoto, A.; Shida, K. Bilateral Continuous Thoracic Paravertebral Block for the Pain Management of Multiple Rib Fractures with Flail Chest: A Case Report. Cureus 2024, 16, e75406. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, R.B.; Kumar, A.; Acharya, A. A Comparative Study of Erector Spinae Plane Block and Thoracic Epidural Block on Respiratory, Analgesic, and Hemodynamic Outcomes in Patients with Traumatic Rib Fractures. Cureus 2025, 17, e84309. [Google Scholar] [CrossRef]
- Pangthipampai, P.; Siriwanarangsun, P.; Pakpirom, J.; Sivakumar, R.K.; Karmakar, M.K. Intertransverse process block (ITPB) at the retro-superior costotransverse ligament (retro-SCTL) space: Evaluation of local anesthetic spread using MRI and sensory blockade in healthy volunteers. J. Clin. Anesth. 2025, 101, 111718. [Google Scholar] [CrossRef]
- Desire, S.M.; Hayward, G. Transversus Thoracic Muscle Plane Block (TTMPB). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK587362/ (accessed on 1 October 2025).
- Serra, S.; Santonastaso, D.P.; Romano, G.; Riccardi, A.; Nigra, S.G.; Russo, E.; Angelini, M.; Agnoletti, V.; Guarino, M.; Cimmino, C.S.; et al. Efficacy and safety of the serratus anterior plane block (SAP block) for pain management in patients with multiple rib fractures in the emergency department: A retrospective study. Eur. J. Trauma Emerg. Surg. 2024, 50, 3177–3188. [Google Scholar] [CrossRef]
- Partyka, C.; Asha, S.; Berry, M.; Ferguson, I.; Burns, B.; Tsacalos, K.; Gaetani, D.; Oliver, M.; Luscombe, G.; Delaney, A.; et al. Serratus Anterior Plane Blocks for Early Rib Fracture Pain Management: The SABRE Randomized Clinical Trial. JAMA Surg. 2024, 159, 810–817. [Google Scholar] [CrossRef]
- Pais, J.V.; Barros, M.S.; Cavalete, S.M.; Cardoso, H.P. Continuous Bilateral Serratus Anterior Plane Block: An Effective Analgesic Strategy for Managing Extensive Bilateral Rib Fractures. Cureus 2025, 17, e77332. [Google Scholar] [CrossRef]
- Jiang, M.; Peri, V.; Ou Yang, B.; Chang, J.; Hacking, D. Erector Spinae Plane Block as an Analgesic Intervention in Acute Rib Fractures: A Scoping Review. Local Reg. Anesth. 2023, 16, 81–90. [Google Scholar] [CrossRef]
- Adhikary, S.D.; Liu, W.M.; Fuller, E.; Cruz-Eng, H.; Chin, K.J. The effect of erector spinae plane block on respiratory and analgesic outcomes in multiple rib fractures: A retrospective cohort study. Anaesthesia 2019, 74, 585–593. [Google Scholar] [CrossRef]
- Liao, C.-A.; Chen, Y.-J.; Shen, S.-J.; Wang, Q.-A.; Chen, S.-A.; Liao, C.-H.; Lin, J.-R.; Lee, C.-W.; Tsai, H.-I. Erector spinae plane block (ESPB) enhances hemodynamic stability decreasing analgesic requirements in surgical stabilization of rib fractures (SSRFs). World J. Emerg. Surg. 2024, 19, 36. [Google Scholar] [CrossRef] [PubMed]
- O’connell, K.M.; Patel, K.V.; Powelson, E.M.; Robinson, B.R.M.; Boyle, K.; Peschman, J.; Blocher-Smith, E.C.D.; Jacobson, L.; Leavitt, J.; McCrum, M.L.; et al. Use of regional analgesia and risk of delirium in older adults with multiple rib fractures: An Eastern Association for the Surgery of Trauma multicenter study. J. Trauma. Acute Care Surg. 2021, 91, 265–271. [Google Scholar] [CrossRef]
- Ju, Q.; Li, P.; Zhang, L.; Zhang, L.; Liu, G.; Yuan, L.; Zhu, M. Parascapular sub-iliocostalis plane block for postoperative analgesia in patients with multiple rib fractures: A randomized controlled trial. BMC Anesthesiol. 2025, 25, 301. [Google Scholar] [CrossRef]
- Tan, H.; Xu, H.; Yu, N.; Yu, Y.; Duan, H.; Fan, Q.; Zhanyu, T. The value of deep learning-based computer aided diagnostic system in improving diagnostic performance of rib fractures in acute blunt trauma. BMC Med. Imaging. 2023, 23, 55. [Google Scholar] [CrossRef]
- Sun, L.; Fan, Y.; Shi, S.; Sun, M.; Ma, Y.; Zhang, K.; Zhang, F.; Liu, H.; Yu, T.; Tong, H.; et al. AI-assisted radiologists vs standard double reading for rib fracture detection on CT images: A real-world clinical study. PLoS ONE 2025, 20, e0316732. [Google Scholar] [CrossRef]
- Vazirizadeh-Mahabadi, M.; Ghaffari Jolfayi, A.; Hosseini, M.; Yarahmadi, M.; Zarei, H.; Masoodi, M.; Sarveazad, A.; Yousefifard, M. Predicting the Presence of Traumatic Chest Injuries Using Machine Learning Algorithm. Arch Acad Emerg Med. 2025, 13, e41. [Google Scholar] [CrossRef]
- Briody, H.; Hanneman, K.; Patlas, M. Applications of artificial intelligence in acute thoracic imaging. Can. Assoc. Radiol. J. 2025, 76, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Kahloul, M.; Kacem, I.; Sboui, M.M.; El Maalel, O.; Daami, H.; Hafsia, M.; Limam, M.; Aissa, S.; Ben Kbaier, I.; Mrizak, N. Chronic Pain following Chest Trauma: Prevalence, Associated Factors, and Psychosocial Impact. Pain Res. Manag. 2020, 2020, 1030463. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angeletti, C.; Zaccagna, G.; Vaccarili, M.; Salve, G.; De Vico, A.; Ciccozzi, A.; Divisi, D. Contemporary and Future Perspectives on Thoracic Trauma Care: Surgical Stabilization, Multidisciplinary Approaches, and the Role of Artificial Intelligence. J. Clin. Med. 2025, 14, 8041. https://doi.org/10.3390/jcm14228041
Angeletti C, Zaccagna G, Vaccarili M, Salve G, De Vico A, Ciccozzi A, Divisi D. Contemporary and Future Perspectives on Thoracic Trauma Care: Surgical Stabilization, Multidisciplinary Approaches, and the Role of Artificial Intelligence. Journal of Clinical Medicine. 2025; 14(22):8041. https://doi.org/10.3390/jcm14228041
Chicago/Turabian StyleAngeletti, Chiara, Gino Zaccagna, Maurizio Vaccarili, Giulia Salve, Andrea De Vico, Alessandra Ciccozzi, and Duilio Divisi. 2025. "Contemporary and Future Perspectives on Thoracic Trauma Care: Surgical Stabilization, Multidisciplinary Approaches, and the Role of Artificial Intelligence" Journal of Clinical Medicine 14, no. 22: 8041. https://doi.org/10.3390/jcm14228041
APA StyleAngeletti, C., Zaccagna, G., Vaccarili, M., Salve, G., De Vico, A., Ciccozzi, A., & Divisi, D. (2025). Contemporary and Future Perspectives on Thoracic Trauma Care: Surgical Stabilization, Multidisciplinary Approaches, and the Role of Artificial Intelligence. Journal of Clinical Medicine, 14(22), 8041. https://doi.org/10.3390/jcm14228041





