Association of FLAIR Positivity and Worse Outcomes After Intravenous Thrombolysis in Known-Onset Strokes: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Information Sources and Search Strategy
2.3. Eligibility Criteria
2.4. Search Strategy
2.5. Selection Process
2.6. Data Collection Form
2.7. Study Risk of Bias Assessment
2.8. Certainty of Evidence
2.9. Statistical Synthesis
2.10. Reporting Bias Assessment
3. Results
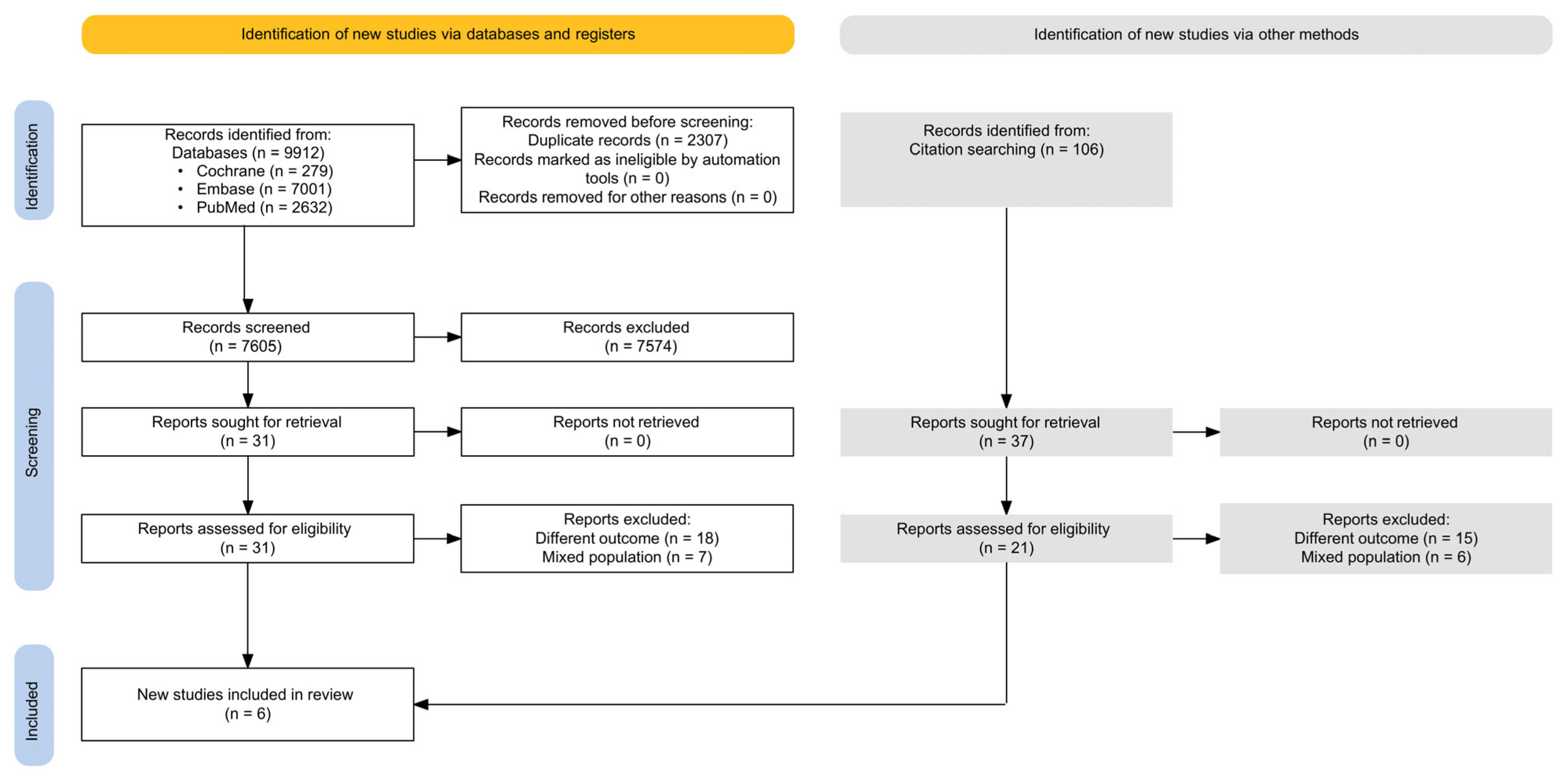
3.1. Literature Search Results
3.2. Basic Characteristics of Included Studies
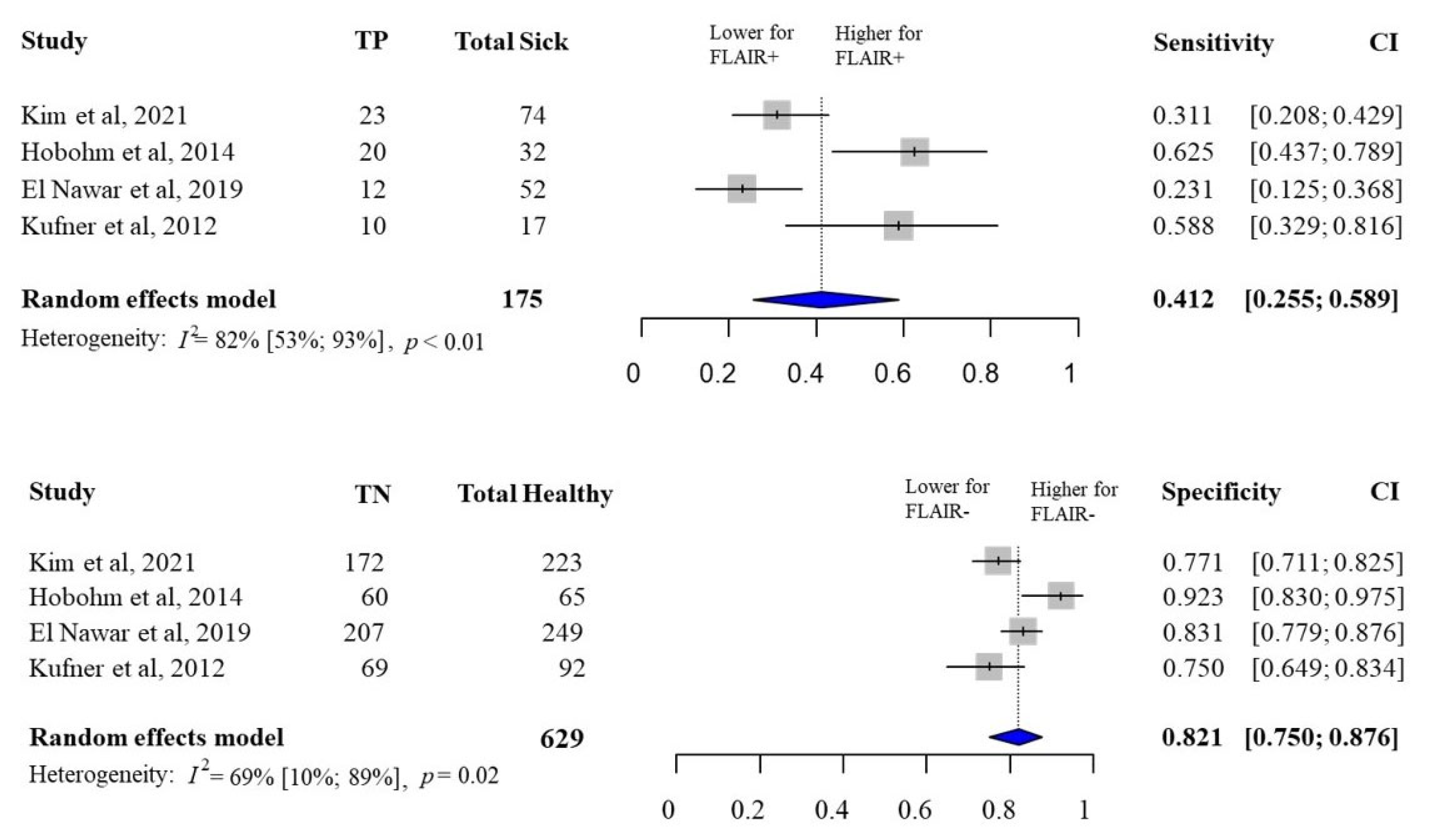
3.3. FLAIR Status and Hemorrhagic Transformation
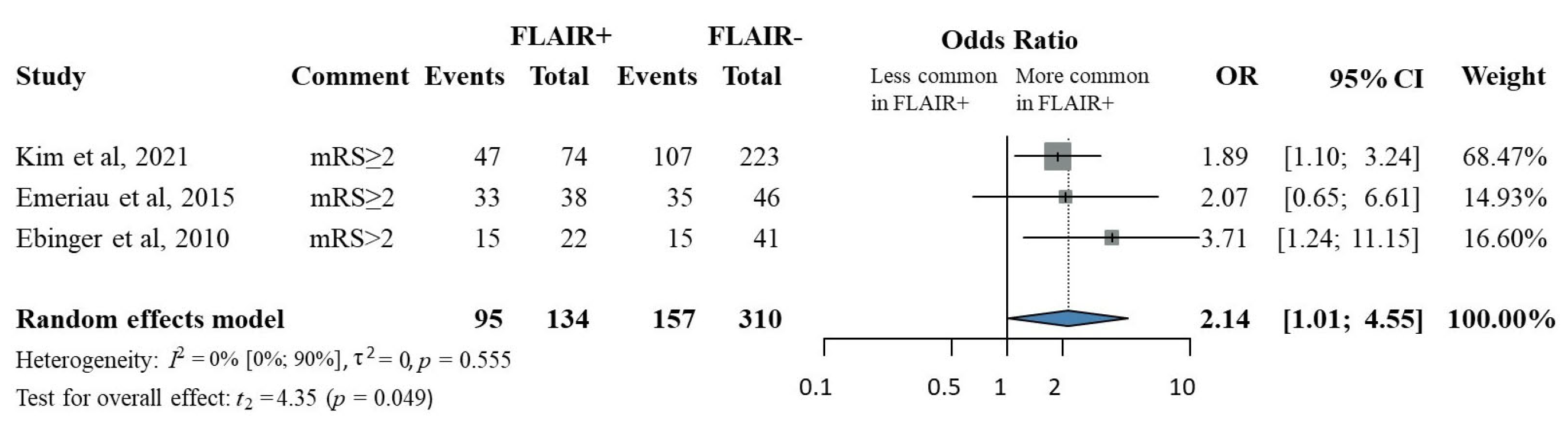
3.4. FLAIR Status and Less Favorable 90-Day Functional Outcome
3.5. Risk of Bias Assessment
3.6. Publication Bias
3.7. Quality of Evidence
4. Discussion
4.1. Strengths and Limitations
4.2. Implications for Clinical Practice and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prendes, C.F.; Rantner, B.; Hamwi, T.; Stana, J.; Feigin, V.L.; Stavroulakis, K.; Tsilimparis, N. Burden of Stroke in Europe: An Analysis of the Global Burden of Disease Study Findings From 2010 to 2019. Stroke 2024, 55, 432–442. [Google Scholar] [CrossRef]
- Berge, E.; Whiteley, W.; Audebert, H.; De Marchis, G.M.; Fonseca, A.C.; Padiglioni, C.; de la Ossa, N.P.; Strbian, D.; Tsivgoulis, G.; Turc, G. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur. Stroke J. 2021, 6, I–LXII. [Google Scholar] [CrossRef] [PubMed]
- van der Merwe, J.; Tsakpounidou, K.; Baskini, M.; Webb, C.; Keramydas, C.; Martins, S.C.O.; Klinke, M.E.; Proios, H. Continuity and Change in Baseline Stroke Knowledge across the world: Second Wave of FAST Heroes campaign implementation. J. Stroke Cerebrovasc. Dis. 2023, 32, 107426. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, G.S. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef]
- Emberson, J.; Lees, K.R.; Lyden, P.; Blackwell, L.; Albers, G.; Bluhmki, E.; Brott, T.; Cohen, G.; Davis, S.; Donnan, G.; et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: A meta-analysis of individual patient data from randomised trials. Lancet 2014, 384, 1929–1935. [Google Scholar] [CrossRef]
- Spronk, E.; Sykes, G.; Falcione, S.; Munsterman, D.; Joy, T.; Kamtchum-Tatuene, J.; Jickling, G.C. Hemorrhagic Transformation in Ischemic Stroke and the Role of Inflammation. Front. Neurol. 2021, 12, 661955. [Google Scholar] [CrossRef]
- Lees, K.R.; Bluhmki, E.; von Kummer, R.; Brott, T.G.; Toni, D.; Grotta, J.C.; Albers, G.W.; Kaste, M.; Marler, J.R.; Hamilton, S.A.; et al. Time to treatment with intravenous alteplase and outcome in stroke: An updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 2010, 375, 1695–1703. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, Y.; Zhou, M.; He, L. Risk factors of haemorrhagic transformation for acute ischaemic stroke in Chinese patients receiving intravenous recombinant tissue plasminogen activator: A systematic review and meta-analysis. Stroke Vasc. Neurol. 2018, 3, 203–208. [Google Scholar] [CrossRef]
- Sun, J.; Lam, C.; Christie, L.; Blair, C.; Li, X.; Werdiger, F.; Yang, Q.; Bivard, A.; Lin, L.; Parsons, M. Risk factors of hemorrhagic transformation in acute ischaemic stroke: A systematic review and meta-analysis. Front. Neurol. 2023, 14, 1079205. [Google Scholar] [CrossRef]
- Howard, G.; Banach, M.; Kissela, B.; Cushman, M.; Muntner, P.; Judd, S.E.; Howard, V.J. Age-Related Differences in the Role of Risk Factors for Ischemic Stroke. Neurology 2023, 100, e1444–e1453. [Google Scholar] [CrossRef]
- Thomalla, G.; Cheng, B.; Ebinger, M.; Hao, Q.; Tourdias, T.; Wu, O.; Kim, J.S.; Breuer, L.; Singer, O.C.; Warach, S.; et al. DWI-FLAIR mismatch for the identification of patients with acute ischaemic stroke within 4·5 h of symptom onset (PRE-FLAIR): A multicentre observational study. Lancet Neurol. 2011, 10, 978–986. [Google Scholar] [CrossRef]
- Thomalla, G.; Simonsen, C.Z.; Boutitie, F.; Andersen, G.; Berthezene, Y.; Cheng, B.; Cheripelli, B.; Cho, T.H.; Fazekas, F.; Fiehler, J.; et al. MRI-Guided Thrombolysis for Stroke with Unknown Time of Onset. N. Engl. J. Med. 2018, 379, 611–622. [Google Scholar] [CrossRef]
- Wouters, A.; Scheldeman, L.; Dupont, P.; Cheng, B.; Ebinger, M.; Jensen, M.; Endres, M.; Gerloff, C.; Muir, K.W.; Nighoghossian, N.; et al. Hyperintense acute reperfusion marker associated with hemorrhagic transformation in the WAKE-UP trial. Eur. Stroke J. 2021, 6, 128–133. [Google Scholar] [CrossRef]
- Kim, Y.; Luby, M.; Burkett, N.S.; Norato, G.; Leigh, R.; Wright, C.B.; Kern, K.C.; Hsia, A.W.; Lynch, J.K.; Adil, M.M.; et al. Fluid-Attenuated Inversion Recovery Hyperintense Ischemic Stroke Predicts Less Favorable 90-Day Outcome after Intravenous Thrombolysis. Cerebrovasc. Dis. 2021, 50, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.; Costello, C.; Christensen, S.; Ebinger, M.; Parsons, M.W.; Desmond, P.M.; Barber, P.A.; Butcher, K.S.; Levi, C.R.; De Silva, D.A.; et al. Fluid-attenuated inversion recovery hyperintensity in acute ischemic stroke may not predict hemorrhagic transformation. Cerebrovasc. Dis. 2011, 32, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.H.; Kim, J.S.; Kim, S.J.; Yun, S.C.; Choi, C.G.; Kim, H.R.; Kwon, S.U.; Lee, D.H.; Kim, E.K.; Suh, D.C.; et al. Focal fluid-attenuated inversion recovery hyperintensity within acute diffusion-weighted imaging lesions is associated with symptomatic intracerebral hemorrhage after thrombolysis. Stroke 2008, 39, 3424–3426. [Google Scholar] [CrossRef] [PubMed]
- Meisterernst, J.; Klinger-Gratz, P.P.; Leidolt, L.; Lang, M.F.; Schroth, G.; Mordasini, P.; Heldner, M.R.; Mono, M.L.; Kurmann, R.; Buehlmann, M.; et al. Focal T2 and FLAIR hyperintensities within the infarcted area: A suitable marker for patient selection for treatment? PLoS ONE 2017, 12, e0185158. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.T.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 2019, ED000142. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Grainger, M.J.; Gray, C.T. Citationchaser: A tool for transparent and efficient forward and backward citation chasing in systematic searching. Res. Synth. Methods 2022, 13, 533–545. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Neyeloff, J.L.; Fuchs, S.C.; Moreira, L.B. Meta-analyses and Forest plots using a microsoft excel spreadsheet: Step-by-step guide focusing on descriptive data analysis. BMC Res. Notes 2012, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Hayden, J.A.; van der Windt, D.A.; Cartwright, J.L.; Côté, P.; Bombardier, C. Assessing bias in studies of prognostic factors. Ann. Intern. Med. 2013, 158, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Phi, L.; Ajaj, R.; Ramchandani, M.H.; Brant, X.M.; Oluwadara, O.; Polinovsky, O.; Moradi, D.; Barkhordarian, A.; Sriphanlop, P.; Ong, M.; et al. Expanding the Grading of Recommendations Assessment, Development, and Evaluation (Ex-GRADE) for Evidence-Based Clinical Recommendations: Validation Study. Open Dent. J. 2012, 6, 31–40. [Google Scholar] [CrossRef]
- Mantel, N.; Haenszel, W. Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl. Cancer Inst. 1959, 22, 719–748. [Google Scholar]
- Robins, J.; Greenland, S.; Breslow, N.E. A general estimator for the variance of the Mantel-Haenszel odds ratio. Am. J. Epidemiol. 1986, 124, 719–723. [Google Scholar] [CrossRef]
- Paule, R.C.; Mandel, J. Consensus Values and Weighting Factors. J. Res. Natl. Bur. Stand. 1982, 87, 377–385. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 28 April 2024).
- Schwarzer, G. Meta-Analysis in R. In Systematic Reviews in Health Research: Meta-Analysis in Context, 3rd ed.; Borenstein, M., Egger, M., Higgins, J.P.T., Smith, G.D., Eds.; John Wiley & Sons: New York, NY, USA, 2022. [Google Scholar]
- Cuijpers, P.F.T.; Ebert, D.D. Dmetar: Companion R Package for the Guide Doing Meta-Analysis in R. 2022. Available online: https://dmetar.protectlab.org (accessed on 10 April 2024).
- Ebinger, M.; Kufner, A.; Galinovic, I.; Brunecker, P.; Malzahn, U.; Nolte, C.H.; Endres, M.; Fiebach, J.B. Fluid-attenuated inversion recovery images and stroke outcome after thrombolysis. Stroke 2012, 43, 539–542. [Google Scholar] [CrossRef] [PubMed]
- El Nawar, R.; Yeung, J.; Labreuche, J.; Chadenat, M.L.; Duong, D.L.; De Malherbe, M.; Cordoliani, Y.S.; Lapergue, B.; Pico, F. MRI-Based Predictors of Hemorrhagic Transformation in Patients With Stroke Treated by Intravenous Thrombolysis. Front. Neurol. 2019, 10, 897. [Google Scholar] [CrossRef] [PubMed]
- Emeriau, S.; Soize, S.; Riffaud, L.; Toubas, O.; Pombourcq, F.; Pierot, L. Parenchymal FLAIR hyperintensity before thrombolysis is a prognostic factor of ischemic stroke outcome at 3 Tesla. J. Neuroradiol. 2015, 42, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Hobohm, C.; Fritzsch, D.; Budig, S.; Classen, J.; Hoffmann, K.T.; Michalski, D. Predicting intracerebral hemorrhage by baseline magnetic resonance imaging in stroke patients undergoing systemic thrombolysis. Acta Neurol. Scand. 2014, 130, 338–345. [Google Scholar] [CrossRef]
- Kufner, A.; Galinovic, I.; Brunecker, P.; Cheng, B.; Thomalla, G.; Gerloff, C.; Campbell, B.C.; Nolte, C.H.; Endres, M.; Fiebach, J.B.; et al. Early infarct FLAIR hyperintensity is associated with increased hemorrhagic transformation after thrombolysis. Eur. J. Neurol. 2012, 20, 281–285. [Google Scholar] [CrossRef]
- Thomalla, G.; Boutitie, F.; Fiebach, J.B.; Simonsen, C.Z.; Nighoghossian, N.; Pedraza, S.; Lemmens, R.; Roy, P.; Muir, K.W.; Ebinger, M.; et al. Stroke With Unknown Time of Symptom Onset: Baseline Clinical and Magnetic Resonance Imaging Data of the First Thousand Patients in WAKE-UP (Efficacy and Safety of MRI-Based Thrombolysis in Wake-Up Stroke: A Randomized, Doubleblind, Placebo-Controlled Trial). Stroke 2017, 48, 770–773. [Google Scholar] [CrossRef]
- Campbell, B.C.V.; Ma, H.; Parsons, M.W.; Churilov, L.; Yassi, N.; Kleinig, T.J.; Hsu, C.Y.; Dewey, H.M.; Butcher, K.S.; Yan, B.; et al. Association of Reperfusion After Thrombolysis With Clinical Outcome Across the 4.5- to 9-Hours and Wake-up Stroke Time Window: A Meta-Analysis of the EXTEND and EPITHET Randomized Clinical Trials. JAMA Neurol. 2021, 78, 236–240. [Google Scholar] [CrossRef]
- Thomalla, G.; Fiebach, J.B.; Østergaard, L.; Pedraza, S.; Thijs, V.; Nighoghossian, N.; Roy, P.; Muir, K.W.; Ebinger, M.; Cheng, B.; et al. A multicenter, randomized, double-blind, placebo-controlled trial to test efficacy and safety of magnetic resonance imaging-based thrombolysis in wake-up stroke (WAKE-UP). Int. J. Stroke 2014, 9, 829–836. [Google Scholar] [CrossRef]
- Jha, R.; Battey, T.W.; Pham, L.; Lorenzano, S.; Furie, K.L.; Sheth, K.N.; Kimberly, W.T. Fluid-attenuated inversion recovery hyperintensity correlates with matrix metalloproteinase-9 level and hemorrhagic transformation in acute ischemic stroke. Stroke 2014, 45, 1040–1045. [Google Scholar] [CrossRef]
- Suh, C.H.; Jung, S.C.; Cho, S.J.; Woo, D.C.; Oh, W.Y.; Lee, J.G.; Kim, K.W. MRI for prediction of hemorrhagic transformation in acute ischemic stroke: A systematic review and meta-analysis. Acta Radiol. 2020, 61, 964–972. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Han, S.K.; Shin, D.H.; Na, J.U.; Lee, H.J.; Choi, P.C.; Lee, J.H. Diffusion-weighted imaging-fluid-attenuated inversion recovery mismatch is associated with better neurologic response to intravenous thrombolytic therapy in acute ischemic stroke patients. Clin. Exp. Emerg. Med. 2015, 2, 31–37. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Quan, G.; Ban, R.; Ren, J.L.; Liu, Y.; Wang, W.; Dai, S.; Yuan, T. FLAIR and ADC Image-Based Radiomics Features as Predictive Biomarkers of Unfavorable Outcome in Patients With Acute Ischemic Stroke. Front. Neurosci. 2021, 15, 730879. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhu, Y.; Zhang, X.; Kong, D.; Duan, S.; Guo, L.; Yin, X.; Jiang, L.; Liu, Z.; Yang, W. Clinical features and FLAIR radiomics nomogram for predicting functional outcomes after thrombolysis in ischaemic stroke. Front. Neurosci. 2023, 17, 1063391. [Google Scholar] [CrossRef] [PubMed]
- Benzakoun, J.; Deslys, M.A.; Legrand, L.; Hmeydia, G.; Turc, G.; Hassen, W.B.; Charron, S.; Debacker, C.; Naggara, O.; Baron, J.C.; et al. Synthetic FLAIR as a Substitute for FLAIR Sequence in Acute Ischemic Stroke. Radiology 2022, 303, 153–159. [Google Scholar] [CrossRef]
- Hamon, G.; Legrand, L.; Hmeydia, G.; Turc, G.; Hassen, W.B.; Charron, S.; Debacker, C.; Naggara, O.; Thirion, B.; Chen, B.; et al. Multicenter validation of synthetic FLAIR as a substitute for FLAIR sequence in acute ischemic stroke. Eur. Stroke J. 2024, 10, 161–171. [Google Scholar] [CrossRef]
- Asuzu, D.; Nystrom, K.; Amin, H.; Schindler, J.; Wira, C.; Greer, D.; Chi, N.F.; Halliday, J.; Sheth, K.N. Comparison of 8 scores for predicting symptomatic intracerebral hemorrhage after IV thrombolysis. Neurocrit. Care 2015, 22, 229–233. [Google Scholar] [CrossRef]
- Adebayo, O.D.; Culpan, G. Diagnostic accuracy of computed tomography perfusion in the prediction of haemorrhagic transformation and patient outcome in acute ischaemic stroke: A systematic review and meta-analysis. Eur. Stroke J. 2020, 5, 4–16. [Google Scholar] [CrossRef]
- Pensato, U.; Rex, N.; Kashani, N.; Yu, A.Y.; Jadhav, A.P.; Rha, J.H.; Puri, A.S.; Burns, P.; Demchuk, A.M.; Hill, M.D.; et al. Association between time and severe hypoperfusion with risk of hemorrhagic transformation in stroke patients. Int. J. Stroke 2025, 17474930251360519. [Google Scholar] [CrossRef]
- Hegyi, P.; Erőss, B.; Izbéki, F.; Párniczky, A.; Szentesi, A. Accelerating the translational medicine cycle: The Academia Europaea pilot. Nat. Med. 2021, 27, 1317–1319. [Google Scholar] [CrossRef]
- Hegyi, P.; Petersen, O.H.; Holgate, S.; Erőss, B.; Garami, A.; Szakács, Z.; Dobszai, D.; Balaskó, M.; Kemény, L.; Peng, S.; et al. Academia Europaea Position Paper on Translational Medicine: The Cycle Model for Translating Scientific Results into Community Benefits. J. Clin. Med. 2020, 9, 1532. [Google Scholar] [CrossRef]



| Author (Year) | Study Site | Study Design | No. of Patients (Female) | Age (Year) ‡ | Outcome | MRI SMF | MRI Protocol | Follow-Up Imaging | NIHSS FLAIR+ ‡ | NIHSS FLAIR- ‡ | LKN-to-IVT FLAIR+ (Min) ‡ | LKN-to-IVT FLAIR− (Min) ‡ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ebinger 2012 [34] | Germany | Prospective cohort | 44 (48.88%) | 72.0 (65–81) | 90-day functional outcome | ND | DWI, FLAIR, MRA | NA | NA | 5 (3.5–8) | ND | 130 (108–154) |
| Kufner 2012 [38] | Germany | Prospective cohort | 60 (55.04%) | 71.3 (±12.5) | HT | 1.5/3T | DWI, FLAIR, T2* (PWI, MRA-optional) | MRI | 10 (6–17) | 6 (4–11) | 120 (95–165) | 124 (98–152) |
| Hobohm 2014 [37] | Germany | Retrospective cohort | 42 (43.29%) | 70.7 (±11.7) | HT | 1.5T | DWI, FLAIR, T2*, PWI, ToF MRA | CT | 14.9 (±6.9) | 12.7 (±7.1) | ND | ND |
| Emeriau 2015 [36] | France | Retrospective cohort | 36 (42.85%) | 64.0 (50–75) | 90-day functional outcome | 3T | DWI, FLAIR | NA | 14 (10–20) | 14 (9–19) | 190 (160–201) | 162 (140–190) |
| El Nawar 2019 [35] | France | Prospective cohort | 145 (48.17%) | 71.3 (±15.9) | HT | 1.5T | DWI, FLAIR, T2*, ADC, ToF MRA | CT/MRI | ND | ND | ND | ND |
| Kim 2021 [15] | USA | Prospective cohort | 143 (48.14%) | 70.0 (59–83) | HT; 90-day functional outcome | 1.5T | DWI, FLAIR, ADC, GRE, MRA (post-contrast FLAIR) | MRI | 7 (4–13) | 6 (3–15) | 169 (120–208) | 142 (107–188) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhubi, E.; Bissenov, A.; Lengyel, A.S.; Tóth, R.; Horváth, A.A.; Kéri, S.; Engh, M.A.; Hegyi, P.; Gunda, B. Association of FLAIR Positivity and Worse Outcomes After Intravenous Thrombolysis in Known-Onset Strokes: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 8031. https://doi.org/10.3390/jcm14228031
Zhubi E, Bissenov A, Lengyel AS, Tóth R, Horváth AA, Kéri S, Engh MA, Hegyi P, Gunda B. Association of FLAIR Positivity and Worse Outcomes After Intravenous Thrombolysis in Known-Onset Strokes: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(22):8031. https://doi.org/10.3390/jcm14228031
Chicago/Turabian StyleZhubi, Esra, Azamat Bissenov, Anna Sára Lengyel, Réka Tóth, András Attila Horváth, Szabolcs Kéri, Marie Anne Engh, Péter Hegyi, and Bence Gunda. 2025. "Association of FLAIR Positivity and Worse Outcomes After Intravenous Thrombolysis in Known-Onset Strokes: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 22: 8031. https://doi.org/10.3390/jcm14228031
APA StyleZhubi, E., Bissenov, A., Lengyel, A. S., Tóth, R., Horváth, A. A., Kéri, S., Engh, M. A., Hegyi, P., & Gunda, B. (2025). Association of FLAIR Positivity and Worse Outcomes After Intravenous Thrombolysis in Known-Onset Strokes: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(22), 8031. https://doi.org/10.3390/jcm14228031








