Modulating the Gut Microbiota via Rectal Ozone Insufflation in Gynecological Cancer Patients with Radiotherapy/Chemotherapy-Induced Pelvic Toxicity: A Proposed Clinical Study Protocol
Abstract
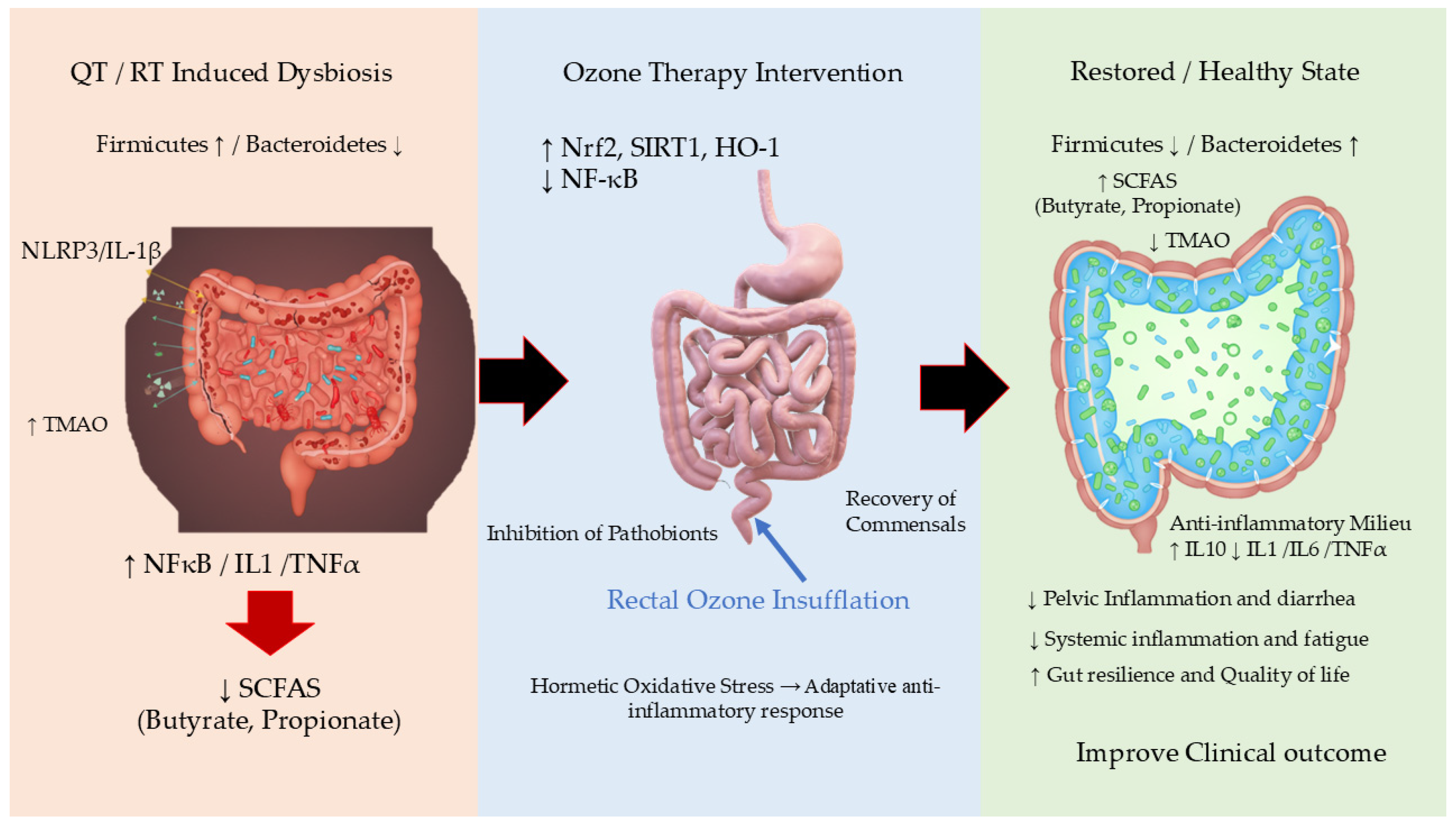
1. Introduction
2. Radiotherapy and Chemotherapy Effects on Gut Microbiota
Evidence of Dysbiosis in Patients Undergoing Pelvic RT/QT
- (a)
- Defining the Insult: How RT and QT Disrupt the Gut Ecosystem.
- –
- Direct Cytotoxicity: Both RT and QT are designed to kill rapidly dividing cells. While their target is cancer, they also attack the rapidly renewing epithelial cells of the intestinal lining [14,15]. This damage compromises the integrity of the mucosal barrier, creating an inflamed environment that is hostile to beneficial, obligate anaerobic bacteria (e.g., Faecalibacterium, Roseburia) and favorable for pathobionts (potentially pathogenic bacteria) [16].
- –
- Indirect Effects via the Host: The destruction of the intestinal epithelium leads to a cascade of events: Mucus Layer Thinning: Goblet cells are damaged, reducing mucin production. This depletes the primary habitat and food source for many commensal bacteria. Inflammation: Cell death triggers a potent inflammatory response, releasing antimicrobial peptides and reactive oxygen species that further reshape the microbial community. Immune Dysregulation: The gut microbiota is in constant cross-talk with the immune system. Therapy-induced damage to immune cells in the gut-associated lymphoid tissue (GALT) disrupts this delicate balance [15,17].
- (b)
- Evidence of Dysbiosis in Patients Undergoing Pelvic RT.
- –
- Loss of Microbial Diversity: A hallmark of a healthy gut is high species richness (alpha-diversity). Multiple studies have demonstrated a significant and rapid decrease in alpha-diversity in patients undergoing pelvic RT. This loss is considered a sign of ecosystem instability and is strongly correlated with the severity of GI symptoms, particularly diarrhea [18,19].
- –
- Shifts in Key Bacterial Taxa. Depletion of Beneficial Genera: There is a consistent and dramatic reduction in beneficial, short-chain fatty acid (SCFA)-producing bacteria. Butyrate producers like Faecalibacterium prausnitzii and Roseburia spp. are particularly vulnerable. Their loss is directly linked to mucosal barrier breakdown and inflammation [18,20].
- –
- –
- Dose–Response Relationship: The degree of dysbiosis is often correlated with the radiation dose delivered to the rectum and bowel. Higher doses are associated with more profound microbial shifts and worse clinical toxicity [20].
- –
- Persistence of Dysbiosis: Critically, these changes are not always transient. Studies following patients for months after RT have found that microbial diversity and the abundance of key beneficial species often fail to return to pre-treatment levels, suggesting long-term alteration of the gut ecosystem [18].
- (c)
- Evidence of Dysbiosis in Patients Undergoing Systemic QT.
- –
- 5-Fluorouracil (5-FU): Strongly associated with mucositis and a decrease in microbial diversity, particularly reducing SCFA producers [23].
- –
- Irinotecan: Causes severe diarrhea and is linked to a bloom of Enterobacteriaceae and a reduction in beneficial Firmicutes [24].
- –
- Cyclophosphamide: Interestingly, some chemotherapies like cyclophosphamide rely on a specific microbiota to stimulate anti-tumor immune responses. It can cause a translocation of specific Gram-positive bacteria to secondary lymphoid organs, priming pathogenic T-helper 17 (Th17) cells [25].
- –
- Reduced Diversity: Similar to RT, a drop in alpha-diversity is a common finding [23].
- –
- Impact on Treatment Outcomes: The microbiota can influence QT efficacy and metabolism. For instance, some gut bacteria can inactivate chemotherapeutic drugs, while others can convert prodrugs into their active forms [26].
- (d)
- Clinical Consequences of Therapy-Induced Dysbiosis.
- –
- RT/CT-Induced Diarrhea and Mucositis: The loss of SCFA producers and the expansion of pro-inflammatory bacteria directly contribute to these debilitating side effects [24].
- –
- Infection Risk: The loss of “colonization resistance” allows for the overgrowth of opportunistic pathogens like C. difficile [27].
- –
- Systemic Inflammation: A leaky gut and an abundance of LPS from Gram-negative bacteria can lead to bacteremia and systemic inflammation.
- –
- Microbiota as a Biomarker: Pre-treatment microbial signatures may predict a patient’s risk of developing severe toxicity [28].
- –
- Microbiome-Targeted Therapies: Interventions like probiotics, prebiotics, and fecal microbiota transplantation (FMT) are being actively investigated to prevent or reverse therapy-induced dysbiosis [28].
3. Biological Mechanisms of Ozone Therapy
4. Evidence Linking Ozone Therapy to Microbiota Modulation
4.1. Preclinical Data: Insights from Animal Studies
4.2. Emerging Clinical Observations and the Current Knowledge Gap
5. Proposed Clinical Study/Protocol
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-FU | 5-Fluorouracil |
| AOPP | Advanced Oxidation Protein Products |
| BAP | Biological Antioxidant Potential |
| CAT | Catalase |
| CRP | C-Reactive Protein |
| CTCAE | Common Terminology Criteria for Adverse Events |
| dROMs | Reactive Oxygen Metabolites |
| EQ-5D-5L™ | EuroQol-5 Dimension-5 Level questionnaire |
| EORTC QLQ-CX24 | European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Cervical Cancer Module 24 |
| EORTC QLQ-C30 | European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 |
| FRAP | Ferric Reducing Ability of Plasma |
| FMT | Fecal Microbiota Transplantation |
| GALT | Gut-Associated Lymphoid Tissue |
| GI | Gastrointestinal |
| GSH | Reduced Glutathione |
| HADS | Hospital Anxiety and Depression Scale |
| LOPs | Lipid Oxidation Products |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| IL-8/CXCL8 | Interleukin-8 / C-X-C Motif Chemokine Ligand 8 |
| NF-κB | Nuclear Factor kappa B |
| Nrf2 | Nuclear Factor Erythroid 2-Related Factor 2 |
| OT | Ozone therapy |
| QT | Chemotherapy |
| QoL | Quality of Life |
| RCT | Randomized Controlled Trial |
| ROOH | Total Hydroperoxides |
| RT | Radiotherapy |
| R/CIPT | Radiotherapy/Chemotherapy-Induced Pelvic Toxicity |
| ROS | Reactive Oxygen Species |
| SCFA | Short-Chain Fatty Acid |
| SOD | Superoxide Dismutase |
| TMA | Trimethylamine |
| TMAO | Trimethylamine N-oxide |
| TNF-α | Tumor Necrosis Factor-alpha |
| VES | Erythrocyte Sedimentation Rate |
References
- Mao, Y.; Gao, Y.; He, Y.; Wan, Z.; Li, S.; Ding, Z.; Hu, B.; Fu, L.; Luo, C.; Zhu, S.; et al. Global burden of cancer in women, 1990-2021: A systematic analysis from the GBD 2021 study. Front. Oncol. 2025, 15, 1633894. [Google Scholar] [CrossRef]
- Ruzindana, K.; Anorlu, R.I. Global disparities in gynecologic cancer outcomes: A call for action. Int. J. Gynaecol. Obstet. 2025, 171 (Suppl. S1), 210–220. [Google Scholar] [CrossRef]
- Lawrie, T.A.; Green, J.T.; Beresford, M.; Wedlake, L.; Burden, S.; Davidson, S.E.; Lal, S.; Henson, C.C.; Andreyev, H.J.N. Interventions to reduce acute and late adverse gastrointestinal effects of pelvic radiotherapy for primary pelvic cancers. Cochrane Database Syst. Rev. 2018, 1, CD012529. [Google Scholar] [CrossRef]
- Spampinato, S.; Jensen, N.B.K.; Potter, R.; Fokdal, L.U.; Chargari, C.; Lindegaard, J.C.; Schmid, M.P.; Sturdza, A.; Jurgenliemk-Schulz, I.M.; Mahantshetty, U.; et al. Severity and Persistency of Late Gastrointestinal Morbidity in Locally Advanced Cervical Cancer: Lessons Learned From EMBRACE-I and Implications for the Future. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.R.; Aggarwal, P.; Costa, R.G.F.; Cole, A.M.; Trinchieri, G. Targeting the gut microbiota for cancer therapy. Nat. Rev. Cancer 2022, 22, 703–722. [Google Scholar] [CrossRef] [PubMed]
- Blake, S.J.; Wolf, Y.; Boursi, B.; Lynn, D.J. Role of the microbiota in response to and recovery from cancer therapy. Nat. Rev. Immunol. 2024, 24, 308–325. [Google Scholar] [CrossRef] [PubMed]
- El Alam, M.B.; Sims, T.T.; Kouzy, R.; Biegert, G.W.G.; Jaoude, J.; Karpinets, T.V.; Yoshida-Court, K.; Wu, X.; Delgado-Medrano, A.Y.; Mezzari, M.P.; et al. A prospective study of the adaptive changes in the gut microbiome during standard-of-care chemoradiotherapy for gynecologic cancers. PLoS ONE 2021, 16, e0247905. [Google Scholar] [CrossRef]
- Bai, J.; Barandouzi, Z.A.; Rowcliffe, C.; Meador, R.; Tsementzi, D.; Bruner, D.W. Gut Microbiome and Its Associations With Acute and Chronic Gastrointestinal Toxicities in Cancer Patients With Pelvic Radiation Therapy: A Systematic Review. Front. Oncol. 2021, 11, 745262. [Google Scholar] [CrossRef]
- Clavo, B.; Santana-Rodríguez, N.; Llontop, P.; Gutiérrez, D.; Suárez, G.; López, L.; Rovira, G.; Martínez-Sánchez, G.; González, E.; Jorge, I.J.; et al. Ozone Therapy as Adjuvant for Cancer Treatment: Is Further Research Warranted? Evid.-Based Complement. Altern. Med. eCAM 2018, 2018, 7931849. [Google Scholar] [CrossRef]
- Viebahn-Haensler, R.; Leon Fernandez, O.S. Mitochondrial Dysfunction, Its Oxidative Stress-Induced Pathologies and Redox Bioregulation through Low-Dose Medical Ozone: A Systematic Review. Molecules 2024, 29, 2738. [Google Scholar] [CrossRef]
- Clavo, B.; Canovas-Molina, A.; Ramallo-Farina, Y.; Federico, M.; Rodriguez-Abreu, D.; Galvan, S.; Ribeiro, I.; Marques da Silva, S.C.; Navarro, M.; Gonzalez-Beltran, D.; et al. Effects of Ozone Treatment on Health-Related Quality of Life and Toxicity Induced by Radiotherapy and Chemotherapy in Symptomatic Cancer Survivors. Int. J. Environ. Res. Public. Health 2023, 20, 1479. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Ji, X.; Han, Q.; He, K.; Zhao, H.; Li, H. Ozone rectal insufflation inhibits the development of atherosclerosis by modulating gut microbiota and short-chain fatty acids in ApoE−/− mice. Front. Microbiol. 2025, 16, 1597695. [Google Scholar] [CrossRef]
- Su, Z.; Lin, J.; Zeng, X.; Li, X.; Hou, Q.; Wang, Q.; Liu, C.; Qin, J.; Li, Y.; Zhang, J.; et al. Ozone water enema activates SIRT1-Nrf2/HO-1 pathway to ameliorate gut dysbiosis in mice receiving COVID-19 patient-derived faecal microbiota. J. Med. Microbiol. 2025, 74, 002038. [Google Scholar] [CrossRef] [PubMed]
- Verginadis, I.I.; Citrin, D.E.; Ky, B.; Feigenberg, S.J.; Georgakilas, A.G.; Hill-Kayser, C.E.; Koumenis, C.; Maity, A.; Bradley, J.D.; Lin, A. Radiotherapy toxicities: Mechanisms, management, and future directions. Lancet 2025, 405, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, D.; Lennernas, H. Review on the effect of chemotherapy on the intestinal barrier: Epithelial permeability, mucus and bacterial translocation. Biomed. Pharmacother. 2023, 162, 114644. [Google Scholar] [CrossRef]
- Li, Y.; Yan, H.; Zhang, Y.; Li, Q.; Yu, L.; Li, Q.; Liu, C.; Xie, Y.; Chen, K.; Ye, F.; et al. Alterations of the Gut Microbiome Composition and Lipid Metabolic Profile in Radiation Enteritis. Front. Cell Infect. Microbiol. 2020, 10, 541178. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, M.; Zhang, S.; Huang, F.; Li, Y.; Chang, P. Radiation-induced gut microbiota dysbiosis and T(reg) cells dysfunction: Mechanistic insights and clinical implications—A review. Life Sci. 2025, 379, 123844. [Google Scholar] [CrossRef]
- Manichanh, C.; Varela, E.; Martinez, C.; Antolin, M.; Llopis, M.; Dore, J.; Giralt, J.; Guarner, F.; Malagelada, J.R. The gut microbiota predispose to the pathophysiology of acute postradiotherapy diarrhea. Am. J. Gastroenterol. 2008, 103, 1754–1761. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, X.; Zhang, G.; Ma, Y.; Zhang, Q.; Li, Z.; Ran, J.; Hou, X.; Geng, Y.; Yang, Z.; et al. The impact of pelvic radiotherapy on the gut microbiome and its role in radiation-induced diarrhoea: A systematic review. Radiat. Oncol. 2021, 16, 187. [Google Scholar] [CrossRef]
- Reis Ferreira, M.; Andreyev, H.J.N.; Mohammed, K.; Truelove, L.; Gowan, S.M.; Li, J.; Gulliford, S.L.; Marchesi, J.R.; Dearnaley, D.P. Microbiota- and Radiotherapy-Induced Gastrointestinal Side-Effects (MARS) Study: A Large Pilot Study of the Microbiome in Acute and Late-Radiation Enteropathy. Clin. Cancer Res. 2019, 25, 6487–6500. [Google Scholar] [CrossRef]
- Guo, H.; Chou, W.C.; Lai, Y.; Liang, K.; Tam, J.W.; Brickey, W.J.; Chen, L.; Montgomery, N.D.; Li, X.; Bohannon, L.M.; et al. Multi-omics analyses of radiation survivors identify radioprotective microbes and metabolites. Science 2020, 370, eaay9097. [Google Scholar] [CrossRef]
- van Lier, Y.F.; Vos, J.; Blom, B.; Hazenberg, M.D. Allogeneic hematopoietic cell transplantation, the microbiome, and graft-versus-host disease. Gut Microbes 2023, 15, 2178805. [Google Scholar] [CrossRef]
- Stringer, A.M.; Gibson, R.J.; Logan, R.M.; Bowen, J.M.; Yeoh, A.S.; Hamilton, J.; Keefe, D.M. Gastrointestinal microflora and mucins may play a critical role in the development of 5-Fluorouracil-induced gastrointestinal mucositis. Exp. Biol. Med. 2009, 234, 430–441. [Google Scholar] [CrossRef]
- Montassier, E.; Gastinne, T.; Vangay, P.; Al-Ghalith, G.A.; Bruley des Varannes, S.; Massart, S.; Moreau, P.; Potel, G.; de La Cochetiere, M.F.; Batard, E.; et al. Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment. Pharmacol. Ther. 2015, 42, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillere, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J.; et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013, 342, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Geller, L.T.; Barzily-Rokni, M.; Danino, T.; Jonas, O.H.; Shental, N.; Nejman, D.; Gavert, N.; Zwang, Y.; Cooper, Z.A.; Shee, K.; et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017, 357, 1156–1160. [Google Scholar] [CrossRef]
- Papanicolas, L.E.; Gordon, D.L.; Wesselingh, S.L.; Rogers, G.B. Improving Risk-Benefit in Faecal Transplantation through Microbiome Screening. Trends Microbiol. 2020, 28, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Ting, N.L.; Lau, H.C.; Yu, J. Cancer pharmacomicrobiomics: Targeting microbiota to optimise cancer therapy outcomes. Gut 2022, 71, 1412–1425. [Google Scholar] [CrossRef]
- Malatesta, M.; Tabaracci, G.; Pellicciari, C. Low-Dose Ozone as a Eustress Inducer: Experimental Evidence of the Molecular Mechanisms Accounting for Its Therapeutic Action. Int. J. Mol. Sci. 2024, 25, 12657. [Google Scholar] [CrossRef]
- Chirumbolo, S.; Tirelli, U.; Franzini, M.; Pandolfi, S.; Ricevuti, G.; Vaiano, F.; Valdenassi, L. Ozone in the adjunct medical treatment. The round personality of a molecule with hormetic properties. Hum. Exp. Toxicol. 2023, 42, 9603271231218926. [Google Scholar] [CrossRef]
- Galie, M.; Covi, V.; Tabaracci, G.; Malatesta, M. The Role of Nrf2 in the Antioxidant Cellular Response to Medical Ozone Exposure. Int. J. Mol. Sci. 2019, 20, 4009. [Google Scholar] [CrossRef]
- Galie, M.; Costanzo, M.; Nodari, A.; Boschi, F.; Calderan, L.; Mannucci, S.; Covi, V.; Tabaracci, G.; Malatesta, M. Mild ozonisation activates antioxidant cell response by the Keap1/Nrf2 dependent pathway. Free Radic. Biol. Med. 2018, 124, 114–121. [Google Scholar] [CrossRef]
- Tricarico, G.; Travagli, V. The Relationship between Ozone and Human Blood in the Course of a Well-Controlled, Mild, and Transitory Oxidative Eustress. Antioxidants 2021, 10, 1946. [Google Scholar] [CrossRef]
- Clavo, B.; Perez, J.L.; Lopez, L.; Suarez, G.; Lloret, M.; Rodriguez, V.; Macias, D.; Santana, M.; Morera, J.; Fiuza, D.; et al. Effect of ozone therapy on muscle oxygenation. J. Altern. Complement. Med. 2003, 9, 251–256. [Google Scholar] [CrossRef]
- Inguscio, C.R.; Cisterna, B.; Carton, F.; Barberis, E.; Manfredi, M.; Malatesta, M. Modifications of Blood Molecular Components after Treatment with Low Ozone Concentrations. Int. J. Mol. Sci. 2023, 24, 17175. [Google Scholar] [CrossRef] [PubMed]
- Cenci, A.; Macchia, I.; La Sorsa, V.; Sbarigia, C.; Di Donna, V.; Pietraforte, D. Mechanisms of Action of Ozone Therapy in Emerging Viral Diseases: Immunomodulatory Effects and Therapeutic Advantages With Reference to SARS-CoV-2. Front. Microbiol. 2022, 13, 871645. [Google Scholar] [CrossRef]
- Viebahn-Haensler, R.; Leon Fernandez, O.S. Ozone in Medicine. The Low-Dose Ozone Concept and Its Basic Biochemical Mechanisms of Action in Chronic Inflammatory Diseases. Int. J. Mol. Sci. 2021, 22, 7890. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, C.Z.; Li, B.T.; Yu, X.Q.; Zhang, J.Y.; Wang, Z.T.; Liao, L.J.; Liu, X.D. Ozone controls the metabolism of tryptophan protecting against sepsis-induced intestinal damage by activating aryl hydrocarbon receptor. World J. Gastroenterol. 2025, 31, 105411. [Google Scholar] [CrossRef] [PubMed]
- Ogut, E. Mechanisms and therapeutic potential of ozone therapy in enhancing vasculogenesis and angiogenesis through growth factor activation and oxidative signaling pathways. Discov. Med. 2025, 2, 240. [Google Scholar] [CrossRef]
- Clavo, B.; Rodriguez-Abreu, D.; Galvan-Ruiz, S.; Federico, M.; Canovas-Molina, A.; Ramallo-Farina, Y.; Antonilli, C.; Benitez, G.; Fabelo, H.; Garcia-Lourve, C.; et al. Long-Term Effects of Ozone Treatment in Patients with Persistent Numbness and Tingling Secondary to Chemotherapy-Induced Peripheral Neuropathy. A Retrospective Study. Integr. Cancer Ther. 2025, 24, 15347354241307038. [Google Scholar] [CrossRef]
- Clavo, B.; Santana-Rodriguez, N.; Llontop, P.; Gutierrez, D.; Ceballos, D.; Mendez, C.; Rovira, G.; Suarez, G.; Rey-Baltar, D.; Garcia-Cabrera, L.; et al. Ozone Therapy in the Management of Persistent Radiation-Induced Rectal Bleeding in Prostate Cancer Patients. Evid. Based Complement. Alternat Med. 2015, 2015, 480369. [Google Scholar] [CrossRef] [PubMed]
- Clavo, B.; Ceballos, D.; Gutierrez, D.; Rovira, G.; Suarez, G.; Lopez, L.; Pinar, B.; Cabezon, A.; Morales, V.; Oliva, E.; et al. Long-term control of refractory hemorrhagic radiation proctitis with ozone therapy. J. Pain. Symptom. Manag. 2013, 46, 106–112. [Google Scholar] [CrossRef]
- Hidalgo-Tallon, J.; Menendez-Cepero, S.; Vilchez, J.S.; Rodriguez-Lopez, C.M.; Calandre, E.P. Ozone therapy as add-on treatment in fibromyalgia management by rectal insufflation: An open-label pilot study. J. Altern. Complement. Med. 2013, 19, 238–242. [Google Scholar] [CrossRef]
- Clavo, B.; Navarro, M.; Federico, M.; Borrelli, E.; Jorge, I.J.; Ribeiro, I.; Rodriguez-Melcon, J.I.; Carames, M.A.; Santana-Rodriguez, N.; Rodriguez-Esparragon, F. Ozone Therapy in Refractory Pelvic Pain Syndromes Secondary to Cancer Treatment: A New Approach Warranting Exploration. J. Palliat. Med. 2021, 24, 97–102. [Google Scholar] [CrossRef]
- Haj, B.; Sukhotnik, I.; Shaoul, R.; Pollak, Y.; Coran, A.G.; Bitterman, A.; Matter, I. Effect of ozone on intestinal recovery following intestinal ischemia-reperfusion injury in a rat. Pediatr. Surg. Int. 2014, 30, 181–188. [Google Scholar] [CrossRef]
- Cheng, J.; Williams, J.P.; Zhou, L.; Wang, P.C.; Sun, L.N.; Li, R.H.; An, J.X. Ozone rectal insufflation mitigates chronic rapid eye movement sleep deprivation-induced cognitive impairment through inflammation alleviation and gut microbiota regulation in mice. Med. Gas. Res. 2024, 14, 213–224. [Google Scholar] [CrossRef]
- Saraswat, I.; Goel, A. Therapeutic Modulation of the Microbiome in Oncology: Current Trends and Future Directions. Curr. Pharm. Biotechnol. 2025, 26, 680–699. [Google Scholar] [CrossRef]
- Pathak, J.L.; Yan, Y.; Zhang, Q.; Wang, L.; Ge, L. The role of oral microbiome in respiratory health and diseases. Respir. Med. 2021, 185, 106475. [Google Scholar] [CrossRef] [PubMed]
- Viebahn-Haensler, R.; Leon Fernandez, O.S. Ozone as Redox Bioregulator in Preventive Medicine: The Molecular and Pharmacological Basis of the Low-Dose Ozone Concept-A Review. Int. J. Mol. Sci. 2023, 24, 15747. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.E.G.; Baeza-Noci, J.; Mendes Abdala, C.V.; Luvisotto, M.M.; Bertol, C.D.; Anzolin, A.P. The role of ozone treatment as integrative medicine. An evidence and gap map. Front. Public. Health 2022, 10, 1112296. [Google Scholar] [CrossRef]
- ISCO3. Madrid Declaration on Ozone Therapy, 3rd ed.; Madrid, G.S.L., Ed.; ISCO3: Madrid, Spain, 2020; p. 103. ISBN 978-84-09-19932-7. [Google Scholar]
- Chrysostomou, D.; Roberts, L.A.; Marchesi, J.R.; Kinross, J.M. Gut Microbiota Modulation of Efficacy and Toxicity of Cancer Chemotherapy and Immunotherapy. Gastroenterology 2023, 164, 198–213. [Google Scholar] [CrossRef] [PubMed]
| Stage | Key Elements | Study Groups & Sampling | Method and Endpoints |
|---|---|---|---|
| Population & Criteria | Objective: Adult women (≥18 years) with gynecological malignancy who have completed pelvic radiotherapy (± chemotherapy). | R/CIPT + OT Group (n = 19): With chronic pelvic toxicity (CTCAE ≥ Grade 2, ≥3 months duration). Control Cohort (n = 19): No pelvic toxicity, matched by age and tumor site. | Inclusion/Exclusion Criteria: Ensuring exclusion of confounding factors (e.g., active inflammatory bowel disease). |
| Intervention & Treatment | Intervention: Ozone Therapy (OT) via rectal insufflation with/without direct local application. | OT Group Only: Receives ≈ 40 sessions of OT over 4 months. | Ozone Regimen: Progressive dosing (10 µg/mL up to 30 µg/mL). |
| Sampling & Follow-Up | Design: Prospective, observational study. Blinding: Sample analysis will be blinded to group assignment. | OT Group: Stool/serum samples at Baseline (pre-ozone) and post-treatment (week 16). Control Group: A single sample taken at routine follow-up. | Confounders: Strict recording of recent use of antibiotics and probiotics. |
| Clinical & Biomarker Endpoints | Objective: Evaluate the effect of Ozone on toxicity and systemic status. | Clinical Assessments: Toxicity: CTCAE v5.0 and EORTC QLQ-CX24. Quality of Life (QoL): EORTC QLQ-C30 and EQ-5D-5L™. Psychological Status: HADS. | Biological Samples: Microbiota analysis (16S rRNA gene sequencing), Inflammatory Cytokines and Oxidative Stress Markers (serum). |
| Analysis & Conclusion | Core Hypothesis: Ozone modulates gut microbiota (weeding and seeding) → reduces inflammation → clinical improvement. | Analysis: Non-parametric tests (Wilcoxon, Mann–Whitney U) due to small sample size (n = 38). Significance: p < 0.05. | Limitation: Observational design and sample size prevent definitive causal conclusions. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clavo, B.; Córdoba-Lanús, E.; Martínez-Sánchez, G.; Federico, M.; Cánovas-Molina, Á.; Piñero, J.E.; Vargas-Prado, A.M.; Ramchandani, A.; Zajac, M.; Ribeiro, I.; et al. Modulating the Gut Microbiota via Rectal Ozone Insufflation in Gynecological Cancer Patients with Radiotherapy/Chemotherapy-Induced Pelvic Toxicity: A Proposed Clinical Study Protocol. J. Clin. Med. 2025, 14, 8015. https://doi.org/10.3390/jcm14228015
Clavo B, Córdoba-Lanús E, Martínez-Sánchez G, Federico M, Cánovas-Molina Á, Piñero JE, Vargas-Prado AM, Ramchandani A, Zajac M, Ribeiro I, et al. Modulating the Gut Microbiota via Rectal Ozone Insufflation in Gynecological Cancer Patients with Radiotherapy/Chemotherapy-Induced Pelvic Toxicity: A Proposed Clinical Study Protocol. Journal of Clinical Medicine. 2025; 14(22):8015. https://doi.org/10.3390/jcm14228015
Chicago/Turabian StyleClavo, Bernardino, Elizabeth Córdoba-Lanús, Gregorio Martínez-Sánchez, Mario Federico, Ángeles Cánovas-Molina, José E. Piñero, Ana M. Vargas-Prado, Avinash Ramchandani, Marta Zajac, Ivone Ribeiro, and et al. 2025. "Modulating the Gut Microbiota via Rectal Ozone Insufflation in Gynecological Cancer Patients with Radiotherapy/Chemotherapy-Induced Pelvic Toxicity: A Proposed Clinical Study Protocol" Journal of Clinical Medicine 14, no. 22: 8015. https://doi.org/10.3390/jcm14228015
APA StyleClavo, B., Córdoba-Lanús, E., Martínez-Sánchez, G., Federico, M., Cánovas-Molina, Á., Piñero, J. E., Vargas-Prado, A. M., Ramchandani, A., Zajac, M., Ribeiro, I., Navarro, M., Jorge, I. J., González-Martín, J. M., Martín-Alfaro, R., Fernández-Tagarro, M., Díaz-Garrido, J. A., Lorenzo-Morales, J., & Rodríguez-Esparragón, F. (2025). Modulating the Gut Microbiota via Rectal Ozone Insufflation in Gynecological Cancer Patients with Radiotherapy/Chemotherapy-Induced Pelvic Toxicity: A Proposed Clinical Study Protocol. Journal of Clinical Medicine, 14(22), 8015. https://doi.org/10.3390/jcm14228015











