Device-Based Therapies for Refractory Angina
Abstract
1. Introduction: Unmet Clinical Needs in Refractory Angina
2. Device-Based Therapies for Refractory Angina
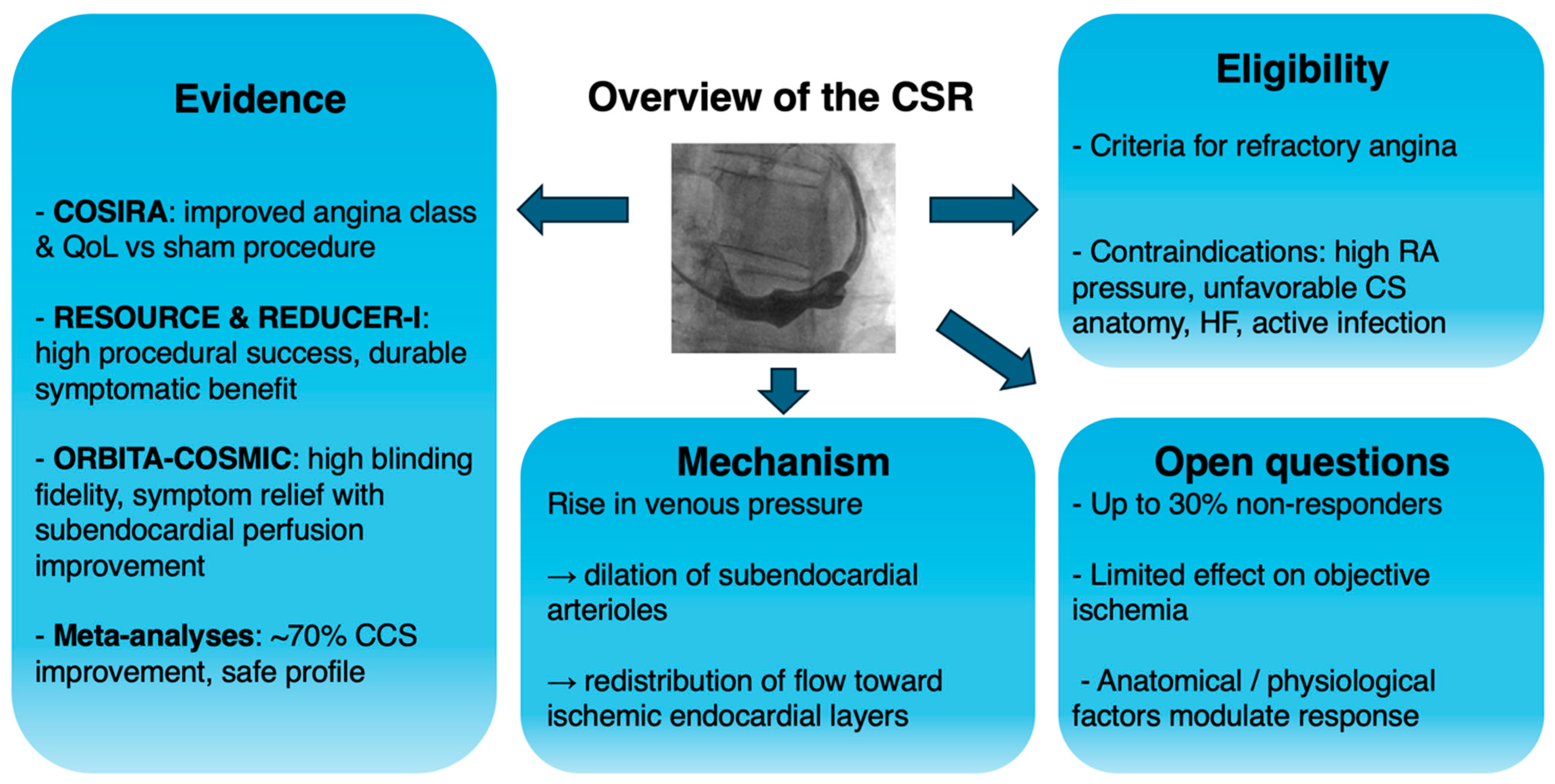
2.1. Coronary Sinus ReducerTM
2.2. Spinal Cord Stimulation (SCS)
2.3. Extracorporeal Shockwave Myocardial Revascularization (ESMR)
2.4. Enhanced External Counter-Pulsation (EECP)
2.5. Outdated Approaches: Transmyocardial Laser Revascularization (TMLR), Percutaneous Myocardial Laser Revascularization
3. Clinical Evidence for the Coronary Sinus Reducer™
4. Clinical Implications and Current Controversies
5. Future Directions
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2019 Diseases and Injuries Collaborators. Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Kaski, J.-C.; Crea, F.; Gersh, B.J.; Camici, P.G. Reappraisal of Ischemic Heart Disease. Circulation 2018, 138, 1463–1480. [Google Scholar] [CrossRef]
- Montone, R.A.; Rinaldi, R.; Niccoli, G.; Andò, G.; Gragnano, F.; Piccolo, R.; Pelliccia, F.; Moscarella, E.; Zimarino, M.; Fabris, E.; et al. Optimizing Management of Stable Angina: A Patient-Centered Approach Integrating Revascularization, Medical Therapy, and Lifestyle Interventions. J. Am. Coll. Cardiol. 2024, 84, 744–760. [Google Scholar] [CrossRef]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the Management of Chronic Coronary Syndromes. Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar] [CrossRef]
- Rinaldi, R.; Kunadian, V.; Crea, F.; Montone, R.A. Management of Angina Pectoris. Trends Cardiovasc. Med. 2025, 35, 341–350. [Google Scholar] [CrossRef]
- Holubkov, R.; Laskey, W.K.; Haviland, A.; Slater, J.C.; Bourassa, M.G.; Vlachos, H.A.; Cohen, H.A.; Williams, D.O.; Kelsey, S.F.; Detre, K.M.; et al. Angina 1 Year after Percutaneous Coronary Intervention: A Report from the NHLBI Dynamic Registry. Am. Heart J. 2002, 144, 826–833. [Google Scholar] [CrossRef]
- Weintraub, W.S.; Spertus, J.A.; Kolm, P.; Maron, D.J.; Zhang, Z.; Jurkovitz, C.; Zhang, W.; Hartigan, P.M.; Lewis, C.; Veledar, E.; et al. Effect of PCI on Quality of Life in Patients with Stable Coronary Disease. N. Engl. J. Med. 2008, 359, 677–687. [Google Scholar] [CrossRef]
- Gallone, G.; Baldetti, L.; Tzanis, G.; Gramegna, M.; Latib, A.; Colombo, A.; Henry, T.D.; Giannini, F. Refractory Angina: From Pathophysiology to New Therapeutic Nonpharmacological Technologies. JACC Cardiovasc. Interv. 2020, 13, 1–19. [Google Scholar] [CrossRef]
- Montone, R.A.; Caffè, A.; Yasumura, K.; Kini, A. Routine Diagnosis of ANOCA/INOCA: Pros and Cons. Eurointerv. J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2025, 21, e293–e295. [Google Scholar] [CrossRef]
- Montone, R.A.; Leone, A.M.; Crea, F. Angina with Non-Obstructive Coronary Arteries: A Success Story. Eur. Heart J. 2025, 46, 4407–4409. [Google Scholar] [CrossRef]
- Montone, R.A.; Cosentino, N.; Graziani, F.; Gorla, R.; Del Buono, M.G.; La Vecchia, G.; Rinaldi, R.; Marenzi, G.; Bartorelli, A.L.; De Marco, F.; et al. Precision Medicine versus Standard of Care for Patients with Myocardial Infarction with Non-Obstructive Coronary Arteries (MINOCA): Rationale and Design of the Multicentre, Randomised PROMISE Trial. EuroInterv. J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2022, 18, e933–e939. [Google Scholar] [CrossRef]
- Montone, R.A.; Cosentino, N.; Gorla, R.; Biscaglia, S.; La Vecchia, G.; Rinaldi, R.; Caffè, A.; Resta, M.; Erriquez, A.; Bedogni, F.; et al. Stratified Treatment of Myocardial Infarction with Non-Obstructive Coronary Arteries: The PROMISE Trial. Eur. Heart J. 2025, ehaf917. [Google Scholar] [CrossRef]
- Davies, A.; Fox, K.; Galassi, A.R.; Banai, S.; Ylä-Herttuala, S.; Lüscher, T.F. Management of Refractory Angina: An Update. Eur. Heart J. 2021, 42, 269–283. [Google Scholar] [CrossRef]
- Henry, T.D.; Satran, D.; Hodges, J.S.; Johnson, R.K.; Poulose, A.K.; Campbell, A.R.; Garberich, R.F.; Bart, B.A.; Olson, R.E.; Boisjolie, C.R.; et al. Long-Term Survival in Patients with Refractory Angina. Eur. Heart J. 2013, 34, 2683–2688. [Google Scholar] [CrossRef]
- Jespersen, L.; Hvelplund, A.; Abildstrøm, S.Z.; Pedersen, F.; Galatius, S.; Madsen, J.K.; Jørgensen, E.; Kelbæk, H.; Prescott, E. Stable Angina Pectoris with No Obstructive Coronary Artery Disease Is Associated with Increased Risks of Major Adverse Cardiovascular Events. Eur. Heart J. 2012, 33, 734–744. [Google Scholar] [CrossRef]
- Verheye, S.; van de Hoef, T.P.; de Silva, R.; van Kuijk, J.-P.; Byrne, J.; Montorfano, M.; Buschmann, E.; Dupont, M.; West, N.E.J.; Banai, S.; et al. Coronary Sinus Narrowing for Treating Refractory Angina: REDUCER-I Multicenter “Real-World” Observational Study Primary Endpoint Analysis. JACC Cardiovasc. Interv. 2024, 17, 2908–2918. [Google Scholar] [CrossRef]
- Medranda, G.A.; Torguson, R.; Waksman, R. Overview of the Virtual 2020 FDA’s Circulatory System Devices Advisory Panel on Neovasc Reducer System. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2021, 98, 1152–1158. [Google Scholar] [CrossRef]
- Ido, A.; Hasebe, N.; Matsuhashi, H.; Kikuchi, K. Coronary Sinus Occlusion Enhances Coronary Collateral Flow and Reduces Subendocardial Ischemia. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H1361–H1367. [Google Scholar] [CrossRef] [PubMed]
- Konigstein, M.; Giannini, F.; Banai, S. The Reducer Device in Patients with Angina Pectoris: Mechanisms, Indications, and Perspectives. Eur. Heart J. 2018, 39, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Konigstein, M.; Moroni, A.; Verheye, S.; Zornitzki, L.; Freund, O.; Banai, S. Efficacy of Coronary Sinus Reducer in Patients with Refractory Angina and Chronic Total Occlusion. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2025; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Mrak, M.; Pavšič, N.; Ponticelli, F.; Beneduce, A.; Palmisano, A.; Guarracini, S.; Esposito, A.; Banai, S.; Žižek, D.; Giannini, F.; et al. Efficacy of Coronary Sinus Reducer Implantation in Patients with Chronic Total Occlusion of the Right Coronary Artery. Kardiol. Pol. 2022, 80, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Verheye, S.; Jolicœur, E.M.; Behan, M.W.; Pettersson, T.; Sainsbury, P.; Hill, J.; Vrolix, M.; Agostoni, P.; Engstrom, T.; Labinaz, M.; et al. Efficacy of a Device to Narrow the Coronary Sinus in Refractory Angina. N. Engl. J. Med. 2015, 372, 519–527. [Google Scholar] [CrossRef]
- Giannini, F.; Tzanis, G.; Ponticelli, F.; Baldetti, L.; Demir, O.M.; Mitomo, S.; Gallone, G.; Banai, S.; Colombo, A. Technical Aspects in Coronary Sinus Reducer Implantation. EuroInterv. J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2020, 15, 1269–1277. [Google Scholar] [CrossRef]
- Foley, M.J.; Rajkumar, C.A.; Ahmed-Jushuf, F.; Simader, F.A.; Chotai, S.; Pathimagaraj, R.H.; Mohsin, M.; Salih, A.; Wang, D.; Dixit, P.; et al. Coronary Sinus Reducer for the Treatment of Refractory Angina (ORBITA-COSMIC): A Randomised, Placebo-Controlled Trial. Lancet 2024, 403, 1543–1553. [Google Scholar] [CrossRef]
- Ponticelli, F.; Khokhar, A.A.; Leenders, G.; Konigstein, M.; Zivelonghi, C.; Agostoni, P.; van Kuijk, J.-P.; Ajmi, I.; Lindsay, S.; Bunc, M.; et al. Safety and Efficacy of Coronary Sinus Narrowing in Chronic Refractory Angina: Insights from the RESOURCE Study. Int. J. Cardiol. 2021, 337, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Cortese, B.; di Palma, G.; Latini, R. Coronary Sinus Perforation during Reducer Implantation. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2018, 91, 1291–1293. [Google Scholar] [CrossRef] [PubMed]
- Imran, T.F.; Malapero, R.; Qavi, A.H.; Hasan, Z.; de la Torre, B.; Patel, Y.R.; Yong, R.J.; Djousse, L.; Gaziano, J.M.; Gerhard-Herman, M.-D. Efficacy of Spinal Cord Stimulation as an Adjunct Therapy for Chronic Refractory Angina Pectoris. Int. J. Cardiol. 2017, 227, 535–542. [Google Scholar] [CrossRef]
- Andréll, P.; Yu, W.; Gersbach, P.; Gillberg, L.; Pehrsson, K.; Hardy, I.; Ståhle, A.; Andersen, C.; Mannheimer, C. Long-Term Effects of Spinal Cord Stimulation on Angina Symptoms and Quality of Life in Patients with Refractory Angina Pectoris—Results from the European Angina Registry Link Study (EARL). Heart Br. Card. Soc. 2010, 96, 1132–1136. [Google Scholar] [CrossRef]
- van Kleef, M.; Staats, P.; Mekhail, N.; Huygen, F. 24. Chronic Refractory Angina Pectoris. Pain Pract. Off. J. World Inst. Pain 2011, 11, 476–482. [Google Scholar] [CrossRef]
- Mannheimer, C.; Eliasson, T.; Augustinsson, L.E.; Blomstrand, C.; Emanuelsson, H.; Larsson, S.; Norrsell, H.; Hjalmarsson, A. Electrical Stimulation versus Coronary Artery Bypass Surgery in Severe Angina Pectoris: The ESBY Study. Circulation 1998, 97, 1157–1163. [Google Scholar] [CrossRef]
- Alunni, G.; Marra, S.; Meynet, I.; D’amico, M.; Elisa, P.; Fanelli, A.; Molinaro, S.; Garrone, P.; Deberardinis, A.; Campana, M.; et al. The Beneficial Effect of Extracorporeal Shockwave Myocardial Revascularization in Patients with Refractory Angina. Cardiovasc. Revasc. Med. Mol. Interv. 2015, 16, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Schmid, J.-P.; Capoferri, M.; Wahl, A.; Eshtehardi, P.; Hess, O.M. Cardiac Shock Wave Therapy for Chronic Refractory Angina Pectoris. A Prospective Placebo-Controlled Randomized Trial. Cardiovasc. Ther. 2013, 31, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.; Ito, K.; Ito, Y.; Shiroto, T.; Tsuburaya, R.; Aizawa, K.; Hao, K.; Fukumoto, Y.; Takahashi, J.; Takeda, M.; et al. Double-Blind and Placebo-Controlled Study of the Effectiveness and Safety of Extracorporeal Cardiac Shock Wave Therapy for Severe Angina Pectoris. Circ. J. Off. J. Jpn. Circ. Soc. 2010, 74, 589–591. [Google Scholar] [CrossRef]
- Čelutkienė, J.; Burneikaitė, G.; Shkolnik, E.; Jakutis, G.; Vajauskas, D.; Čerlinskaitė, K.; Zuozienė, G.; Petrauskienė, B.; Puronaitė, R.; Komiagienė, R.; et al. The Effect of Cardiac Shock Wave Therapy on Myocardial Function and Perfusion in the Randomized, Triple-Blind, Sham-Procedure Controlled Study. Cardiovasc. Ultrasound 2019, 17, 13. [Google Scholar] [CrossRef]
- Vainer, J.; Habets, J.H.M.; Schalla, S.; Lousberg, A.H.P.; de Pont, C.D.J.M.; Vöö, S.A.; Brans, B.T.; Hoorntje, J.C.A.; Waltenberger, J. Cardiac Shockwave Therapy in Patients with Chronic Refractory Angina Pectoris. Neth. Heart J. Mon. J. Neth. Soc. Cardiol. Neth. Heart Found. 2016, 24, 343–349. [Google Scholar] [CrossRef]
- Burneikaitė, G.; Shkolnik, E.; Čelutkienė, J.; Zuozienė, G.; Butkuvienė, I.; Petrauskienė, B.; Šerpytis, P.; Laucevičius, A.; Lerman, A. Cardiac Shock-Wave Therapy in the Treatment of Coronary Artery Disease: Systematic Review and Meta-Analysis. Cardiovasc. Ultrasound 2017, 15, 11. [Google Scholar] [CrossRef]
- Bonetti, P.O.; Barsness, G.W.; Keelan, P.C.; Schnell, T.I.; Pumper, G.M.; Kuvin, J.T.; Schnall, R.P.; Holmes, D.R.; Higano, S.T.; Lerman, A. Enhanced External Counterpulsation Improves Endothelial Function in Patients with Symptomatic Coronary Artery Disease. J. Am. Coll. Cardiol. 2003, 41, 1761–1768. [Google Scholar] [CrossRef]
- Arora, R.R.; Chou, T.M.; Jain, D.; Fleishman, B.; Crawford, L.; McKiernan, T.; Nesto, R.W. The Multicenter Study of Enhanced External Counterpulsation (MUST-EECP): Effect of EECP on Exercise-Induced Myocardial Ischemia and Anginal Episodes. J. Am. Coll. Cardiol. 1999, 33, 1833–1840. [Google Scholar] [CrossRef]
- Soran, O.; Kennard, E.D.; Kfoury, A.G.; Kelsey, S.F. IEPR Investigators Two-Year Clinical Outcomes after Enhanced External Counterpulsation (EECP) Therapy in Patients with Refractory Angina Pectoris and Left Ventricular Dysfunction (Report from the International EECP Patient Registry). Am. J. Cardiol. 2006, 97, 17–20. [Google Scholar] [CrossRef]
- Virani, S.S.; Newby, L.K.; Arnold, S.V.; Bittner, V.; Brewer, L.C.; Demeter, S.H.; Dixon, D.L.; Fearon, W.F.; Hess, B.; Johnson, H.M.; et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients with Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2023, 148, e9–e119. [Google Scholar] [CrossRef] [PubMed]
- Al-Sheikh, T.; Allen, K.B.; Straka, S.P.; Heimansohn, D.A.; Fain, R.L.; Hutchins, G.D.; Sawada, S.G.; Zipes, D.P.; Engelstein, E.D. Cardiac Sympathetic Denervation after Transmyocardial Laser Revascularization. Circulation 1999, 100, 135–140. [Google Scholar] [CrossRef]
- Frazier, O.H.; March, R.J.; Horvath, K.A. Transmyocardial Revascularization with a Carbon Dioxide Laser in Patients with End-Stage Coronary Artery Disease. N. Engl. J. Med. 1999, 341, 1021–1028. [Google Scholar] [CrossRef]
- Leon, M.B.; Kornowski, R.; Downey, W.E.; Weisz, G.; Baim, D.S.; Bonow, R.O.; Hendel, R.C.; Cohen, D.J.; Gervino, E.; Laham, R.; et al. A Blinded, Randomized, Placebo-Controlled Trial of Percutaneous Laser Myocardial Revascularization to Improve Angina Symptoms in Patients with Severe Coronary Disease. J. Am. Coll. Cardiol. 2005, 46, 1812–1819. [Google Scholar] [CrossRef]
- Briones, E.; Lacalle, J.R.; Marin-Leon, I.; Rueda, J.-R. Transmyocardial Laser Revascularization versus Medical Therapy for Refractory Angina. Cochrane Database Syst. Rev. 2015, 2015, CD003712. [Google Scholar] [CrossRef]
- Konigstein, M.; Meyten, N.; Verheye, S.; Schwartz, M.; Banai, S. Transcatheter Treatment for Refractory Angina with the Coronary Sinus Reducer. EuroInterv. J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2014, 9, 1158–1164. [Google Scholar] [CrossRef]
- Giannini, F.; Baldetti, L.; Ponticelli, F.; Ruparelia, N.; Mitomo, S.; Latib, A.; Montorfano, M.; Jabbour, R.J.; Aurelio, A.; Ferri, L.; et al. Coronary Sinus Reducer Implantation for the Treatment of Chronic Refractory Angina: A Single-Center Experience. JACC Cardiovasc. Interv. 2018, 11, 784–792. [Google Scholar] [CrossRef]
- Abawi, M.; Nijhoff, F.; Stella, P.R.; Voskuil, M.; Benedetto, D.; Doevendans, P.A.; Agostoni, P. Safety and Efficacy of a Device to Narrow the Coronary Sinus for the Treatment of Refractory Angina: A Single-Centre Real-World Experience. Neth. Heart J. Mon. J. Neth. Soc. Cardiol. Neth. Heart Found. 2016, 24, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Elshahat, A.; Husain, M.A.; Elbataa, A.; Gadelmawla, A.F.; Makhlouf, H.A.; Ibrahim, R.A.; Hassanin, M.S.; Rzk, F.M.; Mohamed, A.E.; Helmi, A.; et al. The Efficacy and Safety of Coronary Sinus Reducer in Refractory Angina: A Systematic Review and Meta-Analysis. BMC Cardiovasc. Disord. 2025, 25, 530. [Google Scholar] [CrossRef] [PubMed]
- Gallone, G.; Armeni, P.; Verheye, S.; Agostoni, P.; Timmers, L.; Campo, G.; Ielasi, A.; Sgura, F.; Tarantini, G.; Rosseel, L.; et al. Cost-Effectiveness of the Coronary Sinus Reducer and Its Impact on the Healthcare Burden of Refractory Angina Patients. Eur. Heart J. Qual. Care Clin. Outcomes 2020, 6, 32–40. [Google Scholar] [CrossRef]
- Tebaldi, M.; Campo, G.; Ugo, F.; Guarracini, S.; Marrone, A.; Clò, S.; Abdirashid, M.; Di Mauro, M.; Rametta, F.; Di Marco, M.; et al. Coronary Sinus Narrowing Improves Coronary Microcirculation Function in Patients with Refractory Angina: A Multicenter Prospective INROAD Study. Circ. Cardiovasc. Interv. 2024, 17, e013481. [Google Scholar] [CrossRef] [PubMed]
- Tryon, D.; Corban, M.T.; Alkhouli, M.; Prasad, A.; Raphael, C.E.; Rihal, C.S.; Reeder, G.S.; Lewis, B.; Albers, D.; Gulati, R.; et al. Coronary Sinus Reducer Improves Angina, Quality of Life, and Coronary Flow Reserve in Microvascular Dysfunction. JACC Cardiovasc. Interv. 2024, 17, 2893–2904. [Google Scholar] [CrossRef]
- Ullrich, H.; Hammer, P.; Olschewski, M.; Münzel, T.; Escaned, J.; Gori, T. Coronary Venous Pressure and Microvascular Hemodynamics in Patients with Microvascular Angina: A Randomized Clinical Trial. JAMA Cardiol. 2023, 8, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Giannini, F.; Ponticelli, F.; Konigstein, M.; Verheye, S.; Zivelonghi, C.; Agostoni, P.; Cafaro, A.; Patterson, T.; Redwood, S.; Cheng, K.; et al. Impact of Coronary Sinus Reducer on Angina Symptoms in Patients with Myocardial Ischemia Without Obstructive Coronary Artery Disease. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2025, 106, 2313–2320. [Google Scholar] [CrossRef]
- Andò, G.; Montone, R.A. Alleviating Refractory Angina Through Coronary Sinus Narrowing: Consistent Benefits and the Pursuit of Mechanistic Insights. JACC Cardiovasc. Interv. 2024, 17, 2919–2922. [Google Scholar] [CrossRef]
- Giannini, F.; Baldetti, L.; Konigstein, M.; Rosseel, L.; Ruparelia, N.; Gallone, G.; Colombo, A.; Banai, S.; Verheye, S. Safety and Efficacy of the Reducer: A Multi-Center Clinical Registry-REDUCE Study. Int. J. Cardiol. 2018, 269, 40–44. [Google Scholar] [CrossRef]
- Ponticelli, F.; Khokhar, A.A.; Albani, S.; Tzanis, G.; Gallo, F.; Guarracini, S.; Banai, S.; Colombo, A.; Giannini, F. Insights Into Coronary Sinus Reducer Non-Responders. J. Invasive Cardiol. 2021, 33, E884–E889. [Google Scholar] [CrossRef] [PubMed]
- Baldetti, L.; Colombo, A.; Banai, S.; Latib, A.; Esposito, A.; Palmisano, A.; Giannini, F. Coronary Sinus Reducer Non-Responders: Insights and Perspectives. EuroInterv. J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2018, 13, 1667–1669. [Google Scholar] [CrossRef] [PubMed]
- Ojha, U.; Mohsin, M.; Macierzanka, K.; Ahmed-Jushuf, F.; Rajkumar, C.A.; Chotai, S.; Simader, F.A.; Shun-Shin, M.J.; Foley, M.J.; Al-Lamee, R.K. Safety, Efficacy, and Effectiveness of Coronary Sinus Reducer Implantation in Refractory Angina: A Meta-Analysis. JACC Cardiovasc. Interv. 2025, 18, 1864–1877. [Google Scholar] [CrossRef]
- Palmisano, A.; Giannini, F.; Rancoita, P.; Gallone, G.; Benedetti, G.; Baldetti, L.; Tzanis, G.; Vignale, D.; Monti, C.; Ponticelli, F.; et al. Feature Tracking and Mapping Analysis of Myocardial Response to Improved Perfusion Reserve in Patients with Refractory Angina Treated by Coronary Sinus Reducer Implantation: A CMR Study. Int. J. Cardiovasc. Imaging 2021, 37, 291–303. [Google Scholar] [CrossRef]
- Cheng, K.; Alpendurada, F.; Bucciarelli-Ducci, C.; Almeida, J.; Kellman, P.; Hill, J.M.; Pennell, D.J.; de Silva, R. Segmental Redistribution of Myocardial Blood Flow after Coronary Sinus Reducer Implantation Demonstrated by Quantitative Perfusion Cardiovascular Magnetic Resonance. J. Cardiovasc. Magn. Reson. Off. J. Soc. Cardiovasc. Magn. Reson. 2025, 27, 101868. [Google Scholar] [CrossRef]
- Shockwave Medical, Inc. Efficacy of the COronary SInus Reducer in Patients with Refractory Angina II; clinicaltrials.gov; Shockwave Medical, Inc.: Santa Clara, CA, USA, 2025.
- Gori, T. A Multicentric Randomized Open Label Controlled Superiority Trial to Evaluate the Effectiveness of a Therapy with a Coronary Sinus Reducer as Compared to Guideline-Directed Medical Therapy in Patients with Refractory Microvascular Angina. 2023. Available online: https://clinicaltrials.gov/study/NCT04606459 (accessed on 19 September 2025).
- Imperial College London. REducing Microvascular Dysfunction in Patients with Angina, Ischaemia and Unobstructed Coronary Arteries—A PILOT Study; clinicaltrials.gov; Imperial College London: London, UK, 2025.
| Study/Author | Design/Population | Number of Patients/Follow-Up | Ischemia Endpoint | Main Results |
|---|---|---|---|---|
| Konigstein et al. (2014) [45] | Prospective, single-center; RA patients | 23 patients/6 months | Dobutamine stress echo (WMSI, LVEF), thallium stress scan, treadmill | Improved WMSI and LVEF; reduced ischemic burden on thallium; treadmill test not significant. |
| COSIRA (2015) [22] | Randomized, double-blind sham-controlled; RA patients | 104 patients/6 months | Treadmill exercise duration, time to 1 mm ST depression, WMSI (dobutamine) | Significant angina and QoL benefit vs. sham; no consistent improvement in ischemia endpoints. |
| REDUCE (2018) [55] | Multicenter, prospective registry; RA patients | 85 patients (imaging subset)/6–12 months | SPECT, dobutamine stress echo, treadmill, 6-MWT | Reduced ischemia extent on SPECT; lower prevalence of inducible ischemia on echo; WMSI, LVEF, and exercise time not significant. |
| ORBITA-COSMIC (2024) [24] | Randomized, double-blind; RA patients | 50 patients/6 months | Quantitative adenosine-stress perfusion CMR (global & segmental MBF, endo/epi ratio) | Reduced daily angina, improved QoL; no global perfusion gain; evidence of subendocardial redistribution. |
| INROAD (2024) [50] | Multicenter, prospective; post-revascularization RA patients | 24 patients/4 months | Invasive microvascular testing: IMR, CFR, resistive reserve ratio | Significant IMR reduction (71% ≥ 20%); CFR improvement; supports effect on microcirculation. |
| Tryon et al. (2024) [51] | Phase II trial, ANOCA patients with coronary microvascular dysfunction | 30 patients/4 months | Microvascular function (CFR, CBF in response to acetylcholine) | Significant increase in both CFR and CBF in response to acetylcholine |
| Ullrich et al. (2023) [52] | Sham-controlled, crossover, randomized; refractory ANOCA patients with IMR > 25 | 20 patients | Aortic and distal coronary pressure, coronary sinus pressure, right atrial pressure, mean transit time | Significantly reduced IMR and improved coronary blood flow during acute ballon inflation in CS |
| Palmisano et al. (2021) [59] | Multiparametric CMR with feature-tracking & mapping; RA patients | 28 patients (20 analyzed)/4 months | Stress perfusion CMR (MPR), ischemic burden, strain, T1/ECV mapping | Reduced ischemic burden; restored endocardial/epicardial ratio; improved LVEF and strain; no change in T1/ECV. |
| Cheng et al. (2025) [60] | Pilot quantitative perfusion CMR; RA patients | 16 patients/3–4 months | Quantitative perfusion CMR: global & segmental MBF/MPR, endo/epi ratio | No global MBF/MPR change; segmental improvements in most ischemic regions with endocardial redistribution. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caffè, A.; Montone, R.A. Device-Based Therapies for Refractory Angina. J. Clin. Med. 2025, 14, 8013. https://doi.org/10.3390/jcm14228013
Caffè A, Montone RA. Device-Based Therapies for Refractory Angina. Journal of Clinical Medicine. 2025; 14(22):8013. https://doi.org/10.3390/jcm14228013
Chicago/Turabian StyleCaffè, Andrea, and Rocco A. Montone. 2025. "Device-Based Therapies for Refractory Angina" Journal of Clinical Medicine 14, no. 22: 8013. https://doi.org/10.3390/jcm14228013
APA StyleCaffè, A., & Montone, R. A. (2025). Device-Based Therapies for Refractory Angina. Journal of Clinical Medicine, 14(22), 8013. https://doi.org/10.3390/jcm14228013







