Effects of Sodium Glucose Co-Transporter 2 Inhibitors on Atrial Fibrillation Recurrences After Catheter Ablation in Atrial Fibrillation Patients: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Search Strategy
2.3. Eligibility Criteria
2.4. Study Selection
2.5. Data Extraction
Definition of AF Recurrence
2.6. Statistical Analysis
2.7. Risk of Bias
2.8. Publication Bias
2.9. Registration
3. Results
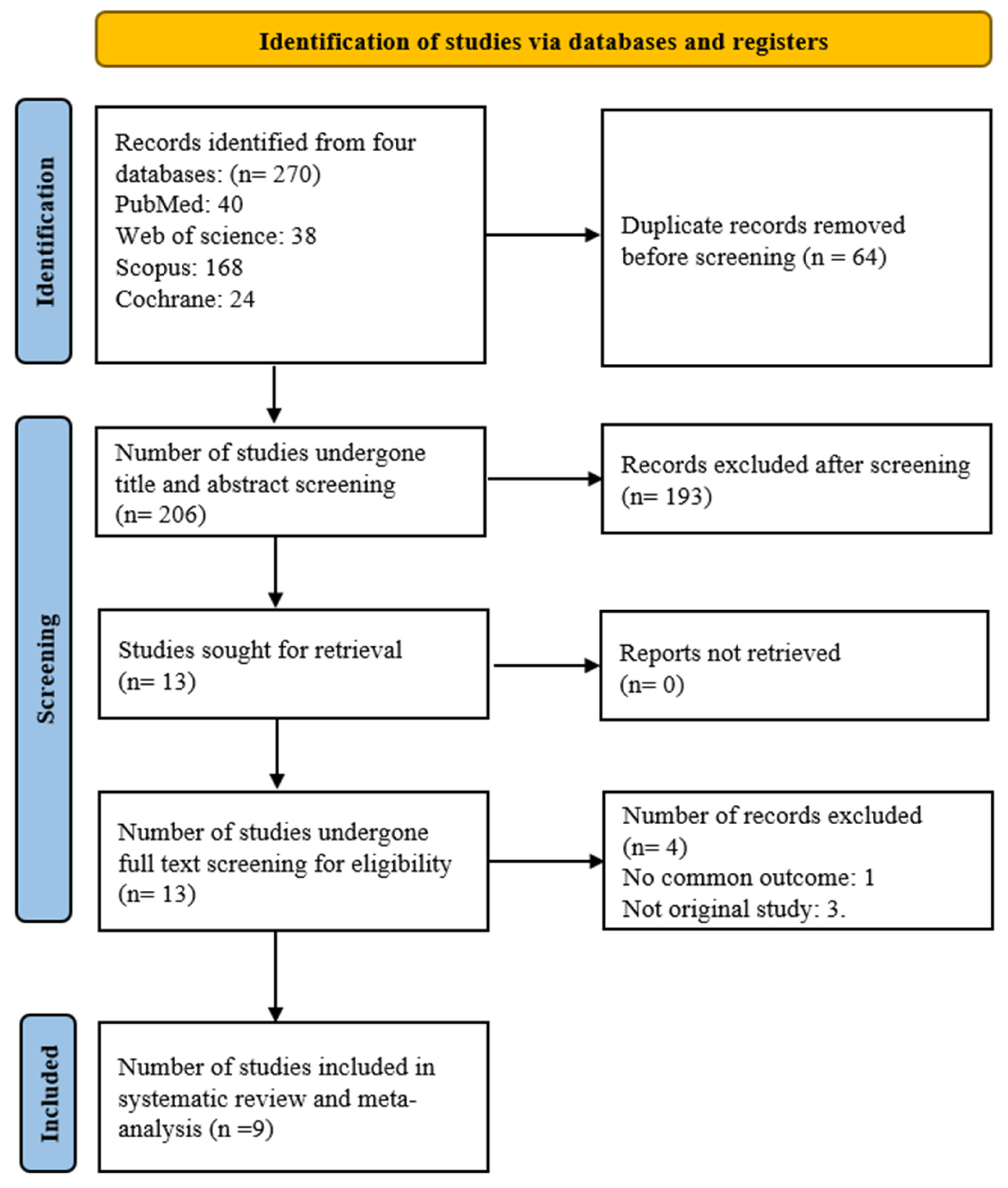
3.1. Literature Search
3.2. Baseline Characteristics
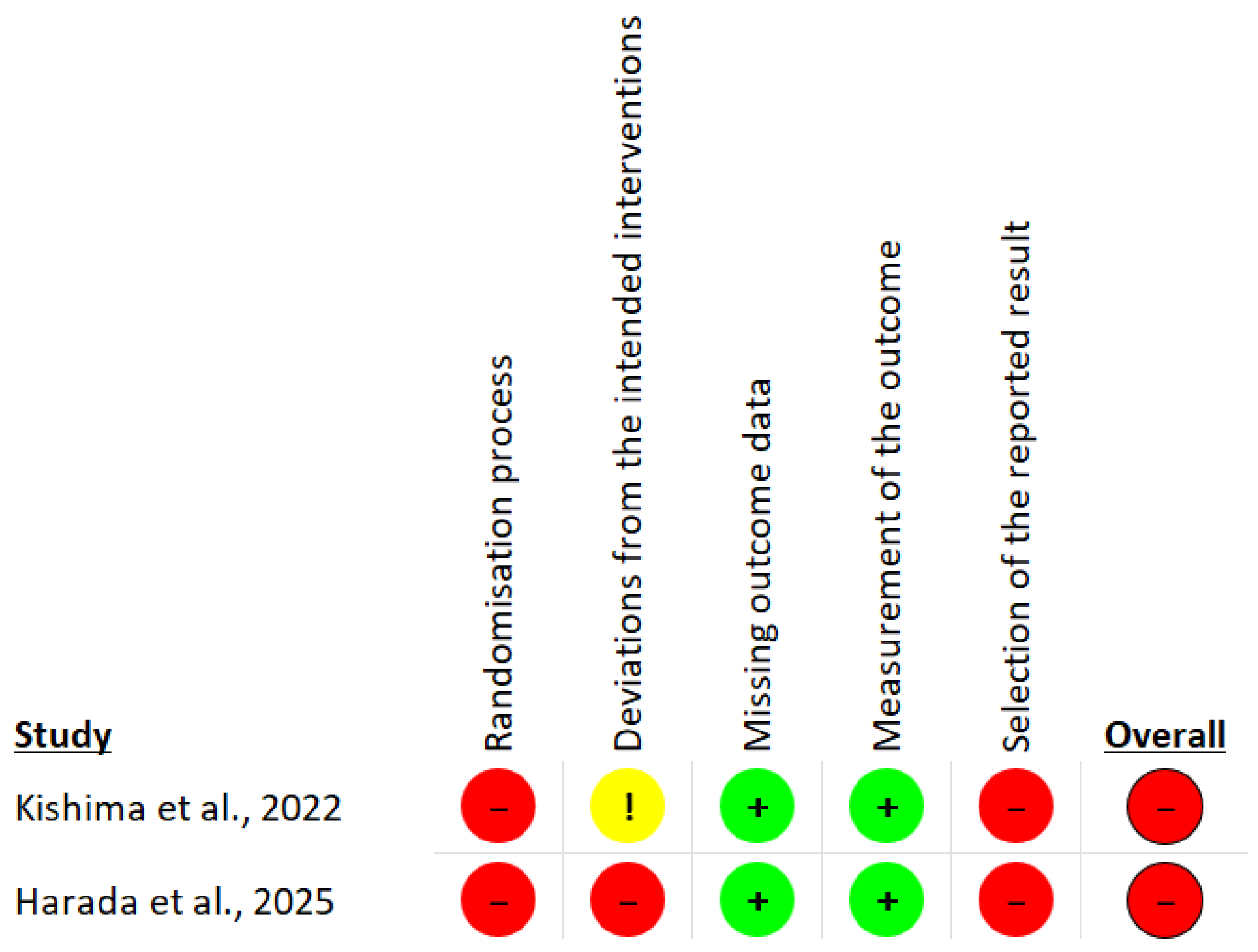
3.3. Risk of Bias
3.4. Clinical Outcomes Analysis
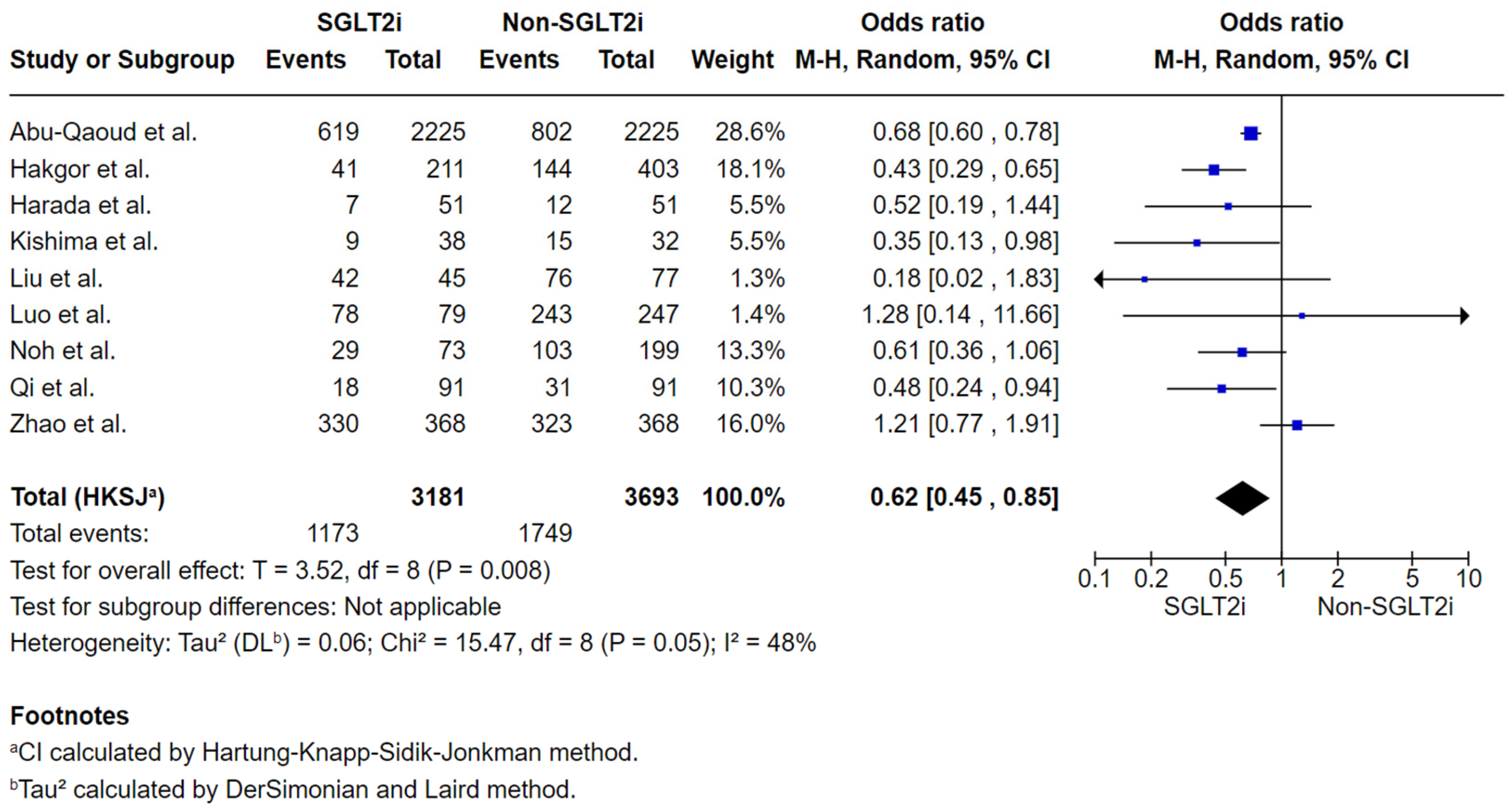
3.4.1. Analysis of the Total Number of Subjects with AF Recurrence by the Final Follow-Up Post-Ablation
3.4.2. Secondary Outcomes
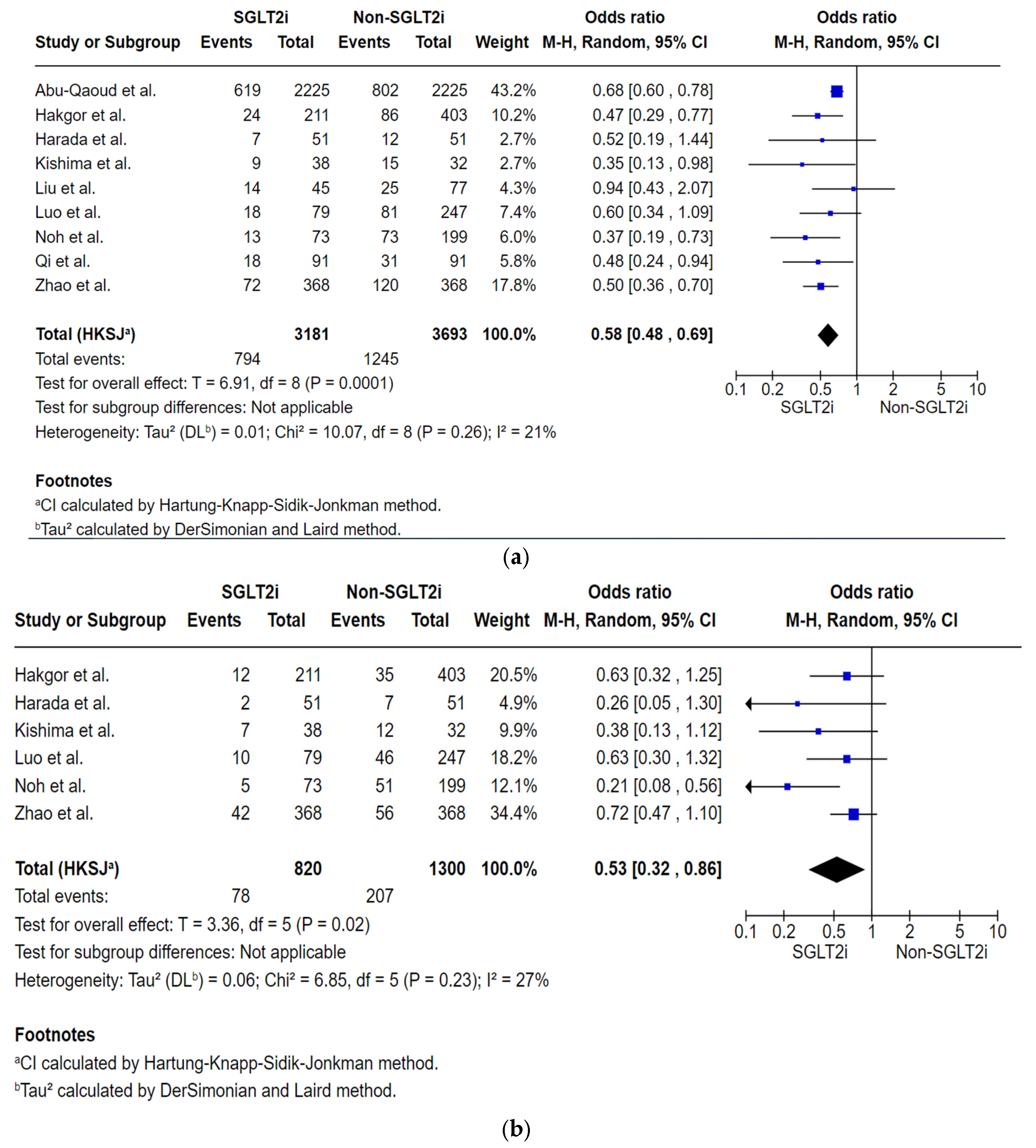
Analysis of Total Number of Subjects with AF Recurrence by the First Follow-Up Within 12 to 24 Months Post-Ablation
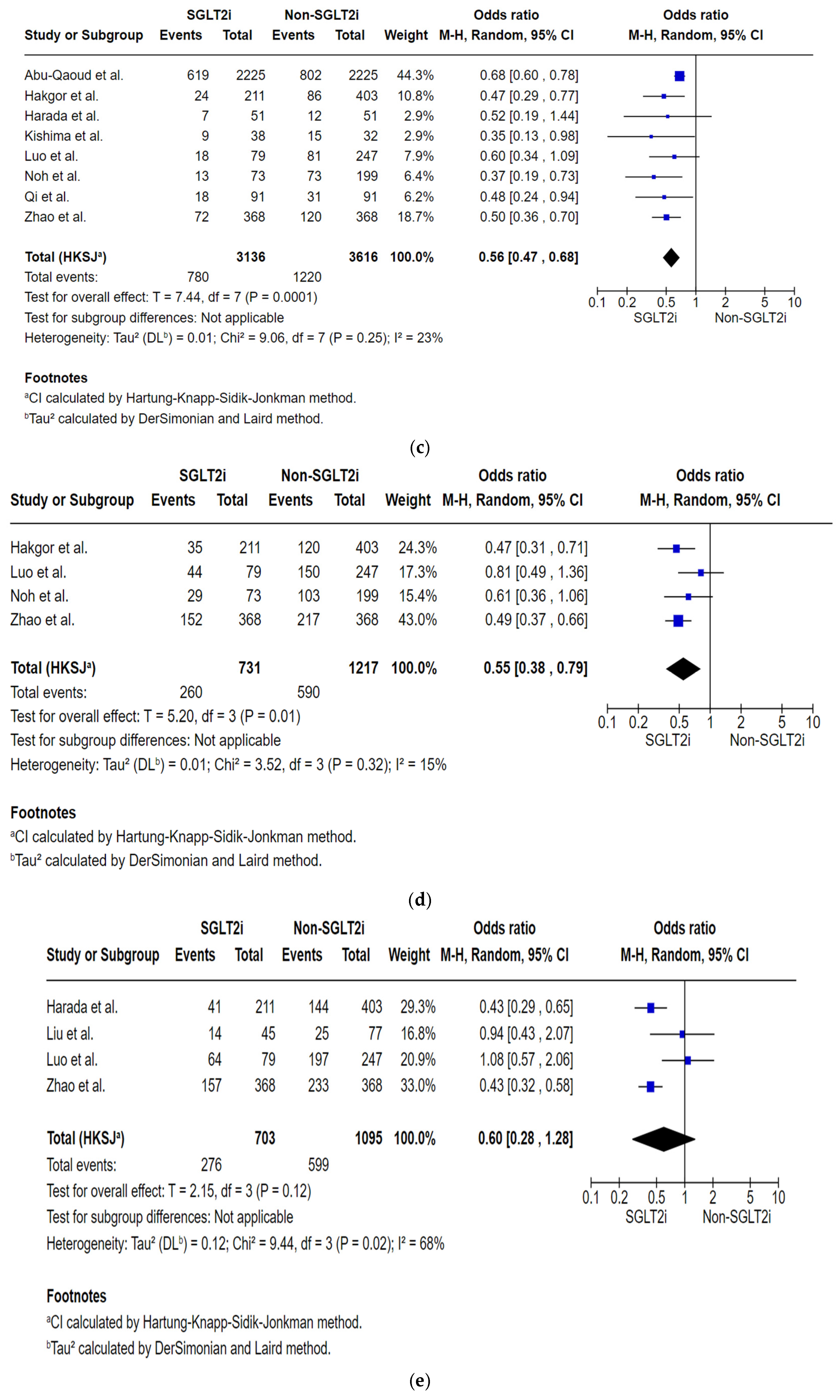
Analysis of the Total Number of Subjects with AF Recurrence by the Specific Follow-Up Intervals
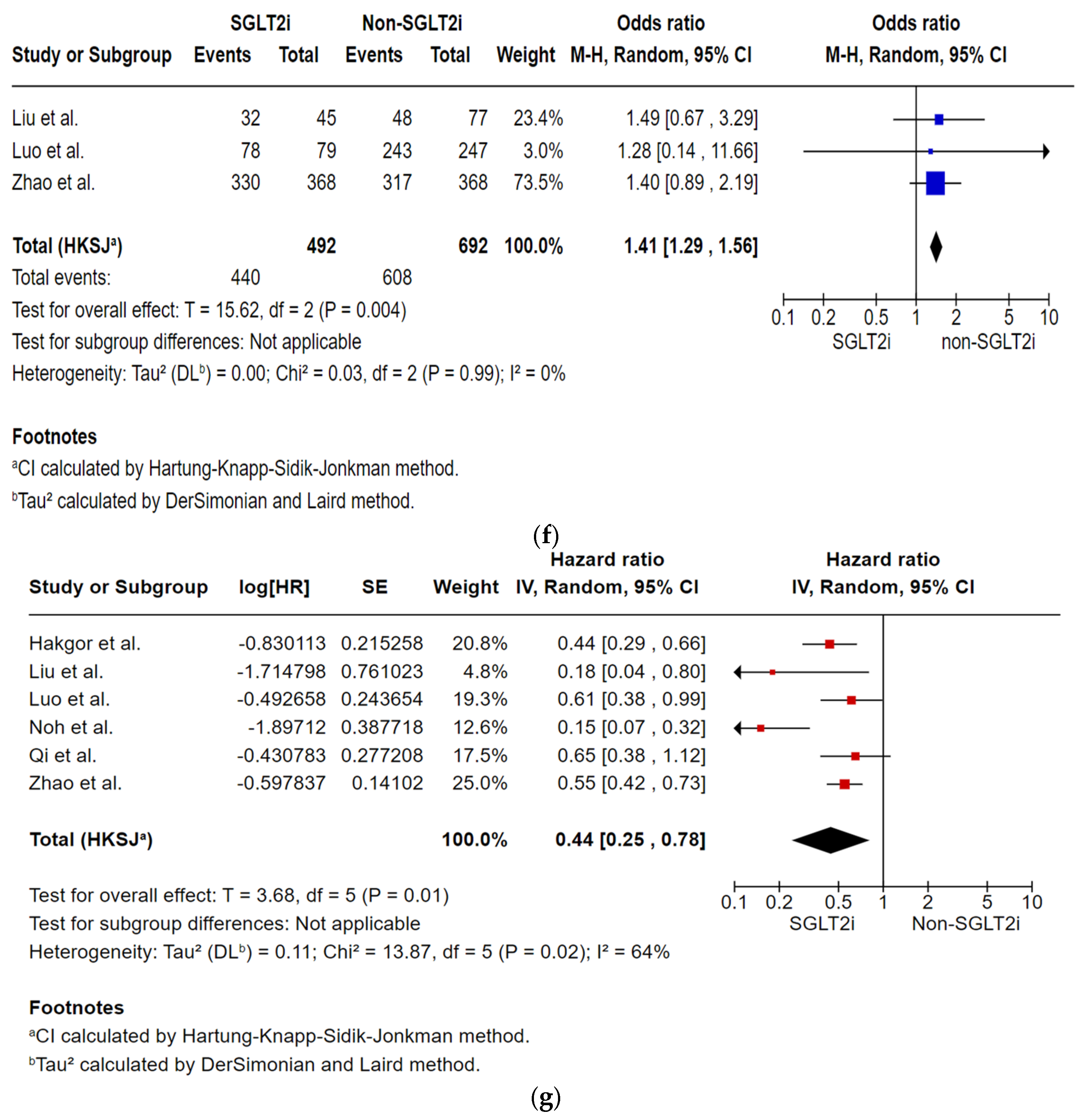
Analysis of Multivariate Risk of AF Recurrence
Analysis of Left Atrial Diameter Change
3.5. Univariate Meta-Regression Model for Primary Outcome
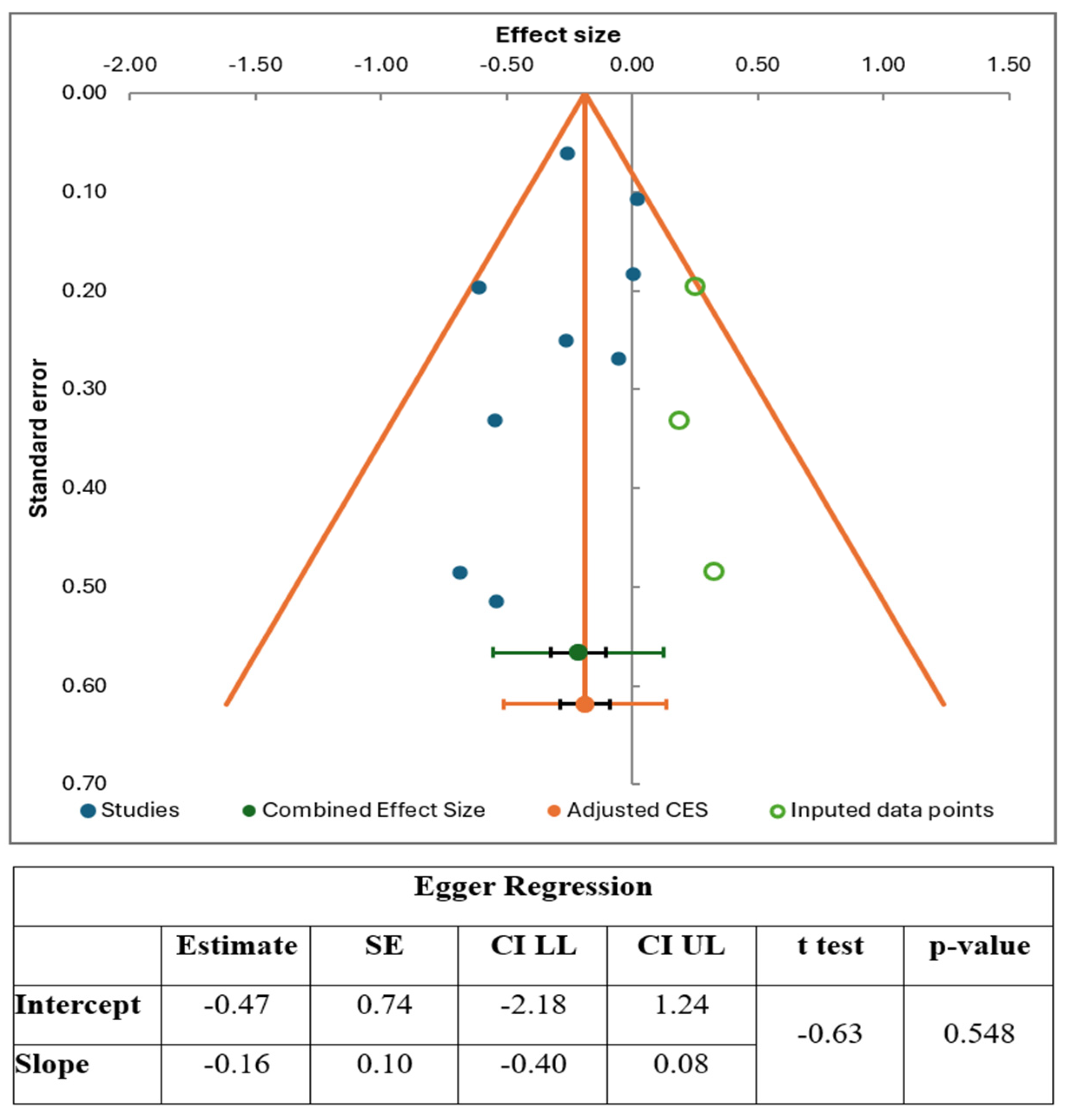
3.6. Publication Bias
3.7. Certainty Assessment Among Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SGLT2i | Sodium glucose co-transporter 2 inhibitors |
| HF | Heart failure |
| AF | Atrial fibrillation |
| RCTs | Randomized controlled trials |
| NIH | National Institutes of Health |
| ROB | Risk of bias |
| OR | Odds ratio |
| CI | Confidence interval |
| LAD | Left atrial diameter |
| CA | Catheter ablation |
| eGFR | Estimated glomerular filtration rate |
| LVEF | Left ventricular ejection fraction |
| HTN | Hypertension |
| T2DM | Type 2 diabetes mellitus |
| COPD | Chronic obstructive pulmonary disease |
| CVA | Cerebrovascular accident |
| CAD | Coronary artery disease |
| HLD | Hyperlipidemia |
| AAD | Anti-arrhythmic drugs |
| GLP1 | Glucagon-like peptide-1 |
| DPP4i | Dipeptidyl peptidase-4 inhibitors |
| PVI | Pulmonary vein isolation |
| CTI | Cavo-tricuspid isthmus |
| SVCI | Superior vena cava isolation |
| RFCA | Radiofrequency catheter ablation |
| TGF | Transforming growth factor |
| NHE | Sodium-hydrogen exchanger |
| NCX | Sodium-calcium exchanger |
| SERCA | Sarco/Endoplasmic Reticulum Calcium ATPase |
| AMPK | Adenosine monophosphate-activated protein kinase |
| SIRT1 | Sirtuin-1 |
| PGC-1α | Peroxisome proliferator-activated receptor-gamma coactivator 1α |
| IL | Interleukin |
| TNF | Tumor necrosis factor |
| CHA2DS2 VASc | Congestive heart failure, Hypertension, Age (≥75, doubled), Diabetes, Stroke/TIA (doubled), Vascular disease, Age (65–74), Sex category (Female) |
References
- Zhao, Z.; Jiang, C.; He, L.; Zheng, S.; Wang, Y.; Gao, M.; Lai, Y.; Zhang, J.; Li, M.; Dai, W.; et al. Impact of Sodium-Glucose Cotransporter 2 Inhibitor on Recurrence After Catheter Ablation for Atrial Fibrillation in Patients with Diabetes: A Propensity-Score Matching Study and Meta-Analysis. J. Am. Heart Assoc. 2023, 12, e031269. [Google Scholar] [CrossRef]
- See, C.; Grubman, S.; Shah, N.; Hu, J.R.; Nanna, M.; Freeman, J.V.; Murugiah, K. Healthcare Expenditure on Atrial Fibrillation in the United States: The Medical Expenditure Panel Survey 2016–2021. JACC Adv. 2025, 4, 101716. [Google Scholar] [CrossRef] [PubMed]
- Berman, A.E.; Kabiri, M.; Wei, T.; Galvain, T.; Sha, Q.; Kuck, K.-H. Economic and Health Value of Delaying Atrial Fibrillation Progression Using Radiofrequency Catheter Ablation. Circ. Arrhythm. Electrophysiol. 2023, 16, e011237. [Google Scholar] [CrossRef] [PubMed]
- Trohman, R.G.; Huang, H.D.; Sharma, P.S. Atrial fibrillation: Primary prevention, secondary prevention, and prevention of thromboembolic complications: Part 1. Front. Cardiovasc. Med. 2023, 10, 1060030. [Google Scholar] [CrossRef] [PubMed]
- Ferro, E.G.; Reynolds, M.R.; Xu, J.; Song, Y.; Cohen, D.J.; Wadhera, R.K.; d’Avila, A.; Zimetbaum, P.J.; Yeh, R.W.; Kramer, D.B. Outcomes of Atrial Fibrillation Ablation Among Older Adults in the United States: A Nationwide Study. JACC Clin. Electrophysiol. 2024, 10 Pt 1, 1341–1350. [Google Scholar] [CrossRef]
- Ganesan, A.N.; Shipp, N.J.; Brooks, A.G.; Kuklik, P.; Lau, D.H.; Lim, H.S.; Sullivan, T.; Roberts-Thomson, K.C.; Sanders, P. Long-term Outcomes of Catheter Ablation of Atrial Fibrillation: A Systematic Review and Meta-analysis. J. Am. Heart Assoc. 2013, 2, e004549. [Google Scholar] [CrossRef]
- Takigawa, M.; Takahashi, A.; Kuwahara, T.; Okubo, K.; Takahashi, Y.; Watari, Y.; Takagi, K.; Fujino, T.; Kimura, S.; Hikita, H.; et al. Long-term follow-up after catheter ablation of paroxysmal atrial fibrillation: The incidence of recurrence and progression of atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2014, 7, 267–273. [Google Scholar] [CrossRef]
- Crowley, R.; Chieng, D.; Sugumar, H.; Ling, L.-H.; Segan, L.; William, J.; Prabhu, S.; Voskoboinik, A.; Wong, G.; Morton, J.B.; et al. Catheter ablation for persistent atrial fibrillation: Patterns of recurrence and impact on quality of life and health care utilization. Eur. Heart J. 2024, 45, 2604–2616. [Google Scholar] [CrossRef]
- Osorio, J.; Miranda-Arboleda, A.F.; Velasco, A.; Varley, A.L.; Rajendra, A.; Morales, G.X.; Hoyos, C.; Matos, C.; Thorne, C.; D’Souza, B.; et al. Real-world data of radiofrequency catheter ablation in paroxysmal atrial fibrillation: Short- and long-term clinical outcomes from the prospective multicenter REAL-AF Registry. Heart Rhythm 2024, 21, 2083–2091. [Google Scholar] [CrossRef]
- Mansour, M.; Karst, E.; Heist, E.K.; Dalal, N.; Wasfy, J.H.; Packer, D.L.; Calkins, H.; Ruskin, J.N.; Mahapatra, S. The Impact of First Procedure Success Rate on the Economics of Atrial Fibrillation Ablation. JACC Clin. Electrophysiol. 2017, 3, 129–138. [Google Scholar] [CrossRef]
- Pandey, A.K.; Okaj, I.; Kaur, H.; Belley-Cote, E.P.; Wang, J.; Oraii, A.; Benz, A.P.; Johnson, L.S.B.; Young, J.; Wong, J.A.; et al. Sodium-Glucose Co-Transporter Inhibitors and Atrial Fibrillation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2021, 10, e022222. [Google Scholar] [CrossRef] [PubMed]
- Mariani, M.V.; Lavalle, C.; Palombi, M.; Pierucci, N.; Trivigno, S.; D’Amato, A.; Filomena, D.; Cipollone, P.; Laviola, D.; Piro, A.; et al. SGLT2i reduce arrhythmic events in heart failure patients with cardiac implantable electronic devices. ESC Heart Fail. 2025, 12, 2125–2133. [Google Scholar] [CrossRef] [PubMed]
- Parsi, S.; Shirsat, P.D.; Mahali, L.P.; Surani, S.; Kashyap, R. Sodium-glucose cotransporter 2 inhibitor in heart failure patients and their outcomes: A meta-analysis of randomized controlled trials. World J. Cardiol. 2025, 17, 109731. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, Y.; Wei, X.; Zhang, X.; Ye, Y.; Li, W.; Su, X. Antiarrhythmic effects and mechanisms of sodium-glucose cotransporter 2 inhibitors: A mini review. Front. Cardiovasc. Med. 2022, 9, 915455. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Y.; Wang, Z.; Liu, D.; Mao, S.; Liang, B. The effectiveness of SGLT2 inhibitor in the incidence of atrial fibrillation/atrial flutter in patients with type 2 diabetes mellitus/heart failure: A systematic review and meta-analysis. J. Thorac. Dis. 2022, 14, 1620–1637. [Google Scholar] [CrossRef]
- Abu-Qaoud, M.R.; Kumar, A.; Tarun, T.; Abraham, S.; Ahmad, J.; Khadke, S.; Husami, R.; Kulbak, G.; Sahoo, S.; Januzzi, J.L., Jr.; et al. Impact of SGLT2 Inhibitors on AF Recurrence After Catheter Ablation in Patients with Type 2 Diabetes. JACC Clin. Electrophysiol. 2023, 9, 2109–2118. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, Y.; Jiang, C.; Yang, Z.; Zhang, J.; Lai, Y.; Wang, J.; Li, S.; Peng, X.; Li, M.; et al. Impact of sodium-glucose cotransporter 2 inhibitor on recurrence and cardiovascular outcomes after catheter ablation for atrial fibrillation in patients with heart failure. Heart Rhythm 2025, 22, 935–943. [Google Scholar] [CrossRef]
- Luo, F.; Sun, L.; Wang, Z.; Zhang, Y.; Li, J.; Chen, Y.; Dong, J. Effect of Dapagliflozin on the Outcome of Radiofrequency Catheter Ablation in Patients with Type 2 Diabetes Mellitus and Atrial Fibrillation. Cardiovasc. Drugs Ther. 2024, 38, 91–98. [Google Scholar] [CrossRef]
- Noh, H.J.; Cha, S.J.; Kim, C.H.; Choi, S.-W.; Lee, C.H.; Hwang, J.K. Efficacy of dapagliflozin in improving arrhythmia-related outcomes after ablation for atrial fibrillation: A retrospective single-center study. Clin. Res. Cardiol. 2024, 113, 924–932. [Google Scholar] [CrossRef]
- Kishima, H.; Mine, T.; Fukuhara, E.; Kitagaki, R.; Asakura, M.; Ishihara, M. Efficacy of Sodium-Glucose Cotransporter 2 Inhibitors on Outcomes After Catheter Ablation for Atrial Fibrillation. JACC Clin. Electrophysiol. 2022, 8, 1393–1404. [Google Scholar] [CrossRef]
- Harada, M.; Motoike, Y.; Nomura, Y.; Nishimura, A.; Koshikawa, M.; Watanabe, E.; Ozaki, Y.; Izawa, H. Impact of sodium-glucose cotransporter 2 inhibitors on catheter ablation for atrial fibrillation in heart failure patients without type-2 diabetes. Int. J. Cardiol. 2025, 422, 132954. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-T.; Wo, H.-T.; Chang, P.-C.; Lee, H.-L.; Wen, M.-S.; Chou, C.-C. Long-term efficacy of sodium-glucose cotransporter 2 inhibitor therapy in preventing atrial fibrillation recurrence after catheter ablation in type 2 diabetes mellitus patients. Heliyon 2023, 9, e16835. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Guan, X.; Liu, X.; Liu, L.; Liu, Z.; Zhang, J. Relationship between sodium-glucose cotransporter 2 inhibitors and atrial fibrillation recurrence after pulmonary vein isolation in patients with type 2 diabetes and persistent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2024, 35, 1799–1805. [Google Scholar] [CrossRef] [PubMed]
- Hakgor, A.; Olgun, F.E.; Dursun, A.; Kahraman, B.C.; Akhundova, A.; Savur, U.; Besiroglu, M.; Kenger, M.Z.; Dervis, E.; Sengor, B.G.; et al. Sodium Glucose Cotransporter 2 Inhibitors Improve Long-term Atrial Fibrillation-free Survival After Catheter Ablation. J. Cardiovasc. Pharmacol. 2025, 85, 225–232. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Menichelli, D.; Pignatelli, P.; Brogi, T.; Pannunzio, A.; Violi, F.; Lip, G.Y.H.; Pastori, D.; Group, A.-A.S. Incidence of All-Cause, Cardiovascular, and Atrial Fibrillation-Related Hospitalizations: Focus on Antiarrhythmic Drugs. JACC Adv. 2024, 3, 101117. [Google Scholar] [CrossRef]
- Morillo, C.A.; Verma, A.; Connolly, S.J.; Kuck, K.H.; Nair, G.M.; Champagne, J.; Sterns, L.D.; Beresh, H.; Healey, J.S.; Natale, A.; et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): A randomized trial. JAMA 2014, 311, 692–700. [Google Scholar] [CrossRef]
- Leong-Sit, P.; Roux, J.F.; Zado, E.; Callans, D.J.; Garcia, F.; Lin, D.; Marchlinski, F.E.; Bala, R.; Dixit, S.; Riley, M.; et al. Antiarrhythmics after ablation of atrial fibrillation (5A Study): Six-month follow-up study. Circ. Arrhythm. Electrophysiol. 2011, 4, 11–14. [Google Scholar] [CrossRef]
- Xu, B.; Peng, F.; Tang, W.; Du, Y.; Guo, H. Short-term Antiarrhythmic Drugs After Catheter Ablation for Atrial Fibrillation: A Meta-analysis of Randomized Controlled Trials. Ann. Pharmacother. 2016, 50, 697–705. [Google Scholar] [CrossRef]
- Steinberg, B.A.; Kim, S.; Fonarow, G.C.; Thomas, L.; Ansell, J.; Kowey, P.R.; Mahaffey, K.W.; Gersh, B.J.; Hylek, E.; Naccarelli, G.; et al. Drivers of hospitalization for patients with atrial fibrillation: Results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Am. Heart J. 2014, 167, 735–742.e2. [Google Scholar] [CrossRef]
- Aggarwal, R.; Vaduganathan, M.; Chiu, N.; Bhatt, D.L. Out-of-Pocket Costs for SGLT-2 (Sodium-Glucose Transport Protein-2) Inhibitors in the United States. Circ. Heart Fail. 2022, 15, e009099. [Google Scholar] [CrossRef] [PubMed]
- Zelniker, T.A.; Braunwald, E. Cardiac and Renal Effects of Sodium-Glucose Co-Transporter 2 Inhibitors in Diabetes. J. Am. Coll. Cardiol. 2018, 72, 1845–1855. [Google Scholar] [CrossRef] [PubMed]
- Fathi, A.; Vickneson, K.; Singh, J.S. SGLT2-inhibitors; more than just glycosuria and diuresis. Heart Fail. Rev. 2021, 26, 623–642. [Google Scholar] [CrossRef] [PubMed]
- Neeland, I.J.; Eliasson, B.; Kasai, T.; Marx, N.; Zinman, B.; Inzucchi, S.E.; Wanner, C.; Zwiener, I.; Wojeck, B.S.; Yaggi, H.K.; et al. The Impact of Empagliflozin on Obstructive Sleep Apnea and Cardiovascular and Renal Outcomes: An Exploratory Analysis of the EMPA-REG OUTCOME Trial. Diabetes Care 2020, 43, 3007–3015. [Google Scholar] [CrossRef]
- Koutentakis, M.; Kuciński, J.; Świeczkowski, D.; Surma, S.; Filipiak, K.J.; Gąsecka, A. The Ketogenic Effect of SGLT-2 Inhibitors—Beneficial or Harmful? J. Cardiovasc. Dev. Dis. 2023, 10, 465. [Google Scholar] [CrossRef]
- Gao, J.; Xue, G.; Zhan, G.; Wang, X.; Li, J.; Yang, X.; Xia, Y. Benefits of SGLT2 inhibitors in arrhythmias. Front. Cardiovasc. Med. 2022, 9, 1011429. [Google Scholar] [CrossRef]
- Kaplan, A.D.; Joca, H.C.; Boyman, L.; Greiser, M. Calcium Signaling Silencing in Atrial Fibrillation: Implications for Atrial Sodium Homeostasis. Int. J. Mol. Sci. 2021, 22, 10513. [Google Scholar] [CrossRef]
- Schönberger, E.; Mihaljević, V.; Steiner, K.; Šarić, S.; Kurevija, T.; Majnarić, L.T.; Bilić Ćurčić, I.; Canecki-Varžić, S. Immunomodulatory Effects of SGLT2 Inhibitors—Targeting Inflammation and Oxidative Stress in Aging. Int. J. Environ. Res. Public Health 2023, 20, 6671. [Google Scholar] [CrossRef]
- Abdelhadi, N.A.; Ragab, K.M.; Elkholy, M.; Koneru, J.; Ellenbogen, K.A.; Pillai, A. Impact of Sodium-Glucose Co-Transporter 2 Inhibitors on Atrial Fibrillation Recurrence Post-Catheter Ablation Among Patients With Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. J. Cardiovasc. Electrophysiol. 2025, 36, 673–682. [Google Scholar] [CrossRef]
- Parsi, S.; Bansal, V.; Rosamystica, M.; Shirsat, P.; Kashyap, R. Impact of Sodium-Glucose Co-Transporter 2 Inhibitors on Atrial Fibrillation Recurrence Post-Catheter Ablation Among Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. J. Cardiovasc. Electrophysiol. 2025. early view. [Google Scholar] [CrossRef]
- Eckstein, J.; Verheule, S.; De Groot, N.; Allessie, M.; Schotten, U. Mechanisms of perpetuation of atrial fibrillation in chronically dilated atria. Prog. Biophys. Mol. Biol. 2008, 97, 435–451. [Google Scholar] [CrossRef]
- Pappone, C.; Rosanio, S.; Augello, G.; Gallus, G.; Vicedomini, G.; Mazzone, P.; Gulletta, S.; Gugliotta, F.; Pappone, A.; Santinelli, V.; et al. Mortality, morbidity, and quality of life after circumferential pulmonary vein ablation for atrial fibrillation: Outcomes from a controlled nonrandomized long-term study. J. Am. Coll. Cardiol. 2003, 42, 185–197. [Google Scholar] [CrossRef]
- Modzelewski, K.L.; Pipilas, A.; Bosch, N.A. Comparative Outcomes of Empagliflozin to Dapagliflozin in Patients With Heart Failure. JAMA Netw. Open 2024, 7, e249305. [Google Scholar] [CrossRef]
| Study | Site | Study Design | Total Sample Size | Study Period | Inclusion Criteria | Blanking Period | AF Recurrence Is Defined as | Follow-Up Schedule |
|---|---|---|---|---|---|---|---|---|
| Abu-Qaoud et al. (2023) [16] | USA | Retrospective cohort study | 4450 | 1 April 2014 to 30 November 2021 | Subjects who were >= 18 years of age, History of T2DM who had undergone AF ablation. Propensity score matching | 3 months | Subject presented for cardioversion, new AAD class I or III, and re-do ablation in 12-month follow-up. | Subject presented for cardioversion, new AAD class I or III, and re-do ablation in 3 to 12 months of follow-up after ablation. |
| Hakgor et al. (2024) [24] | Turkey | Retrospective cohort study | 614 | 2014 to 2021 | Subjects who received catheter ablation for symptomatic AF. | 3 months | AF lasting more than 30 s, either on ECG at follow-ups when symptomatic or 48 h Holter monitoring biannually. | Subjects had an ECG at 1, 3, 6, 12, 18, and 24 months. Also, had an ECG on the same day anytime when subjects had symptoms. Additionally, 48 h ambulatory Holter monitor was done biannually. |
| Harada et al. (2025) [21] | Japan | Prospective, RCT | 102 | June 2022 to November 2023 | Subjects with persistent AF, symptoms/history/findings that suspect HF, and NT-proBNP >=400 pg/mL. | 3 months | Any atrial tachy-arrhythmia lasting >30 s on 24-h Holter ECG monitoring at 6-month follow-up and 7-day Holter ECG monitoring at 12 months follow-up. | Subjects had 12-lead ESG at the cardiologist’s office at 1, 3, 6, 9, and 12 months after ablation. 24-h Holter monitoring was done at 6 months, and 7-day Holter monitoring was done at 12-month follow-up. |
| Kishima et al. (2022) [20] | Japan | Prospective, RCT | 70 | April 2017 and March 2020 | Subjects with age >20 and <80 years, T2DM with non-valvular AF who underwent catheter ablation, and all subjects on oral anti-coagulant, duration of AF <1 year, not taking SGLT2i and/or DPP4i for >2 weeks before enrollment. | 3 months | Episode of atrial tachyarrhythmia lasting for >30 s on Holter monitor. | Subjects followed up at the hospital at 1, 3, 6, 9, and 12 months after the CA or were referred to the emergency department for arrhythmia symptoms in between scheduled visits. |
| Liu et al. (2023) [22] | Taiwan | Retrospective cohort study | 122 | Jan 2016 and December 2021 | Subjects with T2DM undergoing CA for anti-arrhythmic refractory AF. | 3 months | Atrial tachyarrhythmia lasting longer than 30 s on a 12-lead ECG, Holter monitoring, or pacemaker/implantable cardioverter-defibrillator interrogation | Subjects had a 12-lead ECG and a 24-h Holter monitor at follow-ups at 1 week, 1 month, 3 months, 6 months, and every 3 to 6 months after CA or when subjects had symptoms. |
| Luo et al. (2022) [18] | China | Retrospective cohort study | 326 | January 2019 to February 2021 | Subjects with T2DM and drug-refractory AF underwent radiofrequency CA | 1 month | AF, atrial flutter or atrial tachycardia lasting for 30 s recorded on ECG or by 24-h Holter monitoring done at follow-ups or symptoms onset. | Subjects received outpatient or telephone follow-up at 1, 3, 6, 9, and 12 months, then every 6 months after ablation. Subjects had an ECG and a 24-h Holter monitor at each visit, and also when subjects had symptoms. |
| Noh et al. (2024) [19] | Korea | Retrospective cohort study | 272 | January 2018 to December 2022 | Subjects included in this study underwent catheter ablation for symptomatic drug-refractory non-valvular AF. | 3 months | AF, atrial tachycardia, or atypical atrial flutter lasting more than 30 s on a Holter monitor is done at follow-ups. | Subjects’ follow-ups are scheduled at 4 weeks, 3 months, 6 months, and every 3 to 4 months after that. Subjects on Holter monitor at 3 and 6 months and every 12 months after that. |
| Qi et al. (2024) [23] | China | Retrospective cohort study | 182 | December 2021 to January 2023 | Subjects with age >= 18 years old, T2DM, non-valvular AF, 1st radiofrequency CA. | 3 months | Any atrial tachyarrhythmia lasting for more than 30 s on ECG or Holter monitor during follow-up. | Subjects had 12-lead ECGs in the clinic at 1, 3, 6, and 12 months. Also, 24-h Holter monitoring every 6 months. Additionally, further Holter monitoring is done in the event of arrhythmic symptoms. |
| Zhao et al. (2024) [17] | China | Retrospective cohort study. | 736 | January 2017 to December 2022 | Subjects with heart failure undergoing initial catheter ablation for AF. Propensity score matching. | 3 months | Atrial tachyarrhythmia lasting more than 30 s on a 12-lead ECG or 24-h Holter monitoring. | Subjects followed up at clinics or by phone interview at the 3rd month, 6th month, and every 6 months thereafter. |
| Study | Abu-Qaoud et al. (2023) [16] | Hakgor et al. (2024) [24] | Harada et al. (2025) [21] | Kishima et al. (2022) [20] | Liu et al. (2023) [22] | Luo et al. (2022) [18] | Noh et al. (2024) [19] | Qi et al. (2024) [23] | Zhao et al. (2024) [17] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | SGLT2i | Non-SGLT2i | SGLT2i | Non-SGLT2i | SGLT2i | Non-SGLT2i | SGLT2i | Non-SGLT2i | SGLT2i | Non-SGLT2i | SGLT2i | Non-SGLT2i | SGLT2i | Non-SGLT2i | SGLT2i | Non-SGLT2i | SGLT2i | Non-SGLT2i |
| Type of SGLT2i | Empagliflozin | Empaglifozin | Tofogliflozin | Anagliptin | Dapagliflozin | Dapagliflozin | ||||||||||||
| Dapagliflozin | Dapagliflozin | |||||||||||||||||
| No of Participants in each Group | 2225 | 2225 | 211 | 403 | 51 | 51 | 38 | 32 | 45 | 77 | 79 | 247 | 73 | 199 | 91 | 91 | 368 | 368 |
| Age (years) Mean (SD) | 65 | 65 | 59.8 | 57.1 | 70.2 | 72 | 70.3 | 70.3 | 60.1 | 63.2 | 63.4 | 63.8 | 73.47 | 71.72 | 70.9 | 67.3 | 63.5 | 62.7 |
| Male (n) | 1648 | 1642 | 129 | 226 | 33 | 38 | 26 | 22 | 35 | 54 | 48 | 144 | 61 | 175 | 52 | 48 | 242 | 239 |
| BMI (kg/m2) Mean (SD) | 27.5 (5.2) | 26.3 (4.9) | 25 (5.3) | 24 (4.1) | 25.5 (4.6) | 25.3 (4.3) | 28.6 (4.2) | 27.3 (3.9) | 25.9 (3.7) | 26.7 (3.5) | 25.16 (3.64) | 23.73 (5.45) | 26.1 (0.4) | 26.5 (0.3) | 26.4 (3.8) | 26.4 (4.2) | ||
| Paroxysmal (n) | 124 | 291 | 16 | 14 | 32 | 54 | 43 | 107 | ||||||||||
| Persistent (n) | 51 | 51 | 13 | 23 | 35 | 119 | 30 | 92 | 91 | 91 | 279 | 291 | ||||||
| AF duration (years) Mean (SD) | 6.4 (11.4) | 6.7 (5.6) | 3.09 (3.1) | 3.67 (3.73) | 2.19 (0.38) | 3.07 (0.46) | ||||||||||||
| Hemoglobin A1C (%) Mean (SD) | 7.5 (2.5) | 6.2 (1.9) | 5.9 (0.4) | 6 (0.4) | 6.7 (0.6) | 6.8 (0.9) | 6.8 (0.7) | 6.6 (0.8) | 7.7 (1.4) | 7.3 (1.2) | 7.4 (0.1) | 7.2 (0.1) | ||||||
| Creatinine (mg/dL) Mean (SD) | 1.2 (2.9) | 1.3 (2.2) | 1 (0.2) | 0.9 (0.2) | 0.9 (0.2) | 0.9 (0.3) | 0.83 (0.26) | 0.75 (0.2) | 1.01 (0.32) | 0.97 (0.33) | 1.25 (1.16) | 1.1 (0.82) | ||||||
| eGFR (mL/min) Mean (SD) | 81.1 (24.3) | 85.2 (25.1) | 71 (26.7) | 70.8 (29.5) | 70.7 (22.8) | 76.1 (21.1) | 83.3 (24.1) | 85.4 (29.3) | 84.5 (17.3) | 85 (18) | 70.6 (23.02) | 76.65 (25.08) | 99 (28.6) | 103.1 (27.7) | ||||
| CHA2DS2 VASc Score Mean (SD) | 3.1 (1.4) | 1.9 (1.3) | 3.4 (1.6) | 3.3 (1.3) | 2.6 (1.1) | 2.7 (1.3) | 2.8 (1.2) | 2.9 (1.3) | 3.4 (1.4) | 3.5 (1.4) | ||||||||
| LVEF (%) Mean (SD) | 52.3 (13.5) | 60.5 (8.2) | 52.5 (11.8) | 55.1 (7.4) | 61.5 (11.3) | 58 (15.4) | 63.8 (11.7) | 63.8 (10.7) | 60.4 (6.7) | 60 (7.2) | 57.23 (7.26) | 59.68 (6.18) | 63.4 (0.7) | 63.6 (0.6) | 51.4 (10) | 51.4 (11.6) | ||
| LAD (mm) Mean (SD) | 41.6 (7.1) | 40.5 (7.1) | 42.5 (5.9) | 42.9 (5.5) | 45.6 (7.5) | 44.7 (5) | 44.2 (5.7) | 43.5 (6.4) | 40.2 (6.4) | 41 (6.5) | 43.94 (5.45) | 45.35 (5.3) | 41.9 (0.5) | 40.8 (0.4) | 45.8 (7.7) | 45.1 (5.7) | ||
| HTN (n) | 2062 | 2067 | 153 | 276 | 27 | 27 | 26 | 19 | 36 | 57 | 53 | 157 | 50 | 141 | 69 | 79 | 258 | 251 |
| T2DM (n) | 2225 | 2225 | 110 | 38 | 0 | 0 | 38 | 32 | 45 | 77 | 79 | 247 | 21 | 68 | 91 | 91 | 182 | 198 |
| HF (n) | 1316 | 1302 | 77 | 36 | 51 | 51 | 10 | 9 | 6 | 7 | 3 | 5 | 368 | 368 | ||||
| COPD (n) | 26 | 44 | 0 | 2 | 13 | 23 | ||||||||||||
| CVA (n) | 21 | 25 | 5 | 6 | 6 | 9 | 0 | 9 | 9 | 31 | 53 | 49 | ||||||
| CAD (n) | 1480 | 1473 | 98 | 114 | 2 | 2 | 10 | 9 | 31 | 102 | 3 | 33 | 49 | 61 | ||||
| Smoking (n) | 70 | 132 | 6 | 9 | 25 | 53 | ||||||||||||
| Renal disease (n) | 562 | 577 | 2 | 2 | 15 | 25 | ||||||||||||
| HLD (n) | 1892 | 1907 | 26 | 19 | 26 | 47 | 21 | 50 | ||||||||||
| Thyroid disease (n) | 677 | 666 | 7 | 9 | ||||||||||||||
| AAD (n) | 2029 | 2008 | 71 | 112 | 5 | 2 | 4 | 6 | 27 | 63 | 17 | 58 | 73 | 198 | 0 | 0 | ||
| CLASS IC (n) | 14 | 32 | 7 | 14 | 0 | 17 | ||||||||||||
| CLASS III (n) | 1 | 0 | 13 | 31 | 10 | 44 | 73 | 178 | ||||||||||
| Beta-blocker (n) | 2107 | 2110 | 158 | 241 | 44 | 38 | 21 | 15 | 32 | 47 | 32 | 87 | 3 | 26 | 29 | 30 | 197 | 178 |
| Metformin (n) | 1597 | 1642 | 8 | 5 | 36 | 54 | 42 | 139 | 51 | 46 | ||||||||
| Insulin (n) | 1635 | 1604 | 51 | 14 | 1 | 0 | 1 | 5 | 7 | 39 | 16 | 11 | ||||||
| Diuretics (n) | 1824 | 1832 | 54 | 27 | 25 | 27 | 6 | 7 | 12 | 6 | 0 | 9 | 21 | 60 | 6 | 11 | 262 | 286 |
| GLP1 (n) | 1 | 3 | 1 | 2 | ||||||||||||||
| DPP4i (n) | 0 | 32 | 12 | 38 | 7 | 20 | ||||||||||||
| Cryo-ablation PVI (n) | 184 | 361 | 10 | 7 | 8 | 10 | 45 | 77 | 42 | 56 | ||||||||
| CTI ablation (n) | 30 | 20 | 36 | 60 | 19 | 99 | ||||||||||||
| SVCI (n) | 8 | 4 | 14 | 43 | 5 | 9 | ||||||||||||
| Linear ablation (n) | 19 | 32 | 60 | 174 | 279 | 291 | ||||||||||||
| RFCA PVI (n) | 27 | 42 | 41 | 44 | 30 | 22 | 79 | 247 | 31 | 143 | 91 | 91 | 368 | 368 | ||||
| Criteria | Abu-Qaoud et al. | Liu et al. | Luo et al. | Qi et al. | Hakgor et al. | Noh et al. | Zhao et al. |
|---|---|---|---|---|---|---|---|
| Was the research question or objective in this paper clearly stated? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Was the study population clearly specified and defined? | Yes | Yes | Yes | Yes | Yes | yes | Yes |
| Was the participation rate of eligible persons at least 50%? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Were all the subjects selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion criteria for being in the study prespecified and applied uniformly to all participants? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Was a sample size justification, power description, or variance and effect estimates provided? | No | No | No | No | No | No | No |
| For the analyses in this paper, were the exposure(s) of interest measured prior to the outcome(s) being measured? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Was the timeframe sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed? | Yes | Yes | yes | Yes | Yes | Yes | Yes |
| For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (e.g., categories of exposure, or exposure measured as continuous variable)? | No | No | No | Yes | No | No | No |
| Were the exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Was the exposure(s) assessed more than once over time? | NA | NR | NR | Yes | NR | NR | NR |
| Were the outcome measures (dependent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Were the outcome assessors blinded to the exposure status of participants? | No | No | No | No | No | No | No |
| Was loss to follow-up after baseline 20% or less? | CD | Yes | Yes | Yes | Yes | Yes | Yes |
| Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)? | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Domain | Signaling Question | Kishima et al. | Harada et al. |
|---|---|---|---|
| Bias arising from the randomization process | 1.1 Was the allocation sequence random? | Y | PN |
| 1.2 Was the allocation sequence concealed until participants were enrolled and assigned to interventions? | N | NI | |
| 1.3 Did baseline differences between intervention groups suggest a problem with the randomization process? | N | PY | |
| Risk of bias judgement | High | High | |
| Bias due to deviations from intended interventions | 2.1.Were participants aware of their assigned intervention during the trial? | NI | NI |
| 2.2.Were carers and people delivering the interventions aware of participants’ assigned intervention during the trial? | Y | Y | |
| 2.3. If Y/PY/NI to 2.1 or 2.2: Were there deviations from the intended intervention that arose because of the experimental context? | NI | NI | |
| 2.4 If Y/PY to 2.3: Were these deviations likely to have affected the outcome? | NA | NA | |
| 2.5. If Y/PY/NI to 2.4: Were these deviations from intended intervention balanced between groups? | NA | NA | |
| 2.6 Was an appropriate analysis used to estimate the effect of assignment to intervention? | Y | NI | |
| 2.7 If N/PN/NI to 2.6: Was there potential for a substantial impact (on the result) of the failure to analyse participants in the group to which they were randomized? | NA | NI | |
| Risk of bias judgement | Some concerns | High | |
| Bias due to missing outcome data | 3.1 Were data for this outcome available for all, or nearly all, participants randomized? | Y | Y |
| 3.2 If N/PN/NI to 3.1: Is there evidence that result was not biased by missing outcome data? | NA | NA | |
| 3.3 If N/PN to 3.2: Could missingness in the outcome depend on its true value? | NA | NA | |
| 3.4 If Y/PY/NI to 3.3: Is it likely that missingness in the outcome depended on its true value? | NA | NA | |
| Risk of bias judgement | Low | Low | |
| Bias in measurement of the outcome | 4.1 Was the method of measuring the outcome inappropriate? | N | N |
| 4.2 Could measurement or ascertainment of the outcome have differed between intervention groups? | N | N | |
| 4.3 Were outcome assessors aware of the intervention received by study participants? | N | NI | |
| 4.4 If Y/PY/NI to 4.3: Could assessment of the outcome have been influenced by knowledge of intervention received? | NA | PN | |
| 4.5 If Y/PY/NI to 4.4: Is it likely that assessment of the outcome was influenced by knowledge of intervention received? | NA | NA | |
| Risk of bias judgement | Low | Low | |
| Bias in selection of the reported result | 5.1 Were the data that produced this result analysed in accordance with a pre-specified analysis plan that was finalized before unblinded outcome data were available for analysis? | Y | NI |
| 5.2 … multiple eligible outcome measurements (e.g., scales, definitions, time points) within the outcome domain? | Y | Y | |
| 5.3 … multiple eligible analyses of the data? | Y | Y | |
| Risk of bias judgement | High | High | |
| Overall bias | Risk of bias judgement | High | High |
| Covariate | Coefficient | Lower CI | Upper CI | SE | p-Value |
|---|---|---|---|---|---|
| Age | −0.007 | −0.066 | 0.052 | 0.030 | 0.812 |
| Male Proportion | 0.000 | −0.000 | 0.000 | <0.001 | 0.278 |
| BMI | −0.098 | −0.466 | 0.269 | 0.188 | 0.600 |
| LVEF (%) | −0.045 | −0.117 | 0.026 | 0.036 | 0.213 |
| LAD (mm) | 0.176 | 0.049 | 0.304 | 0.065 | 0.007 |
| Hypertension | 0.000 | −0.000 | 0.000 | <0.001 | 0.293 |
| Diabetes mellitus | 0.000 | −0.000 | 0.000 | <0.001 | 0.319 |
| Heart Failure | 0.000 | −0.000 | 0.000 | <0.001 | 0.149 |
| CAD | 0.000 | −0.000 | 0.001 | <0.001 | 0.789 |
| History of Antiarrhythmic Use | 0.000 | −0.000 | 0.000 | <0.001 | 0.54 |
| History of Beta Blocker Use | 0.000 | −0.000 | 0.000 | <0.001 | 0.319 |
| Outcome № of Participants (Studies) | Relative Effect (95% CI) | Anticipated Absolute Effects (95% CI) | Certainty | What Happens | ||
|---|---|---|---|---|---|---|
| Without SGLT2i | With SGLT2i | Difference | ||||
| AF recurrence by the final follow-up post-ablation № of participants: 6874 (9 non-randomised studies) | OR 0.62 (0.45 to 0.85) | 47.4% | 35.8% (28.8 to 43.3) | 11.6% fewer (18.5 fewer to 4 fewer) | ⨁◯◯◯ Very low | Very low certainty due to the majority of studies included are observational studies with a serious risk of bias and inconsistency in results across studies. |
| AF recurrence by the first follow-up within 12 to 24 months post-ablation № of participants: 6874 (9 non-randomised studies) | OR 0.58 (0.48 to 0.69) | 33.7% | 22.8% (19.6 to 26) | 10.9% fewer (14.1 fewer to 7.7 fewer) | ⨁◯◯◯ Very low | Very low certainty due to the majority of studies included are observational studies with a serious risk of bias, and publication bias is strongly suspected. |
| AF recurrences by the 6-month follow-up post-ablation № of participants: 2120 (6 non-randomised studies) | OR 0.53 (0.32 to 0.86) | 15.9% | 9.1% (5.7 to 14) | 6.8% fewer (10.2 fewer to 1.9 fewer) | ⨁◯◯◯ Very low | Very low certainty due to the majority of studies included are observational studies with a serious risk of bias, and publication bias is strongly suspected. |
| AF recurrences by the 12-month follow-up post-ablation № of participants: 6752 (8 non-randomised studies) | OR 0.56 (0.47 to 0.68) | 33.7% | 22.2% (19.3 to 25.7) | 11.6% fewer (14.4 fewer to 8 fewer) | ⨁◯◯◯ Very low | Very low certainty due to the majority of studies included are observational studies with a serious risk of bias, and publication bias is strongly suspected. |
| AF recurrences by the 18-month follow-up post-ablation № of participants: 1948 (4 non-randomised studies) | OR 0.55 (0.38 to 0.79) | 48.5% | 34.1% (26.3 to 42.6) | 14.4% fewer (22.1 fewer to 5.8 fewer) | ⨁◯◯◯ Very low | Very low certainty due to the majority of studies included are observational studies with a serious risk of bias. |
| AF recurrences by the 24-month follow-up post-ablation № of participants: 1798 (4 non-randomised studies) | OR 0.60 (0.28 to 1.28) | 54.7% | 42.0% (25.3 to 60.7) | 12.7% fewer (29.4 fewer to 6 more) | ⨁◯◯◯ Very low | Very low certainty due to the majority of studies included are observational studies with a serious risk of bias and inconsistency in results across studies. |
| AF recurrences by the 36 to 42-month follow-up post-ablation № of participants: 1184 (3 non-randomised studies) | OR 1.41 (1.29 to 1.56) | 87.9% | 91.1% (90.3 to 91.9) | 3.2% more (2.5 more to 4 more) | ⨁◯◯◯ Very low | Very low certainty due to the majority of studies included are observational studies with a serious risk of bias. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parsi, S.; Sonavane, K.; Ravi, U.; Shirsat, P.D.; Chamarthi, V.S.; Gabr, M.; Ponnam, H.C.; Surani, S.; Bansal, V.; Kashyap, R. Effects of Sodium Glucose Co-Transporter 2 Inhibitors on Atrial Fibrillation Recurrences After Catheter Ablation in Atrial Fibrillation Patients: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 8001. https://doi.org/10.3390/jcm14228001
Parsi S, Sonavane K, Ravi U, Shirsat PD, Chamarthi VS, Gabr M, Ponnam HC, Surani S, Bansal V, Kashyap R. Effects of Sodium Glucose Co-Transporter 2 Inhibitors on Atrial Fibrillation Recurrences After Catheter Ablation in Atrial Fibrillation Patients: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(22):8001. https://doi.org/10.3390/jcm14228001
Chicago/Turabian StyleParsi, Saketh, Kunal Sonavane, Usha Ravi, Pallavi D. Shirsat, Venkata S. Chamarthi, Mohamed Gabr, Harikrishna Choudary Ponnam, Salim Surani, Vikas Bansal, and Rahul Kashyap. 2025. "Effects of Sodium Glucose Co-Transporter 2 Inhibitors on Atrial Fibrillation Recurrences After Catheter Ablation in Atrial Fibrillation Patients: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 22: 8001. https://doi.org/10.3390/jcm14228001
APA StyleParsi, S., Sonavane, K., Ravi, U., Shirsat, P. D., Chamarthi, V. S., Gabr, M., Ponnam, H. C., Surani, S., Bansal, V., & Kashyap, R. (2025). Effects of Sodium Glucose Co-Transporter 2 Inhibitors on Atrial Fibrillation Recurrences After Catheter Ablation in Atrial Fibrillation Patients: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(22), 8001. https://doi.org/10.3390/jcm14228001









