Learning Curve of Robotic Pancreaticoduodenectomy with Portal–Superior Mesenteric Vein Resection for Pancreatic Cancers
Abstract
1. Introduction
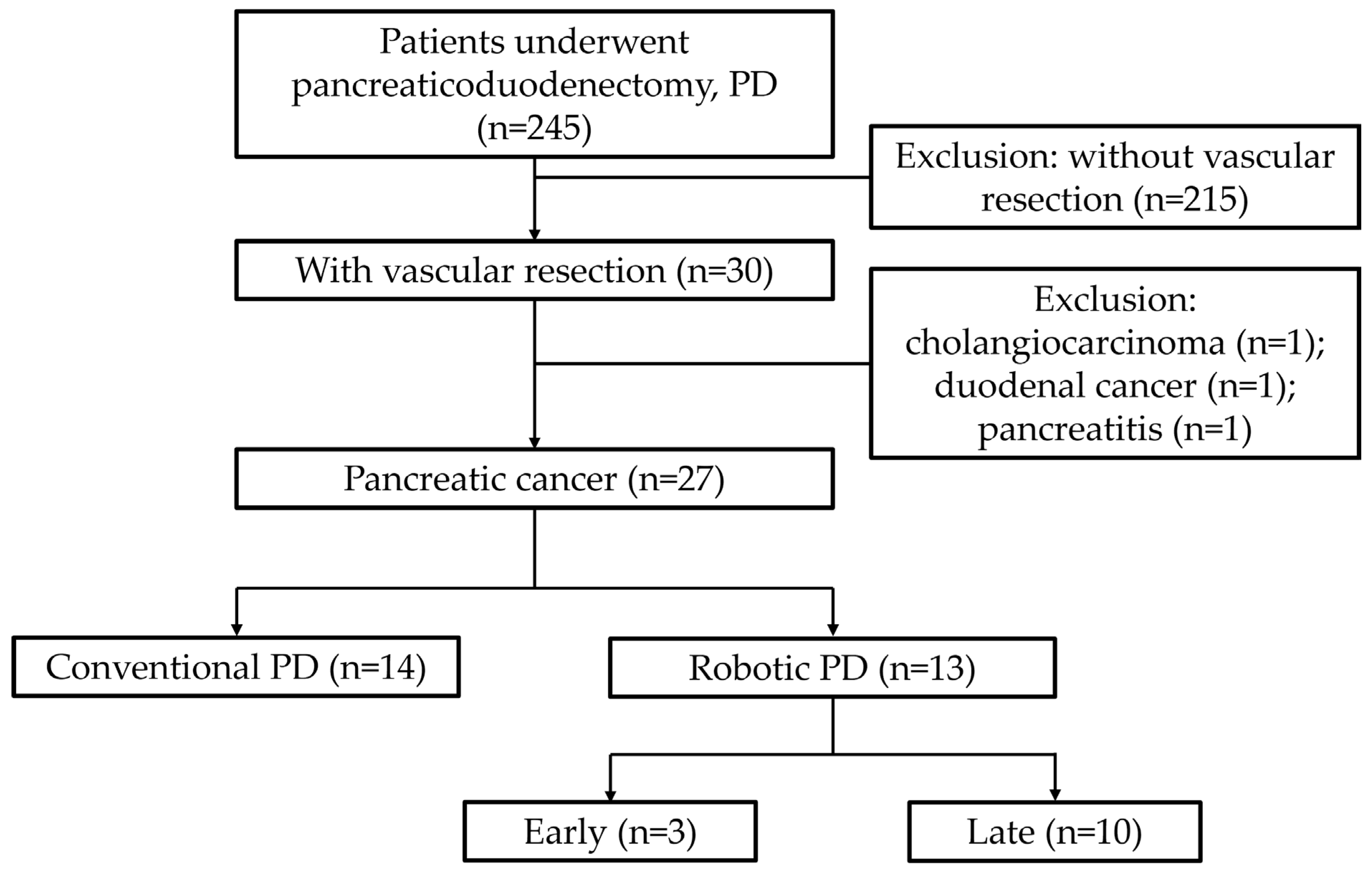
2. Materials and Methods
3. Results
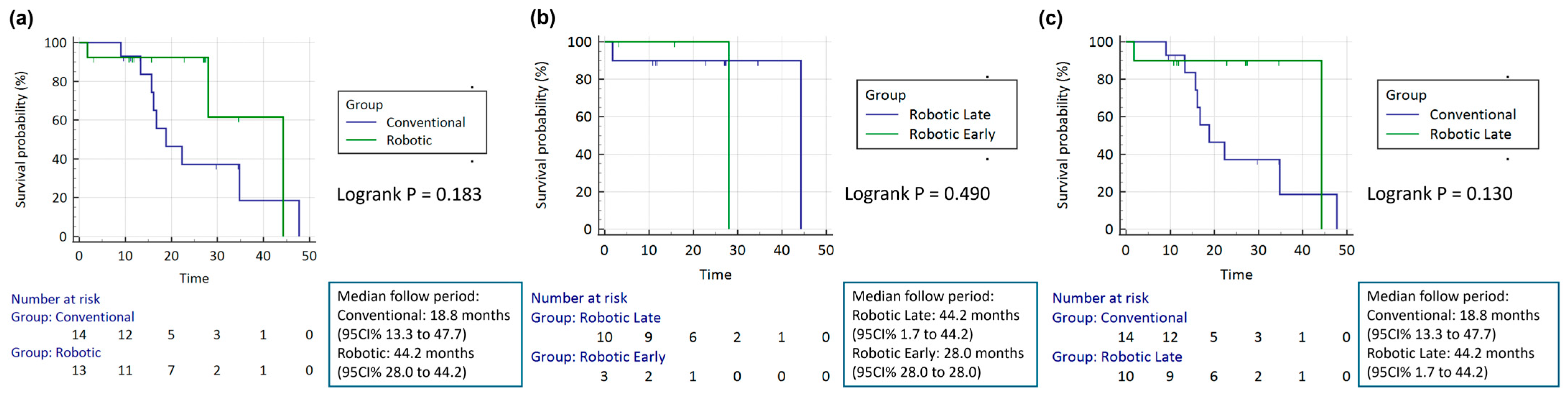
3.1. Conventional Versus Robotic
3.2. Early Stage Robotic Versus Late-Stage Robot
3.3. Conventional Versus Late-Stage Robot
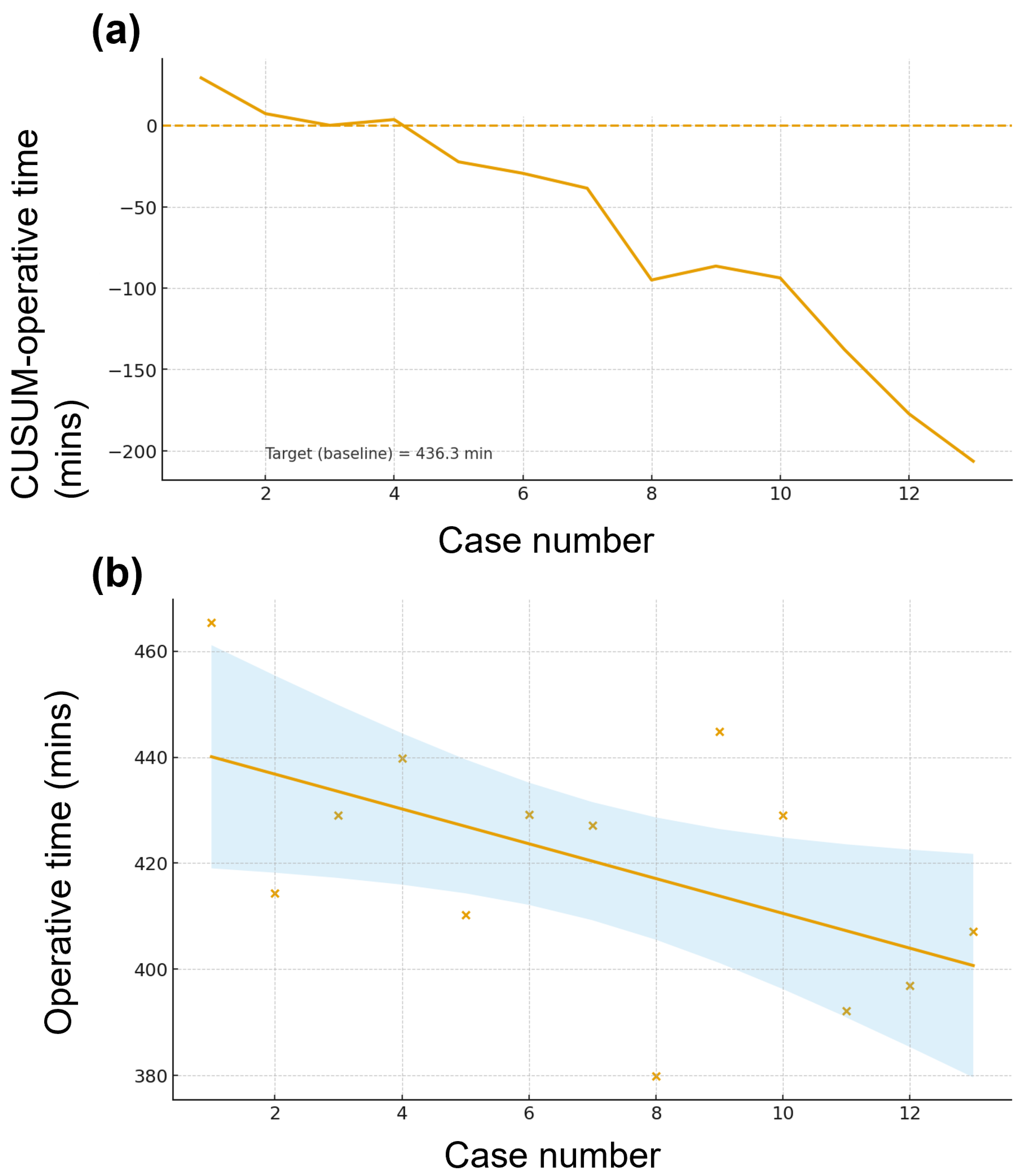
3.4. Learning Curve for Robotic PD
3.5. Comparison Between Different Vein Reconstructions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PD | Pancreaticoduodenectomy |
| PV-SMV | Portal–superior mesenteric vein |
| RPD | Robotic pancreaticoduodenectomy |
| OS | Overall survival |
| MIPD | Minimally invasive pancreatoduodenectomy |
| ASA | American Society of Anesthesiologists |
| BMI | Body mass index |
| POPF | Postoperative pancreatic fistula |
| DGE | Delayed gastric emptying |
| IQR | Interquartile range |
| LOS | Length of stay |
| LN | Lymph node |
| RPD-VR | RPD with vascular resection |
References
- Balaban, E.P.; Mangu, P.B.; Yee, N.S. Locally advanced unresectable pancreatic cancer: American Society of Clinical Oncology Clinical Practice Guideline summary. J. Oncol. Pract. 2017, 13, 265–269. [Google Scholar] [CrossRef]
- Chang, J.S.; Chen, L.T.; Shan, Y.S.; Chu, P.Y.; Tsai, C.R.; Tsai, H.J. The incidence and survival of pancreatic cancer by histology, including rare subtypes: A nation-wide cancer registry-based study from Taiwan. Cancer Med. 2018, 7, 5775–5788. [Google Scholar] [CrossRef] [PubMed]
- Talbot, A.; Talbot, T.; Shaughnessy, E.; Glass, A.; Das, A.; Watanabe, Y.; Cheng, D.; Johansson, M.; Rao, S.; Yusoff, I.; et al. Overall survival and treatment modalities in pancreatic adenocarcinoma: A retrospective analysis of a single centre in Western Australia. J. Gastrointest. Oncol. 2023, 14, 2221–2228. [Google Scholar] [CrossRef]
- Mondo, E.L.; Noel, M.S.; Katz, A.W.; Schoeniger, L.O.; Hezel, A.F. Unresectable locally advanced pancreatic cancer: Treatment with neoadjuvant leucovorin, fluorouracil, irinotecan, and oxaliplatin and assessment of surgical resectability. J. Clin. Oncol. 2013, 31, e37–e39. [Google Scholar] [CrossRef]
- Schäfer, M.; Müllhaupt, B.; Clavien, P.A. Evidence-based pancreatic head resection for pancreatic cancer and chronic pancreatitis. Ann. Surg. 2002, 236, 137–148. [Google Scholar] [CrossRef]
- Moore, G.E.; Sako, Y.; Thomas, L.B. Radical pancreatoduodenectomy with resection and reanastomosis of the superior mesenteric vein. Surgery 1951, 30, 550–553. [Google Scholar] [PubMed]
- Wang, X.; Demir, I.E.; Schorn, S.; Jäger, C.; Scheufele, F.; Friess, H.; Ceyhan, G.O. Venous resection during pancreatectomy for pancreatic cancer: A systematic review. Transl. Gastroenterol. Hepatol. 2019, 4, 46. [Google Scholar] [CrossRef]
- Christians, K.K.; Lal, A.; Pappas, S.; Quebbeman, E.; Evans, D.B. Portal vein resection. Surg. Clin. N. Am. 2010, 90, 309–322. [Google Scholar] [CrossRef]
- Javed, A.A.; Wright, M.J.; Siddique, A.; Blair, A.B.; Ding, D.; Burkhart, R.A.; Makary, M.; Cameron, J.L.; Narang, A.; Herman, J.; et al. Outcome of patients with borderline resectable pancreatic cancer in the contemporary era of neoadjuvant chemotherapy. J. Gastrointest. Surg. 2019, 23, 112–121. [Google Scholar] [CrossRef]
- Yu, X.Z.; Li, J.; Fu, D.L.; Di, Y.; Yang, F.; Hao, S.J.; Jin, C. Benefit from synchronous portal-superior mesenteric vein resection during pancreaticoduodenectomy for cancer: A meta-analysis. Eur. J. Surg. Oncol. 2014, 40, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Dopazo, C.; Sapisochin, G.; Beisani, M.; Blanco, L.; Caralt, M.; Balsells, J.; Charco, R. Long-term results of pancreaticoduodenectomy with superior mesenteric and portal vein resection for ductal adenocarcinoma in the head of the pancreas. Cir. Esp. 2015, 93, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Zecchin Ferrara, V.; Martinino, A.; Toti, F.; Schilirò, D.; Pinto, F.; Giovinazzo, F.; on behalf of the SMAGEICS Group. Robotic vascular resection in pancreatic ductal adenocarcinoma: A systematic review. J. Clin. Med. 2024, 13, 2000. [Google Scholar] [CrossRef] [PubMed]
- Beane, J.D.; Zenati, M.; Hamad, A.; Hogg, M.E.; Zeh, H.J., 3rd; Zureikat, A.H. Robotic pancreatoduodenectomy with vascular resection: Outcomes and learning curve. Surgery 2019, 166, 8–14. [Google Scholar] [CrossRef]
- Ma, M.J.; Cheng, H.; Chen, Y.S.; Yu, X.J.; Liu, C. Laparoscopic pancreaticoduodenectomy with portal or superior mesenteric vein resection and reconstruction for pancreatic cancer: A single-center experience. Hepatobiliary Pancreat. Dis. Int. 2023, 22, 147–153. [Google Scholar] [CrossRef]
- Ouyang, L.; Zhang, J.; Feng, Q.; Zhang, Z.; Ma, H.; Zhang, G. Robotic versus laparoscopic pancreaticoduodenectomy: An up-to-date system review and meta-analysis. Front. Oncol. 2022, 12, 834382. [Google Scholar] [CrossRef] [PubMed]
- Asbun, H.J.; Moekotte, A.L.; Vissers, F.L.; Kunzler, F.; Cipriani, F.; Alseidi, A.; D’aNgelica, M.I.; Balduzzi, A.; Bassi, C.; Björnsson, B.; et al. The Miami international evidence-based guidelines on minimally invasive pancreas resection. Ann. Surg. 2020, 271, 1–14. [Google Scholar] [CrossRef]
- Kauffmann, E.F.; Napoli, N.; Ginesini, M.; Gianfaldoni, C.; Asta, F.; Salamone, A.; Ripolli, A.; Di Dato, A.; Vistoli, F.; Amorese, G.; et al. Tips and tricks for robotic pancreatoduodenectomy with superior mesenteric/portal vein resection and reconstruction. Surg. Endosc. 2023, 37, 3233–3245. [Google Scholar] [CrossRef]
- Horvath, B.; Kloesel, B.; Todd, M.M.; Cole, D.J.; Prielipp, R.C. The evolution, current value, and future of the American Society of Anesthesiologists Physical Status Classification System. Anesthesiology 2021, 135, 904–919. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Bassi, C.; Marchegiani, G.; Dervenis, C.; Sarr, M.; Hilal, M.A.; Adham, M.; Allen, P.; Andersson, R.; Asbun, H.J.; Besselink, M.G.; et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery 2017, 161, 584–591. [Google Scholar] [CrossRef]
- Wente, M.N.; Bassi, C.; Dervenis, C.; Fingerhut, A.; Gouma, D.J.; Izbicki, J.R.; Neoptolemos, J.P.; Padbury, R.T.; Sarr, M.G.; Traverso, L.W.; et al. Delayed gastric emptying (DGE) after pancreatic surgery: A suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007, 142, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Wente, M.N.; Veit, J.A.; Bassi, C.; Dervenis, C.; Fingerhut, A.; Gouma, D.J.; Izbicki, J.R.; Neoptolemos, J.P.; Padbury, R.T.; Sarr, M.G.; et al. Postpancreatectomy hemorrhage (PPH)–an international study group of pancreatic surgery (ISGPS) definition. Surgery 2007, 142, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yu, Z.; Ma, Z.; Deng, H.; Ren, W.; Shi, W.; Jiao, Z. Superior mesenteric artery first approach can improve the clinical outcomes of pancreaticoduodenectomy: A meta-analysis. Int. J. Surg. 2020, 73, 14–24. [Google Scholar] [CrossRef]
- Nagakawa, Y.; Watanabe, Y.; Kozono, S.; Boggi, U.; Palanivelu, C.; Liu, R.; Wang, S.; He, J.; Nishino, H.; Ohtsuka, T.; et al. Surgical approaches to the superior mesenteric artery during minimally invasive pancreaticoduodenectomy: A systematic review. J. Hepatobiliary Pancreat. Sci. 2022, 29, 114–123. [Google Scholar] [CrossRef]
- Liu, R.; Wakabayashi, G.; Palanivelu, C.; Tsung, A.; Yang, K.; Goh, B.K.P.; Chong, C.C.-N.; Kang, C.M.; Peng, C.; Kakiashvili, E.; et al. International consensus statement on robotic pancreatic surgery. Hepatobiliary Surg. Nutr. 2019, 8, 345–360. [Google Scholar] [CrossRef]
- Napoli, N.; Kauffmann, E.F.; Vistoli, F.; Amorese, G.; Boggi, U. State of the art of robotic pancreatoduodenectomy. Updates Surg. 2021, 73, 873–880. [Google Scholar] [CrossRef]
- Shyr, B.-U.; Shyr, B.-S.; Chen, S.-C.; Chang, I.-W.; Shyr, Y.-M.; Wang, S.-E. Operative results and patient satisfaction after robotic pancreaticoduodenectomy. Asian J. Surg. 2020, 43, 519–525. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, F.; Wen, N.; Li, B.; Wei, Y. Patterns, timing, and predictors of recurrence after laparoscopic liver resection for hepatocellular carcinoma: Results from a high-volume HPB center. Surg. Endosc. 2022, 36, 1215–1223. [Google Scholar] [CrossRef]
- Schmidt, C.M.; Turrini, O.; Parikh, P.; House, M.G.; Zyromski, N.J.; Nakeeb, A.; Howard, T.J.; Pitt, H.A.; Lillemoe, K.D. Effect of hospital volume, surgeon experience, and surgeon volume on patient outcomes after pancreaticoduodenectomy: A single-institution experience. Arch. Surg. 2010, 145, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.R.; Zwart, M.J.W.; Molenaar, I.Q.; Koerkamp, B.G.; Hogg, M.E.; Hilal, M.A.; Besselink, M.G. Robotic pancreatoduodenectomy: Patient selection, volume criteria, and training programs. Scand. J. Surg. 2020, 109, 29–33. [Google Scholar] [CrossRef]
| Variable | Conventional PD (n = 14) | Robotic PD (n = 13) | Hodges–Lehmann Median Difference | p Value |
|---|---|---|---|---|
| Sex (male/female) | 8/6 | 10/3 | 0.420 | |
| Age (years) | 57.5 (55.2–67.8) | 62.0 (54.0–76.0) | 4.00 (95%CI: −7.00 to 14.00) | 0.576 |
| ASA 1/2/3 | 0/11/3 | 1/4/8 | 0.054 (dichotomized 1&2 vs. 3) | |
| BMI (kg/m2) | 21.8 (19.6–23.2) | 22.8 (21.4–24.1) | 0.94 (95%CI: −1.09 to 3.20) | 0.356 |
| OP time (mins) | 529.5 (456.5–636.5) | 624.0 (579.0–794.0) | 123.0 (95%CI: 29.5 to 249.0) | 0.024 |
| Blood loss (mL) | 1455.0 (630.0–2940.0) | 350.0 (200.0–1950.0) | −530 (95%CI: −1940.0 to 740.0) | 0.254 |
| LOS (days) | 23.0 (17.0–29.8) | 19.0 (17.0–24.0) | −1 (95%CI: −8.0 to 3.0) | 0.557 |
| Tumor size (cm) | 4.0 (3.0–4.5) | 3.0 (3.0–3.3) | −0.80 (95%CI: −1.75 to 0.30) | 0.230 |
| LNs retrieved | 10.0 (7.2–15.0) | 14.0 (10.0–19.0) | 4.00 (95%CI: −1.00 to 10.00) | 0.151 |
| Conversion | 0 (0%) | 1 (8%) | 0.481 | |
| POPF | 0 (0%) | 0 (0%) | 1.000 | |
| Bile leak | 0 (0%) | 0 (0%) | 1.000 | |
| Chyle leak | 2 (14%) | 2 (15%) | 1.000 | |
| Post pancreatectomy hemorrhage | 0 (0%) | 0 (0%) | 1.000 | |
| Delayed gastric emptying | 5 (36%) | 3 (23%) | 0.678 | |
| Abscess | 1 (7%) | 0 (0%) | 1.000 | |
| Wound infection | 0 (0%) | 1 (8%) | 0.481 | |
| Re-operation | 1 (7%) | 0 (0%) | 1.000 | |
| Clavien–Dindo ≥ 3 | 1 (7%) | 0 (0%) | 1.000 | |
| 90-day readmission | 6 (43%) | 1 (8%) | 0.077 | |
| 90-day op mortality | 0 (0%) | 0 (0%) | 1.000 | |
| R0 resection | 9 (64%) | 9 (69%) | 1.000 | |
| Neoadjuvant | 3 (21%) | 3 (23%) | 1.000 |
| Variable | Robotic Early (n = 3) | Robotic Late (n = 10) | Hodges–Lehmann Median Difference | p Value |
|---|---|---|---|---|
| Sex (male/female) | 2/1 | 8/2 | 1.000 | |
| Age (years) | 76.0 (74.5–81.5) | 61.0 (51.0–62.0) | −20.50 (95%CI: −27.51 to −10.00) | 0.074 |
| ASA (1&2 vs. 3) | 0/3 | 5/5 | 0.231 | |
| BMI (kg/m2) | 21.5 (21.5–22.3) | 23.0 (20.3–24.2) | 1.12 (95%CI: −1.88 to 2.74) | 0.573 |
| OP time (mins) | 794.0 (760.5–799.0) | 601.5 (560.2–646.5) | −171.5 (95%CI: −235.0 to −58.0) | 0.150 |
| Blood loss (mL) | 1950.0 (1675.0–2375.0) | 275.0 (87.5–1362.5) | −1350 (95%CI: −2550.0 to 750.0) | 0.202 |
| LOS (days) | 19.0 (18.0–20.0) | 19.0 (17.0–24.8) | 0.5 (95%CI: −3.0 to 8.0) | 0.864 |
| Tumor size (cm) | 3.0 (3.0–4.0) | 3.0 (2.4–3.3) | −0.25 (95%CI: −2.00 to 0.25) | 0.662 |
| LNs retrieved | 10.0 (9.0–18.0) | 14.5 (12.2–18.0) | 4.00 (95%CI: −12.00 to 11.00) | 0.498 |
| Conversion | 0 (0%) | 1 (10%) | 1.000 | |
| POPF | 0 (0%) | 0 (0%) | 1.000 | |
| Bile leak | 0 (0%) | 0 (0%) | 1.000 | |
| Chyle leak | 1 (33%) | 1 (10%) | 0.423 | |
| Post pancreatectomy hemorrhage | 0 (0%) | 0 (0%) | 1.000 | |
| Delayed gastric emptying | 1 (33%) | 2 (20%) | 1.000 | |
| Abscess | 0 (0%) | 0 (0%) | 1.000 | |
| Wound infection | 0 (0%) | 1 (10%) | 1.000 | |
| Re-operation | 0 (0%) | 0 (0%) | 1.000 | |
| Clavien–Dindo ≥ 3 | 0 (0%) | 0 (0%) | 1.000 | |
| 90-day readmission | 0 (0%) | 1 (10%) | 1.000 | |
| 90-day op mortality | 0 (0%) | 0 (0%) | 1.000 | |
| R0 resection | 3 (100%) | 6 (60%) | 0.497 | |
| Neoadjuvant | 0 (0%) | 3 (30%) | 0.528 |
| Variable | Conventional PD (n = 14) | Robotic Late (n = 10) | Hodges–Lehmann Median Difference | p Value |
|---|---|---|---|---|
| Sex (male/female) | 8/6 | 8/2 | 0.388 | |
| Age (years) | 57.5 (55.2–67.8) | 61.0 (51.0–62.0) | −3.50 (95%CI: −9.00 to 8.00) | 0.703 |
| ASA (1&2 vs. 3) | 11/3 | 5/5 | 0.204 | |
| BMI (kg/m2) | 21.8 (19.6–23.2) | 23.0 (20.3–24.2) | 1.18 (95%CI: −1.40 to 4.11) | 0.319 |
| OP time (mins) | 529.5 (456.5–636.5) | 601.5 (560.2–646.5) | 87.5 (95%CI: −11.5 to 212.0) | 0.095 |
| Blood loss (mL) | 1455.0 (630.0–2940.0) | 275.0 (87.5–1362.5) | −625 (95%CI: −2300.0 to 490.0) | 0.107 |
| LOS (days) | 23.0 (17.0–29.8) | 19.0 (17.0–24.8) | −0.5 (95%CI: −8.0 to 4.0) | 0.701 |
| Tumor size (cm) | 4.0 (3.0–4.5) | 3.0 (2.4–3.3) | −0.80 (95%CI: −1.90 to 0.30) | 0.185 |
| LNs retrieved | 10.0 (7.2–15.0) | 14.5 (12.2–18.0) | 4.00 (95%CI: −1.00 to 11.00) | 0.127 |
| Conversion | 0 (0%) | 1 (10%) | 0.417 | |
| POPF | 0 (0%) | 0 (0%) | 1.000 | |
| Bile leak | 0 (0%) | 0 (0%) | 1.000 | |
| Chyle leak | 2 (14%) | 1 (10%) | 1.000 | |
| Post pancreatectomy hemorrhage | 0 (0%) | 0 (0%) | 1.000 | |
| Delayed gastric emptying | 5 (36%) | 2 (20%) | 0.653 | |
| Abscess | 1 (7%) | 0 (0%) | 1.000 | |
| Wound infection | 0 (0%) | 1 (10%) | 0.417 | |
| Re-operation | 1 (7%) | 0 (0%) | 1.000 | |
| Clavien–Dindo ≥ 3 | 1 (7%) | 0 (0%) | 1.000 | |
| 90-day readmission | 6 (43%) | 1 (10%) | 0.172 | |
| 90-day op mortality | 0 (0%) | 0 (0%) | 1.000 | |
| R0 resection | 9 (64%) | 6 (60%) | 1.000 | |
| Neoadjuvant | 3 (21%) | 3 (30%) | 0.665 |
| Variable | Simple Repair (n = 6) | Interposition Graft (n = 5) | Patch (n = 16) | p Value |
|---|---|---|---|---|
| Sex (male/female) | 3/3 | 3/2 | 12/4 | 0.509 |
| Age (years) | 60.0 (57.2–65.8) | 57.0 (55.0–66.0) | 62.0 (54.8–73.8) | 0.642 |
| ASA (1&2 vs. 3) | 6/0 | 2/3 | 8/8 | 0.065 |
| BMI (kg/m2) | 19.5 (18.7–19.7) | 23.3 (21.1–23.9) | 22.8 (21.5–24.2) | 0.023 |
| OP time (mins) | 499.5 (427.5–531.8) | 494.0 (444.0–668.0) | 639.0 (572.8–743.8) | 0.011 |
| Blood loss (mL) | 840.0 (345.0–1132.5) | 1700.0 (580.0–3890.0) | 1550.0 (237.5–2770.0) | 0.472 |
| LOS (days) | 20.5 (14.0–24.8) | 24.0 (18.0–31.0) | 19.5 (17.0–26.0) | 0.470 |
| Tumor size (cm) | 3.0 (2.6–3.8) | 4.5 (2.7–6.2) | 3.2 (3.0–4.3) | 0.762 |
| LNs retrieved | 15.0 (10.5–15.8) | 8.0 (5.0–10.0) | 13.5 (9.8–23.0) | 0.083 |
| Conversion | 0 (0.0%) | 0 (0.0%) | 1 (6.2%) | 0.991 |
| POPF | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0.835 |
| Bile leak | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0.835 |
| Chyle leak | 1 (16.7%) | 1 (20.0%) | 2 (12.5%) | 0.909 |
| Post pancreatectomy hemorrhage | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0.835 |
| Delayed gastric emptying | 1 (16.7%) | 2 (40.0%) | 5 (31.2%) | 0.683 |
| Abscess | 0 (0.0%) | 0 (0.0%) | 1 (6.2%) | 0.991 |
| Wound infection | 0 (0.0%) | 0 (0.0%) | 1 (6.2%) | 0.991 |
| Re-operation | 0 (0.0%) | 1 (20.0%) | 0 (0.0%) | 0.241 |
| Clavien–Dindo ≥ 3 | 0 (0.0%) | 0 (0.0%) | 1 (6.2%) | 0.991 |
| 90-day readmission | 2 (33.3%) | 2 (40.0%) | 3 (18.8%) | 0.572 |
| 90-day op mortality | 0 (0.0%) | 0 (0.0%) | 0 (0%) | 1.000 |
| R0 resection | 5 (83.3%) | 3 (60.0%) | 10 (62.5%) | 0.614 |
| Neoadjuvant | 2 (33.3%) | 1 (20.0%) | 3 (18.8%) | 0.758 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ku, P.-Y.; Chen, Y.-J.; Lin, H.-C.; Chen, Y.-H.; Huang, S.-Y. Learning Curve of Robotic Pancreaticoduodenectomy with Portal–Superior Mesenteric Vein Resection for Pancreatic Cancers. J. Clin. Med. 2025, 14, 7986. https://doi.org/10.3390/jcm14227986
Ku P-Y, Chen Y-J, Lin H-C, Chen Y-H, Huang S-Y. Learning Curve of Robotic Pancreaticoduodenectomy with Portal–Superior Mesenteric Vein Resection for Pancreatic Cancers. Journal of Clinical Medicine. 2025; 14(22):7986. https://doi.org/10.3390/jcm14227986
Chicago/Turabian StyleKu, Peng-Yu, Yi-Ju Chen, Hui-Chen Lin, Yung-Hsien Chen, and Sheng-Yang Huang. 2025. "Learning Curve of Robotic Pancreaticoduodenectomy with Portal–Superior Mesenteric Vein Resection for Pancreatic Cancers" Journal of Clinical Medicine 14, no. 22: 7986. https://doi.org/10.3390/jcm14227986
APA StyleKu, P.-Y., Chen, Y.-J., Lin, H.-C., Chen, Y.-H., & Huang, S.-Y. (2025). Learning Curve of Robotic Pancreaticoduodenectomy with Portal–Superior Mesenteric Vein Resection for Pancreatic Cancers. Journal of Clinical Medicine, 14(22), 7986. https://doi.org/10.3390/jcm14227986







