Five-Year Mortality of Patients with Perioperative Myocardial Infarction After On-Pump Isolated or Combined Coronary Artery Bypass Graft Surgery: A Retrospective Propensity Score-Weighted Analysis
Abstract
1. Introduction
2. Methods
2.1. Study Population and Ethics
2.2. Ethics
2.3. pMI Definition
2.4. Outcomes
2.5. Data Collection
2.6. Follow-Up
2.7. Statistical Analysis
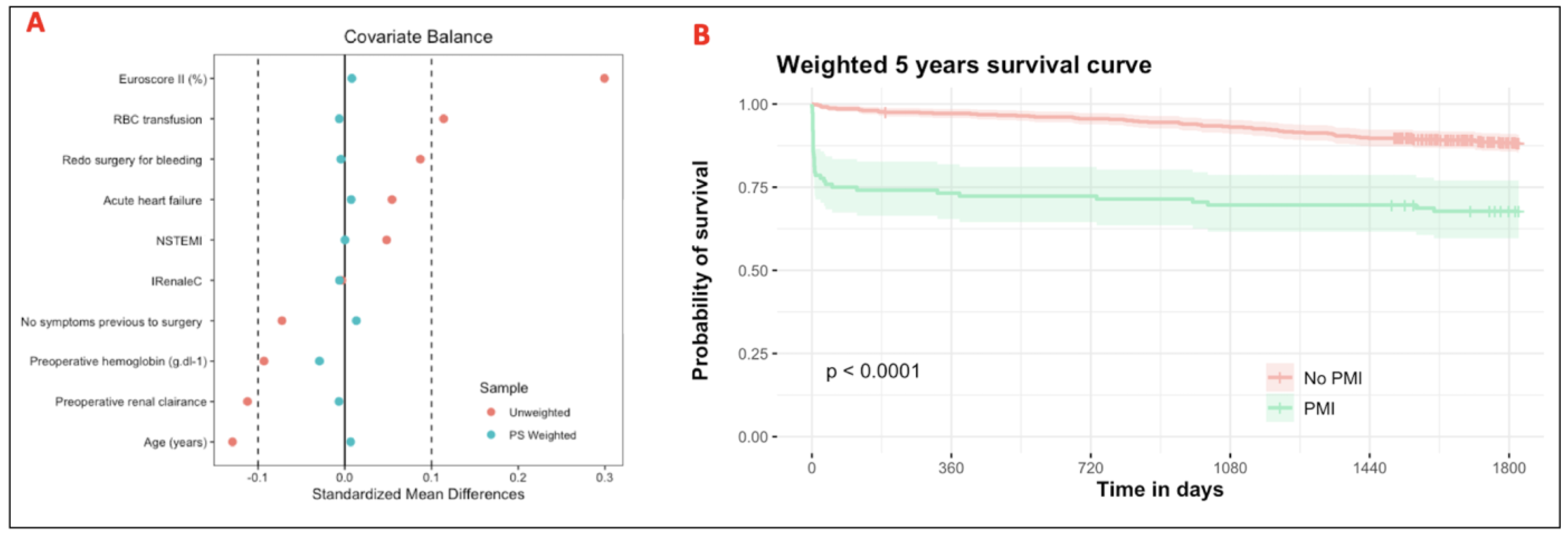
2.8. Missing Data and Propensity Score
2.9. pMI Factors
3. Results
3.1. Patients Characteristic
3.2. Perioperative, Postoperative Data and Follow-Up
3.3. Association Between pMI and 5-Year All-Cause Mortality
3.4. Isolated CABG
3.5. Impact of pMI After 30 Days
3.6. Prognostic Factors of pMI
4. Discussion
4.1. Incidence of pMI
4.2. pMI and 5-Year Mortality
4.3. Factors of pMI
4.4. Clinical Implications
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 4UD | four universal definition of myocardial infarction |
| AKI | acute kidney injury |
| CABG | coronary artery bypass grafting |
| CK-MB | Creatinine kinase MB. |
| CI | confidence interval |
| ECG | electrocardiographic |
| HR | hazard ratio |
| INSEE | National Institute of Statistics and Economic Studies |
| IPTW | inverse probability of treatment weighting |
| MI | myocardial infarction |
| OR | odd ratio |
| TTE | transthoracic echocardiography |
| PMI | perioperative myocardial infarction |
References
- Ohri, S.K.; Benedetto, U.; Luthra, S.; Grant, S.W.; Goodwin, A.T.; Trivedi, U.; Kendall, S.; Jenkins, D.P. Coronary artery bypass surgery in the UK, trends in activity and outcomes from a 15-year complete national series. Eur. J. Cardio-Thorac. Surg. 2022, 61, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Rodriguez Muñoz, D.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC guidelines for the management of acute coronary syndromes: Developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3720–3846. Available online: https://academic.oup.com/eurheartj/article/44/38/3720/7243210?login=true (accessed on 10 November 2023). [CrossRef] [PubMed]
- Gaudino, M.; Dangas, G.D.; Angiolillo, D.J.; Brodt, J.; Chikwe, J.; DeAnda, A.; Hameed, I.; Rodgers, M.L.; Sandner, S.; Sun, L.Y.; et al. Considerations on the Management of Acute Postoperative Ischemia After Cardiac Surgery: A Scientific Statement from the American Heart Association. Circulation 2023, 148, 442–454. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; ESC Scientific Document Group. Fourth universal definition of myocardial infarction (2018). Eur. Heart J. 2019, 40, 237–269. [Google Scholar] [CrossRef]
- Pölzl, L.; Thielmann, M.; Cymorek, S.; Nägele, F.; Hirsch, J.; Graber, M.; Sappler, N.; Eder, J.; Staggl, S.; Theurl, F.; et al. Impact of myocardial injury after coronary artery bypass grafting on long-term prognosis. Eur. Heart J. 2022, 43, 2407–2417. [Google Scholar] [CrossRef]
- Mack, M.J.; Fullerton, D.A.; Fann, J.I. The Answers You Get Depend on the Questions You Ask: Insights from the Recent EXCEL Trial Controversy. Ann. Thorac. Surg. 2021, 111, 1743–1745. [Google Scholar] [CrossRef]
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.-P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Toulouse, E.; Masseguin, C.; Lafont, B.; McGurk, G.; Harbonn, A.; Roberts, J.A.; Granier, S.; Dupeyron, A.; Bazin, J.E. French legal approach to clinical research. Anaesth. Crit. Care Pain Med. 2018, 37, 607–614. [Google Scholar] [CrossRef]
- Yao, X.I.; Wang, X.; Speicher, P.J.; Hwang, E.S.; Cheng, P.; Harpole, D.H.; Berry, M.F.; Schrag, D.; Pang, H.H. Reporting and Guidelines in Propensity Score Analysis: A Systematic Review of Cancer and Cancer Surgical Studies. J. Natl. Cancer Inst. 2017, 109, djw323. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Silverborn, M.; Nielsen, S.; Karlsson, M. The performance of EuroSCORE II in CABG patients in relation to sex, age, and surgical risk: A nationwide study in 14,118 patients. J. Cardiothorac. Surg. 2023, 18, 40. [Google Scholar] [CrossRef] [PubMed]
- Jammer, I.; Wickboldt, N.; Sander, M.; Smith, A.; Schultz, M.J.; Pelosi, P.; Leva, B.; Rhodes, A.; Hoeft, A.; Walder, B.; et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions. Eur. J. Anaesthesiol. 2015, 32, 88–105. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Chang, C.; Ido, M.S.; Long, Q. Multiple Imputation for General Missing Data Patterns in the Presence of High-dimensional Data. Sci. Rep. 2016, 6, 21689. [Google Scholar] [CrossRef]
- Austin, P.C.; Stuart, E.A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat. Med. 2015, 34, 3661–3679. [Google Scholar] [CrossRef]
- Zindrou, D.; Taylor, K.M.; Bagger, J.P. Preoperative haemoglobin concentration and mortality rate after coronary artery bypass surgery. Lancet 2002, 359, 1747–1748. [Google Scholar] [CrossRef]
- Madhavan, S.; Chan, S.-P.; Tan, W.-C.; Eng, J.; Li, B.; Luo, H.-D.; Teoh, L.-K.K. Cardiopulmonary bypass time: Every minute counts. J. Cardiovasc. Surg. 2018, 59, 274–281. [Google Scholar] [CrossRef]
- Al-Sarraf, N.; Thalib, L.; Hughes, A.; Houlihan, M.; Tolan, M.; Young, V.; McGovern, E. Cross-clamp time is an independent predictor of mortality and morbidity in low- and high-risk cardiac patients. Int. J. Surg. Lond. Engl. 2011, 9, 104–109. [Google Scholar] [CrossRef]
- Nalysnyk, L.; Fahrbach, K.; Reynolds, M.W.; Zhao, S.Z.; Ross, S. Adverse events in coronary artery bypass graft (CABG) trials: A systematic review and analysis. Heart 2003, 89, 767–772. [Google Scholar] [CrossRef]
- Pretto, P.; Martins, G.F.; Biscaro, A.; Kruczan, D.D.; Jessen, B. Perioperative myocardial infarction in patients undergoing myocardial revascularization surgery. Rev. Bras. Cir. Cardiovasc. 2015, 30, 49–54. [Google Scholar] [CrossRef][Green Version]
- Litwinowicz, R.; Mazur, P.; Śliwiński, P.; Bryndza, M.; Bartuś, K.; Filip, G.; Bartoszcze, A.; Piątek, J.; Konstanty-Kalandyk, J.; Kowalewski, M.; et al. Long-term survival following postoperative myocardial infraction after coronary artery bypass surgery. J. Thorac. Dis. 2022, 14, 102–112. [Google Scholar] [CrossRef]
- Carr, B.M.; Romeiser, J.; Ruan, J.; Gupta, S.; Seifert, F.C.; Zhu, W.; Shroyer, A.L. Long-Term Post-CABG Survival: Performance of Clinical Risk Models Versus Actuarial Predictions. J. Card. Surg. 2016, 31, 23–30. [Google Scholar] [CrossRef]
- Rupprecht, L.; Schmid, C.; Debl, K.; Lunz, D.; Flörchinger, B.; Keyser, A. Impact of coronary angiography early after CABG for suspected postoperative myocardial ischemia. J. Cardiothorac. Surg. 2019, 14, 54. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Iqbal, J.; Van Klaveren, D.; Campos, C.M.; Holmes, D.R.; Kappetein, A.P.; Morice, M.-C.; Banning, A.P.; Grech, E.D.; Bourantas, C.V.; et al. Smoking Is Associated with Adverse Clinical Outcomes in Patients Undergoing Revascularization with PCI or CABG. J. Am. Coll. Cardiol. 2015, 65, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e21–e129. [Google Scholar] [CrossRef] [PubMed]
- Mejía, O.A.V.; Lisboa, L.A.F.; Tiveron, M.G.; Santiago, J.A.D.; Tineli, R.A.; Dallan, L.A.O.; Jatene, F.B.; Stolf, N.A.G. Coronary artery bypass grafting in acute myocardial infarction: Analysis of predictors of in-hospital mortality. Braz. J. Cardiovasc. Surg. 2012, 27, 66–74. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abou-Arab, O.; Kamel, S.; Beyls, C.; Huette, P.; Bar, S.; Lorne, E.; Galmiche, A.; Guinot, P.-G. Vasoplegia After Cardiac Surgery Is Associated with Endothelial Glycocalyx Alterations. J. Cardiothorac. Vasc. Anesth. 2020, 34, 900–905. [Google Scholar] [CrossRef]
- Gaudino, M.; Flather, M.; Capodanno, D.; Milojevic, M.; Bhatt, D.L.; Biondi Zoccai, G.; Boden, W.E.; Devereaux, P.J.; Doenst, T.; Farkouh, M.; et al. European Association of Cardio-Thoracic Surgery (EACTS) expert consensus statement on perioperative myocardial infarction after cardiac surgery. Eur. J. Cardiothorac. Surg. 2024, 65, ezad415. [Google Scholar] [CrossRef]
- Grant, S.W.; Kendall, S.; Goodwin, A.T.; Cooper, G.; Trivedi, U.; Page, R.; Jenkins, D.P. Trends and outcomes for cardiac surgery in the United Kingdom from 2002 to 2016. JTCVS Open 2021, 7, 259–269. [Google Scholar] [CrossRef]
- Armillotta, M.; Bergamaschi, L.; Paolisso, P.; Belmonte, M.; Angeli, F.; Sansonetti, A.; Stefanizzi, A.; Bertolini, D.; Bodega, F.; Amicone, S.; et al. Prognostic Relevance of Type 4a Myocardial Infarction and Periprocedural Myocardial Injury in Patients with Non–ST-Segment–Elevation Myocardial Infarction. Circulation 2025, 151, 760–772. [Google Scholar] [CrossRef]
- Beyls, C.; Huette, P.; Huang, P.; Kowalik, H.; Andreamifidi-Berti, C.; Guilbart, M.; Bernasinsiki, M.; Besserve, P.; Touati, G.; Caus, T.; et al. Five-year mortality of patients with perioperative myocardial infarction after on-pump isolated or combined coronary artery bypass graft surgery: A retrospective propensity score-weighted analysis. Arch. Cardiovasc. Dis. 2024, 117, S25–S26. [Google Scholar] [CrossRef]

| Variables | No pMI (n = 600) | pMI (n = 112) | p Value | SMD |
|---|---|---|---|---|
| Age (years) | 68 ± 9 | 67 ± 11 | 0.29 | 0.129 |
| BMI (kg.m−2) | 29 ± 11 | 29 ± 5 | 0.28 | 0.024 |
| Male gender (n; %) | 488 (81) | 85 (76) | 0.22 | 0.133 |
| Medical history, n (%) | ||||
| No symptoms | 97 (16) | 10 (9) | 0.05 | 0.220 |
| Angina severity according to CCS > 2 | 304 (51) | 57 (51) | 1.00 | 0.005 |
| Recent myocardial infarction | 140 (23) | 16 (14) | 0.71 | 0.038 |
| Previous PCI | 107 (18) | 28 (17) | 1.000 | 0.001 |
| Peripheral vascular disease | 87 (14) | 19 (17) | 0.59 | 0.068 |
| Hypertension | 477 (79) | 83 (75) | 0.21 | 0.128 |
| Active Smoking | 94 (16) | 29 (27) | 0.008 | 0.277 |
| Diabetes mellitus | 240 (40) | 44 (39) | 0.91 | 0.015 |
| Dyslipidemia | 446 (74) | 78 (70) | 0.29 | 0.105 |
| Chronic renal disease | 61 (10) | 11 (10) | 1.000 | 0.012 |
| Stroke | 36 (6) | 6 (5) | 1.000 | 0.042 |
| Atrial fibrillation | 81 (13) | 16 (14) | 0.89 | 0.018 |
| Chronic obstructive pulmonary disease | 59 (10) | 6 (5) | 0.15 | 0.170 |
| Prior cardiac surgery | 6 (1) | 2 (2) | 0.36 | 0.067 |
| EuroSCORE 2 (%) | 5.3 ± 3 | 6.5 ± 4 | 0.01 | 0.302 |
| Hemoglobin (g/dL) | 13.3 ± 1.8 | 13.1 ± 1.9 | 0.43 | 0.113 |
| Glomerular filtration rate (mL/min/1.73 m2) | 80 ± 27 | 72 ± 29 | 0.41 | 0.120 |
| Preoperative TTE | ||||
| Left ventricular ejection fraction, (%) | 56 ± 11 | 54 ± 13 | 0.19 | 0.183 |
| Acute coronary syndrome, n (%) | ||||
| NSTEMI | 121 (23) | 28 (29) | 0.24 | 0.116 |
| STEMI | 38 (7) | 8 (8) | 0.67 | 0.035 |
| Coronagraphy | ||||
| One diseased vessel | 58 (10) | 1 (5) | 0.20 | 0.127 |
| Two diseased vessels | 134 (22) | 20 (18) | 0.31 | 0.090 |
| Three diseased vessels | 405 (68) | 84 (75) | 0.14 | 0.164 |
| Variables | No pMI (n = 600) | pMI (n = 112) | p Value | SMD |
|---|---|---|---|---|
| Cardiac surgery procedure, n (%) | ||||
| Isolated CABG | 425 (71) | 81 (72) | 0.91 | 0.025 |
| Complete arterial grafting | 333 (55) | 62 (55) | 1 | 0.003 |
| Right internal mammary artery | 326 (54) | 59 (53) | 0.75 | 0.033 |
| Left internal mammary artery | 560 (93) | 108 (96) | 0.28 | 0.140 |
| Radial-artery graft | 33 (5) | 6 (5) | 1 | 0.006 |
| Saphenous vein graft | 251 (42) | 47 (42) | 0.83 | 0.021 |
| Combined CABG, n (%) | 175 (29) | 31 (28) | 0.82 | 0.152 |
| Valve replacement | 173/175 (98) | 30/31 (97) | 0.73 | 0.046 |
| Aortic root replacement | 25/175 (14) | 3/31 (10) | 0.61 | 0.082 |
| Number of grafts, n (%) | ||||
| >3 | 336 (56) | 61 (54) | 0.68 | 0.040 |
| CPB time, (min) | 89 ± 41 | 104 ± 56 | 0.04 | 0.270 |
| Cross aortic clamp, (min) | 61 ± 34 | 64 ± 41 | 0.97 | 0.084 |
| Administration of RBC products, n (%) | 103 (17) | 31 (28) | 0.01 | 0.257 |
| Administration of vasopressor, n (%) | 129 (21) | 39 (35) | 0.003 | 0.395 |
| Mechanical circulatory support, n (%) | 10 (2) | 9 (8) | <0.0001 | 0.302 |
| ECMO | 3 (30) | 3 (33) | 0.05 | 0.174 |
| IABP | 7 (70) | 8 (88) | 0.001 | 0.291 |
| Cardiac troponin at day 2 (µg/L) | 4.6 ± 10.8 | 20.5 ± 75.8 | 0.001 | 0.295 |
| Postoperative complications, n (%) | ||||
| Atrial fibrillation | 152 (25) | 28 (25) | 1 | 0.003 |
| Stroke | 9 (1) | 3 (3) | 0.41 | 0.084 |
| Mechanical ventilation > 24 h | 49 (8) | 29 (26) | <0.0001 | 0.491 |
| Pneumonia | 92 (15) | 21 (19) | 0.32 | 0.099 |
| Acute kidney injury | 97 (16) | 36 (32) | <0.0001 | 0.385 |
| Vasoplegic syndrome | 179 (30) | 44 (39) | 0.05 | 0.218 |
| Revised surgery | 28 (5) | 15 (14) | 0.002 | 0.307 |
| Outcomes | ||||
| Intra-hospital death, n (%) | 8 (1) | 28 (25) | <0.0001 | 0.718 |
| Five-year death, n (%) | 66 (11) | 36 (32) | <0.0001 | 0.509 |
| Length of ICU, days | 5 ± 20 | 5 ± 10 | 0.04 | 0.117 |
| Length of hospital, days | 13 ± 10 | 13 ± 13 | 0.08 | 0.021 |
| Variable | OR (95% CI) by Logistic Regression | |||
|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||
| OR (95%CI) | p | OR (95%CI) | p | |
| EuroSCORE II > 4% | 1.51 (1.01–2.25) | 0.048 | 1.45 (0.94–2.25) | 0.08 |
| Diabetes mellitus | 0.93 (0.64–1.34) | 0.71 | - | |
| Dyslipidemia | 0.79 (0.51–1.23) | 0.32 | - | |
| Active Smoking | 1.97 (1.12–3.19) | 0.006 | 2.24 (1.36–3.69) | 0.001 |
| Preoperative Hemoglobin < 10 g/dL | 1.54 (0.89–2.65) | 0.11 | - | |
| Combined surgery | 0.61 (0.29–1.23) | 0.16 | - | |
| CPB time > 180 min | 2.51 (1.19–5.25) | 0.01 | 2.57 (1.19–5.34) | 0.015 |
| Cross clamping time > 60 min | 1.23 (0.79–1.91) | 0.35 | - | |
| Administration of RBC products | 1.56 (1.06–2.35) | 0.03 | 1.41 (0.91–2.19) | 0.12 |
| Mechanical hemodynamic support | 5.41 (1.09–27.9) | 0.04 | 3.85 (0.69–21.2) | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beyls, C.; Huette, P.; Luang, P.; Kowalik, H.; Andriamifidy-Berti, C.; Guilbart, M.; Bernasinski, M.; Besserve, P.; Touati, G.; Caus, T.; et al. Five-Year Mortality of Patients with Perioperative Myocardial Infarction After On-Pump Isolated or Combined Coronary Artery Bypass Graft Surgery: A Retrospective Propensity Score-Weighted Analysis. J. Clin. Med. 2025, 14, 7970. https://doi.org/10.3390/jcm14227970
Beyls C, Huette P, Luang P, Kowalik H, Andriamifidy-Berti C, Guilbart M, Bernasinski M, Besserve P, Touati G, Caus T, et al. Five-Year Mortality of Patients with Perioperative Myocardial Infarction After On-Pump Isolated or Combined Coronary Artery Bypass Graft Surgery: A Retrospective Propensity Score-Weighted Analysis. Journal of Clinical Medicine. 2025; 14(22):7970. https://doi.org/10.3390/jcm14227970
Chicago/Turabian StyleBeyls, Christophe, Pierre Huette, Paul Luang, Hélène Kowalik, Chloé Andriamifidy-Berti, Mathieu Guilbart, Mickael Bernasinski, Patricia Besserve, Gilles Touati, Thierry Caus, and et al. 2025. "Five-Year Mortality of Patients with Perioperative Myocardial Infarction After On-Pump Isolated or Combined Coronary Artery Bypass Graft Surgery: A Retrospective Propensity Score-Weighted Analysis" Journal of Clinical Medicine 14, no. 22: 7970. https://doi.org/10.3390/jcm14227970
APA StyleBeyls, C., Huette, P., Luang, P., Kowalik, H., Andriamifidy-Berti, C., Guilbart, M., Bernasinski, M., Besserve, P., Touati, G., Caus, T., Dupont, H., Mahjoub, Y., & Abou-Arab, O. (2025). Five-Year Mortality of Patients with Perioperative Myocardial Infarction After On-Pump Isolated or Combined Coronary Artery Bypass Graft Surgery: A Retrospective Propensity Score-Weighted Analysis. Journal of Clinical Medicine, 14(22), 7970. https://doi.org/10.3390/jcm14227970






