Lymphocyte-Associated Inflammation Markers Predict Bleomycin-Induced Pulmonary Toxicity in Testicular Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Patient Characteristics
2.2. Inclusion and Exclusion Criteria of the Study
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
| Presence of a second malignancy diagnosis Presence of diabetes mellitus Presence of chronic renal failure Presence of chronic rheumatic disease Chronic lung disease | Obesity Accompanying pulmonary infection History of pulmonary radiotherapy and surgery Not having received bleomycin in treatment |
2.3. The Purposes and Calculation of Prognostic Markers
Lymphocyte-Related Inflammation Markers (NLR, PLR, LMR, CLR, SII, SIRI)
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TC | Testicular cancer |
| NSGCT | Nonseminomatous germ cell carcinoma |
| PFT | Pulmonary function test |
| BAL | Bronchoalveolar lavage |
| DLCO | Carbon monoxide diffusion capacity |
| NLR | Neutrophil/lymphocyte ratio |
| PLR | Platelet/lymphocyte ratio |
| LMR | Lymphocyte/monocyte ratio |
| CLR | CRP/lymphocyte ratio |
| SII | Systemic immune inflammation score |
| SIRI | Systemic inflammation response index |
| mGCT | Mixed germ cell tumor |
| mOS | Median overall survival |
| GCSF | Granulocyte colony-stimulating factor |
| FVC | Forced vital capacity |
| TLC | Total lung capacity |
| BEP | Bleomycin, etoposide, cisplatin |
References
- Ozkan, M.; Dweik, R.A.; Ahmad, M. Drug-induced lung disease. Clevel. Clin. J. Med. 2001, 68, 782–785. [Google Scholar] [CrossRef] [PubMed]
- Nebeker, J.R.; Barach, P.; Samore, M.H. Clarifying adverse drug events: A clinician’s guide to terminology, documentation, and reporting. Ann. Intern. Med. 2004, 140, 795–801. [Google Scholar] [CrossRef]
- Dieckmann, K.P.; Richter-Simonsen, H.; Kulejewski, M.; Ikogho, R.; Zecha, H.; Anheuser, P.; Pichlmeier, U.; Isbarn, H. Testicular Germ-Cell Tumours: A Descriptive Analysis of Clinical Characteristics at First Presentation. Urol. Int. 2018, 100, 409–419. [Google Scholar] [CrossRef]
- Shah, Y.B.; Goldberg, H.; Hu, B.; Daneshmand, S.; Chandrasekar, T. Metastatic Testicular Cancer Patterns and Predictors: A Contemporary Population-based SEER Analysis. Urology 2023, 180, 182–189. [Google Scholar] [CrossRef]
- Haugnes, H.S.; Aass, N.; Fosså, S.D.; Dahl, O.; Brydøy, M.; Aasebø, U.; Wilsgaard, T.; Bremnes, R.M. Pulmonary function in long-term survivors of testicular cancer. J. Clin. Oncol. 2009, 27, 2779–2786. [Google Scholar] [CrossRef] [PubMed]
- Lauritsen, J.; Kier, M.G.; Mortensen, M.S.; Bandak, M.; Gupta, R.; Holm, N.V.; Agerbaek, M.; Daugaard, G. Germ Cell Cancer and Multiple Relapses: Toxicity and survival. J. Clin. Oncol. 2015, 33, 3116–3123. [Google Scholar] [CrossRef]
- Vahid, B.; Marik, P.E. Pulmonary complications of novel antineoplastic agents for solid tumors. Chest 2008, 133, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Limper, A.H. Chemotherapy-induced lung disease. Clin. Chest Med. 2004, 25, 53–64. [Google Scholar] [CrossRef]
- Possick, J.D. Pulmonary Toxicities from Checkpoint Immunotherapy for Malignancy. Clin. Chest Med. 2017, 38, 223–232. [Google Scholar] [CrossRef]
- Sleijfer, S. Bleomycin-induced pneumonitis. Chest 2001, 120, 617–624. [Google Scholar] [CrossRef]
- Yerushalmi, R.; Kramer, M.R.; Rizel, S.; Sulkes, A.; Gelmon, K.; Granot, T.; Neiman, V.; Stemmer, S.M. Decline in pulmonary function in patients with breast cancer receiving dose-dense chemotherapy: A prospective study. Ann. Oncol. 2009, 20, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Camus, P.; Bonniaud, P.; Fanton, A.; Camus, C.; Baudaun, N.; Foucher, P. Drug-induced and iatrogenic infiltrative lung disease. Clin. Chest Med. 2004, 25, 479–519. [Google Scholar] [CrossRef] [PubMed]
- Schrier, D.J.; Phan, S.H.; McGarry, B.M. The effects of the nude (nu/nu) mutation on bleomycin-induced pulmonary fibrosis. A biochemical evaluation. Am. Rev. Respir. Dis. 1983, 127, 614–617. [Google Scholar] [CrossRef]
- Otsuka, R.; Hayashi, H.; Uesato, M.; Hayano, K.; Murakami, K.; Toyozumi, T.; Matsumoto, Y.; Kurata, Y.; Nakano, A.; Takahashi, Y.; et al. Inflammatory and Nutritional Indices as Prognostic Markers in Elderly Patients With Gastric Cancer. Anticancer Res. 2023, 43, 5261–5267. [Google Scholar] [CrossRef]
- Ruan, G.T.; Xie, H.L.; Yuan, K.T.; Lin, S.Q.; Zhang, H.Y.; Liu, C.A.; Shi, J.Y.; Ge, Y.Z.; Song, M.M.; Hu, C.L.; et al. Prognostic value of systemic inflammation and for patients with colorectal cancer cachexia. J. Cachexia Sarcopenia Muscle 2023, 14, 2813–2823. [Google Scholar] [CrossRef] [PubMed]
- Nøst, T.H.; Alcala, K.; Urbarova, I.; Byrne, K.S.; Guida, F.; Sandanger, T.M.; Johansson, M. Systemic inflammation markers and cancer incidence in the UK Biobank. Eur. J. Epidemiol. 2021, 36, 841–848. [Google Scholar] [CrossRef]
- Zhang, S.; Cheng, T. Prognostic and clinicopathological value of systemic inflammation response index (SIRI) in patients with breast cancer: A meta-analysis. Ann. Med. 2024, 56, 2337729. [Google Scholar] [CrossRef]
- Blumenschein, G.R., Jr.; Gatzemeier, U.; Fossella, F.; Stewart, D.J.; Cupit, L.; Cihon, F.; O’Leary, J.; Reck, M. Phase II, multicenter, uncontrolled trial of single-agent sorafenib in patients with relapsed or refractory, advanced non-small-cell lung cancer. J. Clin. Oncol. 2009, 27, 4274–4280. [Google Scholar] [CrossRef]
- Nicolls, M.R.; Terada, L.S.; Tuder, R.M.; Prindiville, S.A.; Schwarz, M.I. Diffuse alveolar hemorrhage with underlying pulmonary capillaritis in the retinoic acid syndrome. Am. J. Respir. Crit. Care. Med. 1998, 158, 1302–1305. [Google Scholar] [CrossRef]
- Lee, C.; Gianos, M.; Klaustermeyer, W.B. Diagnosis and management of hypersensitivity reactions related to common cancer chemotherapy agents. Ann. Allergy Asthma Immunol. 2009, 102, 179–187. [Google Scholar] [CrossRef]
- Meadors, M.; Floyd, J.; Perry, M.C. Pulmonary toxicity of chemotherapy. Semin. Oncol. 2006, 33, 98–105. [Google Scholar] [CrossRef]
- Jules-Elysee, K.; White, D.A. Bleomycin-induced pulmonary toxicity. Clin. Chest Med. 1990, 11, 1–20. [Google Scholar] [CrossRef]
- O’Sullivan, J.M.; Huddart, R.A.; Norman, A.R.; Nicholls, J.; Dearnaley, D.P.; Horwich, A. Predicting the risk of bleomycin lung toxicity in patients with germ-cell tumours. Ann. Oncol. 2003, 14, 91–96. [Google Scholar] [CrossRef]
- Lazo, J.S.; Merrill, W.W.; Pham, E.T.; Lynch, T.J.; McCallister, J.D.; Ingbar, D.H. Bleomycin hydrolase activity in pulmonary cells. J. Pharmacol. Exp. Ther. 1984, 231, 583–588. [Google Scholar] [CrossRef]
- Martin, W.G.; Ristow, K.M.; Habermann, T.M.; Colgan, J.P.; Witzig, T.E.; Ansell, S.M. Bleomycin pulmonary toxicity has a negative impact on the outcome of patients with Hodgkin’s lymphoma. J. Clin. Oncol. 2005, 23, 7614–7620. [Google Scholar] [CrossRef] [PubMed]
- Stamatoullas, A.; Brice, P.; Bouabdallah, R.; Mareschal, S.; Camus, V.; Rahal, I.; Franchi, P.; Lanic, H.; Tilly, H. Outcome of patients older than 60 years with classical Hodgkin lymphoma treated with front line ABVD chemotherapy: Frequent pulmonary events suggest limiting the use of bleomycin in the elderly. Br. J. Haematol. 2015, 170, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Fosså, S.D.; Kaye, S.B.; Mead, G.M.; Cullen, M.; de Wit, R.; Bodrogi, I.; van Groeningen, C.J.; De Mulder, P.H.; Stenning, S.; Lallemand, E.; et al. Filgrastim during combination chemotherapy of patients with poor-prognosis metastatic germ cell malignancy. European Organization for Research and Treatment of Cancer, Genito-Urinary Group, and the Medical Research Council Testicular Cancer Working Party, Cambridge, United Kingdom. J. Clin. Oncol. 1998, 16, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Saxman, S.B.; Nichols, C.R.; Einhorn, L.H. Pulmonary toxicity in patients with advanced-stage germ cell tumors receiving bleomycin with and without granulocyte colony stimulating factor. Chest 1997, 111, 657–660. [Google Scholar] [CrossRef]
- Evens, A.M.; Cilley, J.; Ortiz, T.; Gounder, M.; Hou, N.; Rademaker, A.; Miyata, S.; Catsaros, K.; Augustyniak, C.; Bennett, C.L.; et al. G-CSF is not necessary to maintain over 99% dose-intensity with ABVD in the treatment of Hodgkin lymphoma: Low toxicity and excellent outcomes in a 10-year analysis. Br. J. Haematol. 2007, 137, 545–552. [Google Scholar] [CrossRef]
- Nichols, C.R.; Catalano, P.J.; Crawford, E.D.; Vogelzang, N.J.; Einhorn, L.H.; Loehrer, P.J. Randomized comparison of cisplatin and etoposide and either bleomycin or ifosfamide in treatment of advanced disseminated germ cell tumors: An Eastern Cooperative Oncology Group, Southwest Oncology Group, and Cancer and Leukemia Group B Study. J. Clin. Oncol. 1998, 16, 1287–1293. [Google Scholar] [CrossRef]
- Lauritsen, J.; Kier, M.G.; Bandak, M.; Mortensen, M.S.; Thomsen, F.B.; Mortensen, J.; Daugaard, G. Pulmonary Function in Patients With Germ Cell Cancer Treated With Bleomycin, Etoposide, and Cisplatin. J. Clin. Oncol. 2016, 34, 1492–1499. [Google Scholar] [CrossRef]
- Chaudhary, U.B.; Haldas, J.R. Long-term complications of chemotherapy for germ cell tumours. Drugs 2003, 63, 1565–1577. [Google Scholar] [CrossRef]
- Lower, E.E.; Strohofer, S.; Baughman, R.P. Bleomycin causes alveolar macrophages from cigarette smokers to release hydrogen peroxide. Am. J. Med. Sci. 1988, 295, 193–197. [Google Scholar] [CrossRef]
- Thomas, T.S.; Luo, S.; Reagan, P.M.; Keller, J.W.; Sanfilippo, K.M.; Carson, K.R. Advancing age and the risk of bleomycin pulmonary toxicity in a largely older cohort of patients with newly diagnosed Hodgkin Lymphoma. J. Geriatr. Oncol. 2020, 11, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.B.; Paul, J.; Graham, J.; Kaye, S.B. Fatal bleomycin pulmonary toxicity in the west of Scotland 1991-95: A review of patients with germ cell tumours. Br. J. Cancer 1998, 78, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Uzel, I.; Ozguroglu, M.; Uzel, B.; Kaynak, K.; Demirhan, O.; Akman, C.; Oz, F.; Yaman, M. Delayed onset bleomycin-induced pneumonitis. Urology 2005, 66, 195. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulou, I.; Efstathiou, E.; Samakovli, A.; Dafni, U.; Moulopoulos, L.A.; Papadimitriou, C.; Lyberopoulos, P.; Kastritis, E.; Roussos, C.; Dimopoulos, M.A. A prospective study on lung toxicity in patients treated with gemcitabine and carboplatin: Clinical, radiological and functional assessment. Ann. Oncol. 2004, 15, 1250–1255. [Google Scholar] [CrossRef]
- Rivera, M.P.; Detterbeck, F.C.; Socinski, M.A.; Moore, D.T.; Edelman, M.J.; Jahan, T.M.; Ansari, R.H.; Luketich, J.D.; Peng, G.; Monberg, M.; et al. Impact of preoperative chemotherapy on pulmonary function tests in resectable early-stage non-small cell lung cancer. Chest 2009, 135, 1588–1595. [Google Scholar] [CrossRef]
- Buchler, T.; Bomanji, J.; Lee, S.M. FDG-PET in bleomycin-induced pneumonitis following ABVD chemotherapy for Hodgkin’s disease--a useful tool for monitoring pulmonary toxicity and disease activity. Haematologica 2007, 92, 120–121. [Google Scholar] [CrossRef]
- von Rohr, L.; Klaeser, B.; Joerger, M.; Kluckert, T.; Cerny, T.; Gillessen, S. Increased pulmonary FDG uptake in bleomycin-associated pneumonitis. Onkologie 2007, 30, 320–323. [Google Scholar] [CrossRef]
- Cleverley, J.R.; Screaton, N.J.; Hiorns, M.P.; Flint, J.D.; Müller, N.L. Drug-induced lung disease: High-resolution CT and histological findings. Clin. Radiol. 2002, 57, 292–299. [Google Scholar] [CrossRef]
- Torrisi, J.M.; Schwartz, L.H.; Gollub, M.J.; Ginsberg, M.S.; Bosl, G.J.; Hricak, H. CT findings of chemotherapy-induced toxicity: What radiologists need to know about the clinical and radiologic manifestations of chemotherapy toxicity. Radiology 2011, 258, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Sikdar, T.; MacVicar, D.; Husband, J.E. Pneumomediastinum complicating bleomycin related lung damage. Br. J. Radiol. 1998, 71, 1202–1204. [Google Scholar] [CrossRef] [PubMed]
- Bossi, G.; Cerveri, I.; Volpini, E.; Corsico, A.; Baio, A.; Corbella, F.; Klersy, C.; Arico, M. Long-term pulmonary sequelae after treatment of childhood Hodgkin’s disease. Ann. Oncol. 1997, 8, 19–24. [Google Scholar] [CrossRef]
- Fujimoto, D.; Kato, R.; Morimoto, T.; Shimizu, R.; Sato, Y.; Kogo, M.; Ito, J.; Teraoka, S.; Nagata, K.; Nakagawa, A.; et al. Characteristics and Prognostic Impact of Pneumonitis during Systemic Anti-Cancer Therapy in Patients with Advanced Non-Small-Cell Lung Cancer. PLoS ONE 2016, 11, 0168465. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.A.; De La Peña, H.; Tsakok, M.T.; Joseph, J.; Stoneham, S.; Shamash, J.; Joffe, J.; Mazhar, D.; Traill, Z.; Ho, L.P.; et al. Development of a best-practice clinical guideline for the use of bleomycin in the treatment of germ cell tumours in the UK. Br. J. Cancer 2018, 119, 1044–1051. [Google Scholar] [CrossRef]
- Comis, R.L.; Kuppinger, M.S.; Ginsberg, S.J.; Crooke, S.T.; Gilbert, R.; Auchincloss, J.H.; Prestayko, A.W. Role of single-breath carbon monoxide-diffusing capacity in monitoring the pulmonary effects of bleomycin in germ cell tumor patients. Cancer Res. 1979, 39, 5076–5080. [Google Scholar]
- Pascual, R.S.; Mosher, M.B.; Sikand, R.S.; De Conti, R.C.; Bouhuys, A. Effects of bleomycin on pulmonary function in man. Am. Rev. Respir. Dis. 1973, 108, 211–217. [Google Scholar]
- Villani, F.; De Maria, P.; Bonfante, V.; Viviani, S.; Laffranchi, A.; Dell’oca, I.; Dirusso, A.; Zanini, M. Late pulmonary toxicity after treatment for Hodgkin’s disease. Anticancer Res. 1997, 17, 4739–4742. [Google Scholar]
- McKeage, M.J.; Evans, B.D.; Atkinson, C.; Perez, D.; Forgeson, G.V.; Dady, P.J. Carbon monoxide diffusing capacity is a poor predictor of clinically significant bleomycin lung. New Zealand Clinical Oncology Group. J. Clin. Oncol. 1990, 8, 779–783. [Google Scholar] [CrossRef]
- Ng, A.K.; Li, S.; Neuberg, D.; Chi, R.; Fisher, D.C.; Silver, B.; Mauch, P.M. A prospective study of pulmonary function in Hodgkin’s lymphoma patients. Ann. Oncol. 2008, 19, 1754–1758. [Google Scholar] [CrossRef] [PubMed]
- Shamash, J.; Sarker, S.J.; Huddart, R.; Harland, S.; Joffe, J.K.; Mazhar, D.; Birtle, A.; White, J.; Chowdhury, K.; Wilson, P.; et al. A randomized phase III study of 72 h infusional versus bolus bleomycin in BEP (bleomycin, etoposide and cisplatin) chemotherapy to treat IGCCCG good prognosis metastatic germ cell tumours (TE-3). Ann. Oncol. 2017, 28, 1333–1338. [Google Scholar] [CrossRef]
- Kolb, P.; Upagupta, C.; Vierhout, M.; Ayaub, E.; Bellaye, P.S.; Gauldie, J.; Shimbori, C.; Inman, M.; Ask, K.; Kolb, M.R.J. The importance of interventional timing in the bleomycin model of pulmonary fibrosis. Eur. Respir. J. 2020, 55, 1901105. [Google Scholar] [CrossRef]
- Kato, S.; Inui, N.; Hakamata, A.; Suzuki, Y.; Enomoto, N.; Fujisawa, T.; Nakamura, Y.; Watanabe, H.; Suda, T. Changes in pulmonary endothelial cell properties during bleomycin-induced pulmonary fibrosis. Respir. Res. 2018, 19, 127. [Google Scholar] [CrossRef]
- Ryu, W.K.; Moon, Y.; Park, M.H.; Lim, J.H.; Kim, Y.S.; Lee, K.H.; Kwak, S.M.; Kim, C.; Nam, H.S. A Preliminary Study on the Prognostic Impact of Neutrophil to Lymphocyte Ratio of the Bronchoalveolar Lavage Fluid in Patients with Lung Cancer. Diagnostics 2021, 11, 2201. [Google Scholar] [CrossRef] [PubMed]
- Crowley, L.E.; Stockley, R.A.; Thickett, D.R.; Dosanjh, D.; Scott, A.; Parekh, D. Neutrophil dynamics in pulmonary fibrosis: Pathophysiological and therapeutic perspectives. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2024, 33, 240139. [Google Scholar] [CrossRef]
- Parajuli, N.; Yao, Y.; Khalasawi, N.; Yin, C.; Zhang, Q.; Adrianto, I.; Hans, A.; Zhou, L.; Mi, Q.S. MicroRNAs regulate alveolar macrophage homeostasis and its function in lung fibrosis. Front. Immunol. 2025, 16, 1598306. [Google Scholar] [CrossRef]
- Liu, X.; Qin, X.; Qin, H.; Jia, C.; Yuan, Y.; Sun, T.; Chen, B.; Chen, C.; Zhang, H. Characterization of the heterogeneity of endothelial cells in bleomycin-induced lung fibrosis using single-cell RNA sequencing. Angiogenesis 2021, 24, 809–821. [Google Scholar] [CrossRef]
- Strunz, M.; Simon, L.M.; Ansari, M.; Kathiriya, J.J.; Angelidis, I.; Mayr, C.H.; Tsidiridis, G.; Lange, M.; Mattner, L.F.; Yee, M.; et al. Alveolar regeneration through a Krt8+ transitional stem cell state that persists in human lung fibrosis. Nat. Commun. 2020, 11, 3559. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.C.; Ryffel, B. The Chemokine System as a Key Regulator of Pulmonary Fibrosis: Converging Pathways in Human Idiopathic Pulmonary Fibrosis (IPF) and the Bleomycin-Induced Lung Fibrosis Model in Mice. Cells 2024, 13, 2058. [Google Scholar] [CrossRef]

| Variables | N (%) | |
|---|---|---|
| Age at diagnosis (median ± SS) | 32.19 ± 9.62 | 30.79 (15.73–65.33) |
| Age at diagnosis | 15–29 years | 53 (44.92) |
| 30–39 years | 43 (36.44) | |
| 40–49 years | 15 (12.71) | |
| ≥50 years | 7 (5.93) | |
| Eastern Cooperative Oncology Group (ECOG) performance status | 0 | 112 (94.92) |
| 1 | 3 (2.54) | |
| 2 | 1 (0.85) | |
| 3 | 2 (1.69) | |
| Histopathology | Seminoma | 43 (36.44) |
| Embryonal carcinoma | 20 (16.95) | |
| Mixed germ cell carcinoma | 54 (45.76) | |
| Epididymal invasion | No | 112 (94.92) |
| Yes | 6 (5.08) | |
| Tunica albuginea invasion | No | 101 (85.59) |
| Yes | 17 (14.41) | |
| Tunica vaginalis invasion | No | 112 (94.92) |
| Yes | 6 (5.08) | |
| Lymphovascular invasion | No | 52 (44.07) |
| Yes | 66 (55.93) | |
| Tumor localization | Right | 56 (47.46) |
| Left | 59 (50) | |
| Bilateral | 3 (2.54) | |
| Diagnostic symptom | Mass | 53 (44.92) |
| Swelling | 35 (29.66) | |
| Pain | 30 (25.42) | |
| Stage | Stage 1 | 69 (58.47) |
| Stage 2 | 29 (24.58) | |
| Stage 3 | 20 (16.95) | |
| Tumor size | 42.61 ± 24.7 | 35 (6–120) |
| Radiotherapy | No | 113 (95.76) |
| Yes | 5 (4.24) | |
| Surgery | No | 4 (3.39) |
| Yes | 114 (96.61) | |
| Retroperitoneal lymph node dissection | No | 86 (72.88) |
| Yes | 32 (27.12) | |
| Adjuvant therapy | No | 19 (16.1) |
| Yes | 99 (83.9) | |
| Number of adjuvant cycles | 2.96 ± 0.94 | 3 (1–6) |
| Pulmonary toxicity | No | 95 (80.51) |
| Yes | 23 (19.49) | |
| Decrease in DLCO in patients with pulmonary toxicity | ≤10% | 7 (33.33) |
| >10% | 14 (66.67) | |
| Use of GCSF in patients developing pulmonary toxicity | No | 10 (45.45) |
| Yes | 12 (54.55) | |
| Progression | No | 93 (78.81) |
| Yes | 25 (21.19) | |
| Living situation | Alive | 107 (90.68) |
| Exitus | 11 (9.32) | |
| Median overall survival (mOS) | 159.86 ± 4.34 (min/max:151.346–168.364) | |
| NLR | ≤1.64 | 50 (42.37) |
| >1.64 | 68 (57.63) | |
| PLR | ≤93.92 | 32 (27.12) |
| >93.92 | 86 (72.88) | |
| LMR | ≤4.85 | 70 (59.32) |
| >4.85 | 48 (40.68) | |
| CLR | ≤0.49 | 66 (55.93) |
| >0.49 | 52 (44.07) | |
| SII | ≤444.25 | 55 (46.61) |
| >444.25 | 63 (53.39) | |
| SIRI | ≤0.66 | 43 (36.44) |
| >0.66 | 75 (63.56) | |
| Median forced vital capacity (FVC) | 85.96 ± 7.41 | 87 (73–105) |
| Median carbon monoxide diffusion capacity (DLCO) | 68.18 ± 13.44 | 70 (34–88) |
| Variables | Pulmonary Toxicity | p | ||
|---|---|---|---|---|
| No | Yes | |||
| N (%) | N (%) | |||
| Age at diagnosis | 15–29 years | 43 (45.26) | 10 (43.48) | 0.880 |
| 30–39 years | 35 (36.84) | 8 (34.78) | ||
| 40–49 years | 12 (12.63) | 3 (13.04) | ||
| ≥50 years | 5 (5.26) | 2 (8.7) | ||
| Smoking history | No | 66 (69.47) | 10 (43.48) | 0.028 * |
| Yes | 29 (30.53) | 13 (56.52) | ||
| Eastern Cooperative Oncology Group (ECOG) performance status | 0 | 89 (93.68) | 23 (100) | 1.000 |
| 1 | 3 (3.16) | 0 (0) | ||
| 2 | 1 (1.05) | 0 (0) | ||
| 3 | 2 (2.11) | 0 (0) | ||
| Histopathology | Seminoma | 34 (35.79) | 9 (39.13) | 0.716 |
| Embryonal carcinoma | 15 (15.79) | 5 (21.74) | ||
| Mixed germ cell carcinoma | 45 (47.37) | 9 (39.13) | ||
| Epididymal invasion | No | 90 (94.74) | 22 (95.65) | 1.000 |
| Yes | 5 (5.26) | 1 (4.35) | ||
| Tunica albuginea invasion | No | 82 (86.32) | 19 (82.61) | 0.741 |
| Yes | 13 (13.68) | 4 (17.39) | ||
| Tunica vaginalis invasion | No | 89 (93.68) | 23 (100) | 0.596 |
| Yes | 6 (6.32) | 0 (0) | ||
| Lymphovascular invasion | No | 43 (45.26) | 9 (39.13) | 0.646 |
| Yes | 52 (54.74) | 14 (60.87) | ||
| Tumor localization | Right | 46 (48.42) | 10 (43.48) | 0.818 |
| Left | 46 (48.42) | 13 (56.52) | ||
| Bilateral | 3 (3.16) | 0 (0) | ||
| Diagnostic symptom | Mass | 40 (42.11) | 13 (56.52) | 0.362 |
| Swelling | 31 (32.63) | 4 (17.39) | ||
| Pain | 24 (25.26) | 6 (26.09) | ||
| Stage | Stage 1 | 56 (58.95) | 13 (56.52) | 0.381 |
| Stage 2 | 25 (26.32) | 4 (17.39) | ||
| Stage 3 | 14 (14.74) | 6 (26.09) | ||
| Radiotherapy | No | 90 (94.74) | 23 (100) | 0.582 |
| Yes | 5 (5.26) | 0 (0) | ||
| Surgery | No | 4 (4.21) | 0 (0) | 1.000 |
| Yes | 91 (95.79) | 23 (100) | ||
| Retroperitoneal lymph node dissection | No | 69 (72.63) | 17 (73.91) | 1.000 |
| Yes | 26 (27.37) | 6 (26.09) | ||
| Adjuvant therapy | No | 15 (15.79) | 4 (17.39) | 1.000 |
| Yes | 80 (84.21) | 19 (82.61) | ||
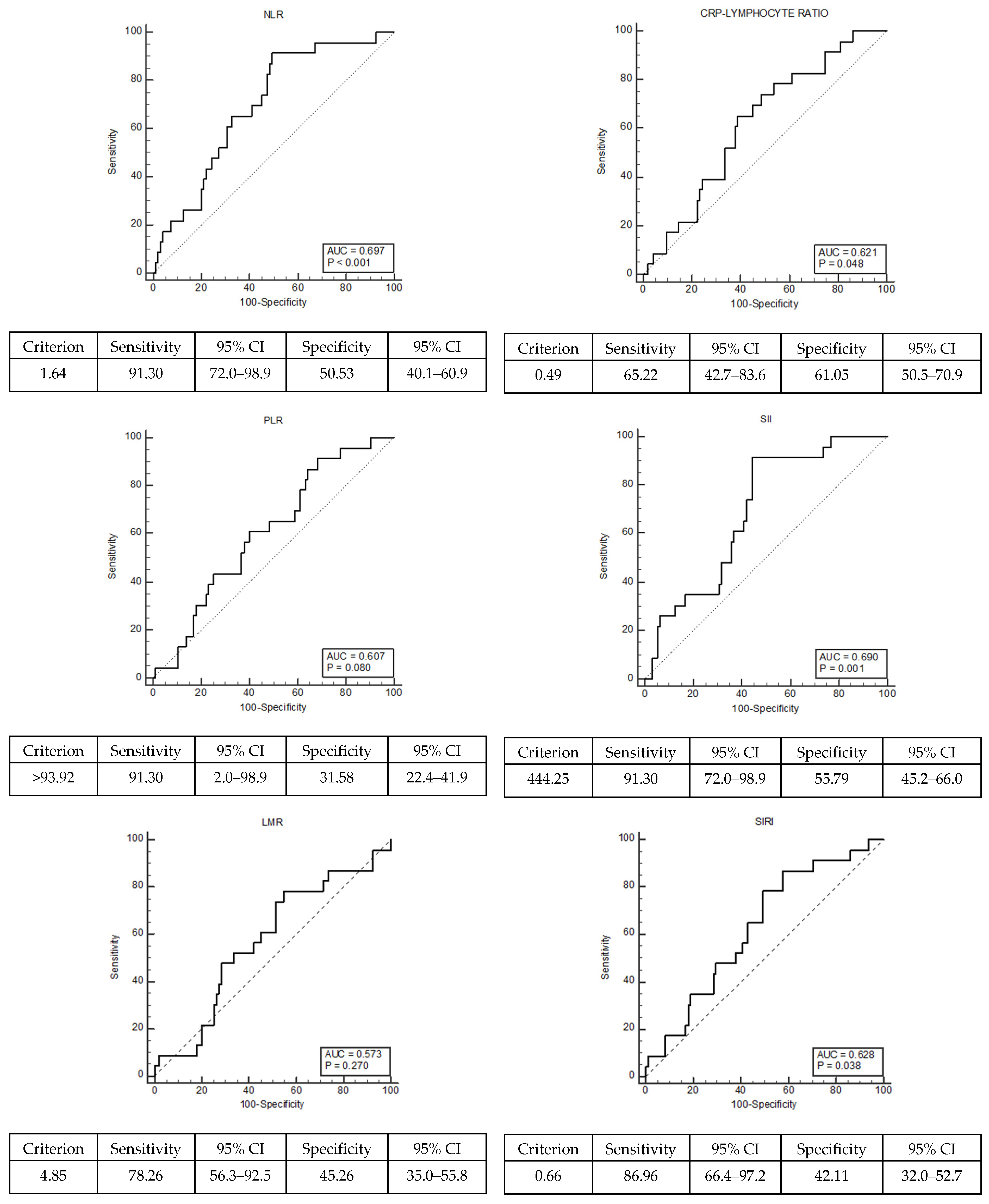
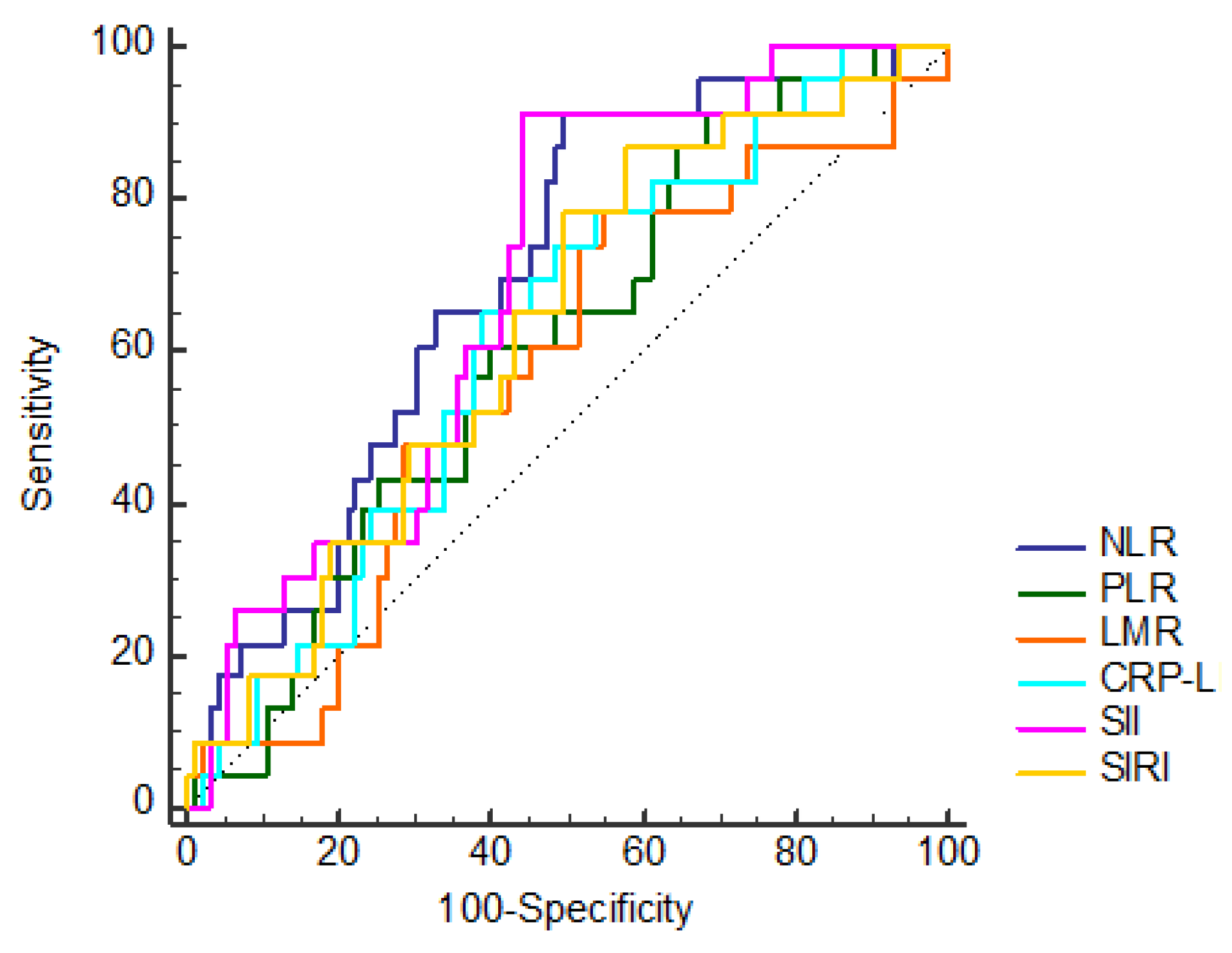
| NLR | ≤1.64 | 48 (50.53) | 2 (8.7) | 0.001 * |
| >1.64 | 47 (49.47) | 21 (91.3) | ||
| PLR | ≤93.92 | 30 (31.58) | 2 (8.7) | 0.035 * |
| >93.92 | 65 (68.42) | 21 (91.3) | ||
| LMR | ≤4.85 | 52 (54.74) | 18 (78.26) | 0.057 |
| >4.85 | 43 (45.26) | 5 (21.74) | ||
| CLR | ≤0.49 | 58 (61.05) | 8 (34.78) | 0.034 * |
| >0.49 | 37 (38.95) | 15 (65.22) | ||
| SII | ≤444.25 | 53 (55.79) | 2 (8.7) | 0.001 * |
| >444.25 | 42 (44.21) | 21 (91.3) | ||
| SIRI | ≤0.66 | 40 (42.11) | 3 (13.04) | 0.014 * |
| >0.66 | 55 (57.89) | 20 (86.96) | ||
| Variables | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | ||
| Age at diagnosis | 15–29 years | 1 (reference) | 0.941 | ||
| 30–39 years | 0.983 (0.35–2.756) | 0.974 | |||
| 40–49 years | 1.075 (0.255–4.538) | 0.922 | |||
| ≥50 years | 1.72 (0.291–10.182) | 0.550 | |||
| Smoking history | Yes | 2.959 (1.164–7.52) | 0.023 * | 5.23 (1.536–17.814) | 0.008 * |
| Histopathology | Seminoma | 1 (reference) | 0.872 | ||
| Embryonal carcinoma | 1.259 (0.361–4.398) | 0.718 | |||
| Mixed germ cell carcinoma | 0.756 (0.271–2.107) | 0.592 | |||
| Lymphovascular invasion | No | 1 (reference) | |||
| Yes | 1.286 (0.508–3.259) | 0.596 | |||
| Tumor localization | Right | 1 (reference) | 0.855 | ||
| Left | 1.3 (0.518–3.263) | 0.576 | |||
| Diagnostic symptom | Mass | 1 (reference) | 0.329 | ||
| Swelling | 0.397 (0.118–1.338) | 0.136 | |||
| Pain | 0.769 (0.258–2.292) | 0.638 | |||
| Stage | Stage 1 | 1 (reference) | 0.374 | ||
| Stage 2 | 0.689 (0.204–2.325) | 0.549 | |||
| Stage 3 | 1.846 (0.596–5.72) | 0.288 | |||
| Retroperitoneal lymph node dissection | No | 1 (reference) | |||
| Yes | 0.937 (0.333–2.635) | 0.901 | |||
| NLR | ≤1.64 | 1 (reference) | |||
| >1.64 | 10.723 (2.38–48.306) | 0.002 * | |||
| PLR | ≤93.92 | 1 (reference) | |||
| >93.92 | 4.846 (1.067–22.015) | 0.041 * | |||
| LMR | ≤4.85 | 1 (reference) | |||
| >4.85 | 0.336 (0.115–0.979) | 0.046* | |||
| CLR | ≤0.49 | 1 (reference) | |||
| >0.49 | 2.939 (1.134–7.615) | 0.026 * | |||
| SII | ≤444.25 | 1 (reference) | |||
| >444.25 | 13.25 (2.939–59.731) | 0.001 * | |||
| SIRI | ≤0.66 | 1 (reference) | |||
| >0.66 | 4.848 (1.348–17.439) | 0.016 * | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özdemir, M.; Gököz Doğu, G.; Yapar Taşköylü, B.; Demiray, A.G.; Yaren, A.; Değirmencioğlu, S. Lymphocyte-Associated Inflammation Markers Predict Bleomycin-Induced Pulmonary Toxicity in Testicular Cancer. J. Clin. Med. 2025, 14, 7926. https://doi.org/10.3390/jcm14227926
Özdemir M, Gököz Doğu G, Yapar Taşköylü B, Demiray AG, Yaren A, Değirmencioğlu S. Lymphocyte-Associated Inflammation Markers Predict Bleomycin-Induced Pulmonary Toxicity in Testicular Cancer. Journal of Clinical Medicine. 2025; 14(22):7926. https://doi.org/10.3390/jcm14227926
Chicago/Turabian StyleÖzdemir, Melek, Gamze Gököz Doğu, Burcu Yapar Taşköylü, Atike Gökçen Demiray, Arzu Yaren, and Serkan Değirmencioğlu. 2025. "Lymphocyte-Associated Inflammation Markers Predict Bleomycin-Induced Pulmonary Toxicity in Testicular Cancer" Journal of Clinical Medicine 14, no. 22: 7926. https://doi.org/10.3390/jcm14227926
APA StyleÖzdemir, M., Gököz Doğu, G., Yapar Taşköylü, B., Demiray, A. G., Yaren, A., & Değirmencioğlu, S. (2025). Lymphocyte-Associated Inflammation Markers Predict Bleomycin-Induced Pulmonary Toxicity in Testicular Cancer. Journal of Clinical Medicine, 14(22), 7926. https://doi.org/10.3390/jcm14227926





