Hyperuricemia as an Early Indicator of Cardiovascular Risk in the General Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Recruitment
2.3. Parameters Controlled
2.4. Cardiovascular Risk Factors
- BMI ≥ 30 kg/m2
- LDL > 130 mg/dL
- Smoking
- Diabetes Mellitus: HbA1c > 6.5% or pharmacological treatment
- Blood pressure ≥ 140/90 mmHg
2.5. Physical Activity
- Regular physical activity: ≥ 1× daily or 2–3× per week
- Moderate activity: 1×/week or every 2 weeks
- Rare activity/Inactivity: 1×/month or less
2.6. Cognitive Impairment (DemTect)
- Physiological: >13 points
- Pathological: 0–12 points
2.7. Laboratory Parameters
2.8. Follow-Up
2.9. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | Atrial Fibrillation |
| BMI | Body mass index |
| CAD | Coronary artery disease |
| CKD | Chronic kidney disease |
| CVRF | Cardiovascular risk factors |
| eGFR | Estimated glomerular filtration rate |
| HbA1c | Glycated hemoglobin |
| HDL | High-density lipoprotein |
| HF | Heart failure |
| HU | Hyperuricemia |
| KDIGO | Kidney Disease: Improving Global Outcomes |
| LDL | Low-density lipoprotein |
| Lp(a) | Lipoprotein (a) |
| MI | Myocardial Infarction |
| PAD | Peripheral artery disease |
| SD | Standard deviation |
| SPSS | Statistical Package for the Social Sciences |
| XOI | Xanthine oxidase inhibitor |
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Tan, S.C.W.; Zheng, B.-B.; Tang, M.-L.; Chu, H.; Zhao, Y.-T.; Weng, C. Global Burden of Cardiovascular Diseases and its Risk Factors, 1990–2021: A Systematic Analysis for the Global Burden of Disease Study 2021. QJM Int. J. Med. 2025, 118, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Boehme, A.K.; Esenwa, C.; Elkind, M.S. Stroke Risk Factors, Genetics, and Prevention. Circ. Res. 2017, 120, 472–495. [Google Scholar] [CrossRef]
- O’Donnell, M.J.; Xavier, D.; Liu, L.; Zhang, H.; Chin, S.L.; Rao-Melacini, P.; Rangarajan, S.; Islam, S.; Pais, P.; McQueen, M.J.; et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): A case-control study. Lancet 2010, 376, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Garrod, A.B. A Treatise on Gout and Rheumatic Gout (Rheumatoid Arthritis); Longmans, Green: London, UK, 1876. [Google Scholar]
- Brand, F.N.; McGee, D.L.; Kannel, W.B.; Stokes, J., 3rd; Castelli, W.P. Hyperuricemia as a risk factor of coronary heart disease: The Framingham Study. Am. J. Epidemiol. 1985, 121, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Shan, R.; Ning, Y.; Ma, Y.; Gao, X.; Zhou, Z.; Jin, C.; Wu, J.; Lv, J.; Li, L. Incidence and Risk Factors of Hyperuricemia among 2.5 Million Chinese Adults during the Years 2017–2018. Int. J. Environ. Res. Public Health 2021, 18, 2360. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Cheng, J.; Huangfu, N.; Zhao, R.; Xu, Z.; Zhang, F.; Zheng, W.; Zhang, D. Hyperuricemia and Cardiovascular Disease. Curr. Pharm. Des. 2019, 25, 700–709. [Google Scholar] [CrossRef]
- Deutsche Gesellschaft für Rheumatologie. Leitlinie: Diagnostik und Therapie der Gicht. 2024. Available online: https://register.awmf.org/de/leitlinien/detail/060-005 (accessed on 12 July 2025).
- Matsuo, H.; Nakayama, A.; Sakiyama, M.; Chiba, T.; Shimizu, S.; Kawamura, Y.; Nakashima, H.; Nakamura, T.; Takada, Y.; Oikawa, Y.; et al. ABCG2 dysfunction causes hyperuricemia due to both renal urate underexcretion and renal urate overload. Sci. Rep. 2014, 4, 3755. [Google Scholar] [CrossRef]
- Waheed, Y.; Yang, F.; Sun, D. Role of asymptomatic hyperuricemia in the progression of chronic kidney disease and cardiovascular disease. Korean J. Intern. Med. 2021, 36, 1281–1293. [Google Scholar] [CrossRef]
- Yanai, H.; Adachi, H.; Hakoshima, M.; Katsuyama, H. Molecular Biological and Clinical Understanding of the Pathophysiology and Treatments of Hyperuricemia and Its Association with Metabolic Syndrome, Cardiovascular Diseases and Chronic Kidney Disease. Int. J. Mol. Sci. 2021, 22, 9221. [Google Scholar] [CrossRef]
- He, Y.; Chen, D.; Xu, J.-P.; Jin, J.; Wang, J.; Geng, C.; He, Y.-M. Association between Serum Uric Acid and Hypertension in a Large Cross-Section Study in a Chinese Population. J. Cardiovasc. Dev. Dis. 2022, 9, 346. [Google Scholar] [CrossRef]
- Landmesser, U.; Spiekermann, S.; Dikalov, S.; Tatge, H.; Wilke, R.; Kohler, C.; Harrison, D.G.; Hornig, B.; Drexler, H. Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: Role of xanthine-oxidase and extracellular superoxide dismutase. Circulation 2002, 106, 3073–3078. [Google Scholar] [CrossRef]
- Packer, M. Uric Acid Is a Biomarker of Oxidative Stress in the Failing Heart: Lessons Learned from Trials with Allopurinol and SGLT2 Inhibitors. J. Card. Fail. 2020, 26, 977–984. [Google Scholar] [CrossRef]
- Kleber, M.E.; Delgado, G.; Grammer, T.B.; Silbernagel, G.; Huang, J.; Krämer, B.K.; Ritz, E.; März, W. Uric Acid and Cardiovascular Events: A Mendelian Randomization Study. J. Am. Soc. Nephrol. 2015, 26, 2831–2838. [Google Scholar] [CrossRef]
- Bos, M.J.; Koudstaal, P.J.; Hofman, A.; Witteman, J.C.; Breteler, M.M. Uric acid is a risk factor for myocardial infarction and stroke: The Rotterdam study. Stroke 2006, 37, 1503–1507. [Google Scholar] [CrossRef]
- Ekundayo, O.J.; Dell’Italia, L.J.; Sanders, P.W.; Arnett, D.; Aban, I.; Love, T.E.; Filippatos, G.; Anker, S.D.; Lloyd-Jones, D.M.; Bakris, G.; et al. Association between hyperuricemia and incident heart failure among older adults: A propensity-matched study. Int. J. Cardiol. 2010, 142, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Guevara, J.P.; Kim, K.M.; Choi, H.K.; Heitjan, D.F.; Albert, D.A. Hyperuricemia and risk of stroke: A systematic review and meta-analysis. Arthritis Care Res. 2009, 61, 885–892. [Google Scholar] [CrossRef]
- Nyrnes, A.; Toft, I.; Njølstad, I.; Mathiesen, E.B.; Wilsgaard, T.; Hansen, J.B.; Løchen, M.-L. Uric acid is associated with future atrial fibrillation: An 11-year follow-up of 6308 men and women--the Tromso Study. Europace 2014, 16, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Feig, D.I.; Stack, A.G.; Kang, D.H.; Lanaspa, M.A.; Ejaz, A.A.; Sánchez-Lozada, L.G.; Kuwabara, M.; Borghi, C.; Johnson, R.J. The case for uric acid-lowering treatment in patients with hyperuricaemia and CKD. Nat. Rev. Nephrol. 2019, 15, 767–775. [Google Scholar] [CrossRef]
- Gupta, M.K.; Singh, J.A. Cardiovascular Disease in Gout and the Protective Effect of Treatments Including Urate-Lowering Therapy. Drugs 2019, 79, 531–541. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, J.D.; Dalbeth, N.; Mikuls, T.; Brignardello-Petersen, R.; Guyatt, G.; Abeles, A.M.; Gelber, A.C.; Harrold, L.R.; Khanna, D.; King, C.; et al. 2020 American College of Rheumatology Guideline for the Management of Gout. Arthritis Care Res. 2020, 72, 744–760. [Google Scholar] [CrossRef]
- Holubarsch, C. Deutsche Gesellschaft für Kardiologie: Harnsäuresenkung zur Kardiovaskulären Risikoreduktion? 2016. Available online: https://dgk.org/pressemitteilungen/2016-ht-pm/2016-ht-statements/2016-ht-statements-tag2/harnsaeuresenkung-zur-kardiovaskulaeren-risikoreduktion/ (accessed on 21 January 2025).
- Shubietah, A.; Awashra, A.; Milhem, F.; Ghannam, M.; Hattab, M.; Rajab, I.; Neiroukh, H.; Zahdeh, M.; Nouri, A.; Assaassa, A.; et al. Hyperuricemia and Cardiovascular Risk: Insights and Implications. Crit. Pathw. Cardiol. 2025, 24, e0388. [Google Scholar] [CrossRef]
- Zhiwei, W.; Guiping, Y.; Bing, Y.; Chenghao, Z. The relationship between hyperuricemia and hypertension: A short review of current evidence. Metab. Target Organ Damage 2024, 4, 3. [Google Scholar] [CrossRef]
- Zheng, L.; Zhu, Y.; Ma, Y.; Zhang, H.; Zhao, H.; Zhang, Y.; Yang, Z.; Liu, Y. Relationship between hyperuricemia and the risk of cardiovascular events and chronic kidney disease in both the general population and hypertensive patients: A systematic review and meta-analysis. Int. J. Cardiol. 2024, 399, 131779. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, M.; Hisatome, I.; Ae, R.; Kosami, K.; Aoki, Y.; Andres-Hernando, A.; Kanbay, M.; Lanaspa, M.A. Hyperuricemia, A new cardiovascular risk. Nutr. Metab. Cardiovasc. Dis. 2025, 35, 103796. [Google Scholar] [CrossRef] [PubMed]
- Lüders, S.; Schrader, B.; Bäsecke, J.; Haller, H.; Elsässer, A.; Koziolek, M.; Schrader, J. ELITE study—Nutrition, lifestyle and individual information for the prevention of stroke, dementia and heart attack—Study design and cardiovascular status. Dtsch. Med. Wochenschr. 2019, 144, e42–e50. [Google Scholar]
- Schrader, B.; Schrader, J.; Koziolek, M.; Elsässer, A.; Bünker, A.M.; Hillmann, B.; Vaske, B.; Haller, H.; Lüders, S. Influence of individualized prevention recommendations after one year on the control of hypertension in 3,868 follow-up participants of the ELITE study. Cent. Eur. J. Public Health 2021, 29, 305–310. [Google Scholar] [CrossRef]
- Zitt, E.; Fischer, A.; Lhotta, K.; Concin, H.; Nagel, G. Sex- and age-specific variations, temporal trends and metabolic determinants of serum uric acid concentrations in a large population-based Austrian cohort. Sci. Rep. 2020, 10, 7578. [Google Scholar] [CrossRef]
- Liu, D.; Zheng, X.; Zhu, J.; Yang, J.; Lu, L.; Ji, X.; Hui, J.; Luo, Y. Gender-specific association between serum uric acid levels and hypertension in East China: A cross-sectional study. BMC Public Health 2025, 25, 944. [Google Scholar] [CrossRef]
- Nakahashi, T.; Tada, H.; Sakata, K.; Takamura, M. Gout, Uric Acid, and Coronary Artery Disease. J. Atheroscler. Thromb. 2025. [Google Scholar] [CrossRef]
- Choi, H.K.; Ford, E.S. Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am. J. Med. 2007, 120, 442–447. [Google Scholar] [CrossRef]
- Yuan, H.; Yu, C.; Li, X.; Sun, L.; Zhu, X.; Zhao, C.; Zhang, Z.; Yang, Z. Serum Uric Acid Levels and Risk of Metabolic Syndrome: A Dose-Response Meta-Analysis of Prospective Studies. J. Clin. Endocrinol. Metab. 2015, 100, 4198–4207. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, T.; Liu, Y.; Chang, Q.; Zhao, Y.; Guo, C.; Xia, Y. Prevalence of Diabetes in Patients with Hyperuricemia and Gout: A Systematic Review and Meta-analysis. Curr. Diabetes Rep. 2023, 23, 103–117. [Google Scholar] [CrossRef]
- Sowers, J.R.; Whaley-Connell, A.; Hayden, M.R. The Role of Overweight and Obesity in the Cardiorenal Syndrome. Cardiorenal Med. 2011, 1, 5–12. [Google Scholar] [CrossRef]
- Jin, M.; Yang, F.; Yang, I.; Yin, Y.; Luo, J.J.; Wang, H.; Yang, X.-F. Uric acid, hyperuricemia and vascular diseases. Front. Biosci. J. Virtual Libr. 2012, 17, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, L.F.; Navas-Acien, A.; Brady, T.M.; Miller, E.R., 3rd; Fadrowski, J.J. Uric acid level and elevated blood pressure in US adolescents: National Health and Nutrition Examination Survey, 1999–2006. Hypertension 2012, 59, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Suliman, M.E.; Johnson, R.J.; García-López, E.; Qureshi, A.R.; Molinaei, H.; Carrero, J.J.; Heimbürger, O.; Bárány, P.; Axelsson, J.; Lindholm, B.; et al. J-shaped mortality relationship for uric acid in CKD. Am. J. Kidney Dis. 2006, 48, 761–771. [Google Scholar] [CrossRef]
- Regionalkomitee für Europa T. Fünfundsechzigste Tagung des Regionalkomitees für Europa: Vilnius, 14–17 September 2015: Strategie der Europäischen Region der WHO zur Bewegungsförderung (2016–2025); Weltgesundheitsorganisation; Regionalbüro für Europa: Vilnius, Lithuania, 2015. [Google Scholar]
- Zhang, W.Z.; Peng, Q.; Cai, X.S.; Jiang, G.L.; Huang, J.J.; Lu, L.L.; Feng, W.-Z.B.; Yan, P.-Y.; Gu, J.-R. A study on the correlation between hyperuricemia and lifestyle and dietary habits. Medicine 2025, 104, e41399. [Google Scholar] [CrossRef]
- Chen, J.H.; Wen, C.P.; Wu, S.B.; Lan, J.L.; Tsai, M.K.; Tai, Y.P.; Lee, J.H.; Hsu, C.C.; Tsao, C.K.; Wai, J.P.M.; et al. Attenuating the mortality risk of high serum uric acid: The role of physical activity underused. Ann. Rheum. Dis. 2015, 74, 2034–2042. [Google Scholar] [CrossRef]
- Lobelo, F.; Rohm Young, D.; Sallis, R.; Garber, M.D.; Billinger, S.A.; Duperly, J.; Hutber, A.; Pate, R.R.; Thomas, R.J.; Widlansky, M.E.; et al. Routine Assessment and Promotion of Physical Activity in Healthcare Settings: A Scientific Statement from the American Heart Association. Circulation 2018, 137, e495–e522. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Cen, Y.; Yang, B.; Zhang, H.; Pu, Z.; Feng, J.; Pan, H.; Zhang, Y. Effects of dietary factors on hyperuricaemia and gout: A systematic review and meta-analysis of observational studies. Int. J. Food Sci. Nutr. 2024, 75, 753–773. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Zheng, A.; Xu, P.; Wang, J.; Xue, T.; Dai, S.; Pan, S.; Guo, Y.; Xie, X.; Li, L.; et al. High-Protein Diet Induces Hyperuricemia in a New Animal Model for Studying Human Gout. Int. J. Mol. Sci. 2020, 21, 2147. [Google Scholar] [CrossRef]
- Kawakami, Y.; Mazuka, M.; Yasuda, A.; Sato, M.; Hosaka, T.; Arai, H. Acute effect of fructose, sucrose, and isomaltulose on uric acid metabolism in healthy participants. J. Clin. Biochem. Nutr. 2023, 72, 61–67. [Google Scholar] [CrossRef]
- Olofsson, C.; Anderstam, B.; Bragfors-Helin, A.C.; Eriksson, M.; Qureshi, A.R.; Lindholm, B.; Hilding, A.; Wiczkowski, W.; Orsini, N.; Stenvinkel, P.; et al. Effects of acute fructose loading on levels of serum uric acid-a pilot study. Eur. J. Clin. Investig. 2019, 49, e13040. [Google Scholar] [CrossRef]
- Tana, C.; Ticinesi, A.; Prati, B.; Nouvenne, A.; Meschi, T. Uric Acid and Cognitive Function in Older Individuals. Nutrients 2018, 10, 975. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, S.; Ni, J.; Wei, M.; Li, T.; Long, S.; Shi, J.; Tian, J. Gout or Hyperuricemia and Dementia Risk: A Meta-Analysis of Observational Studies. J. Alzheimers Dis. 2024, 101, 417–427. [Google Scholar] [CrossRef]
- Latourte, A.; Soumaré, A.; Bardin, T.; Perez-Ruiz, F.; Debette, S.; Richette, P. Uric acid and incident dementia over 12 years of follow-up: A population-based cohort study. Ann. Rheum. Dis. 2018, 77, 328–335. [Google Scholar] [CrossRef]
- Krittanawong, C.; Maitra, N.S.; Qadeer, Y.K.; Wang, Z.; Fogg, S.; Storch, E.A.; Celano, C.M.; Huffman, J.C.; Jha, M.; Charney, D.S.; et al. Association of Depression and Cardiovascular Disease. Am. J. Med. 2023, 136, 881–895. [Google Scholar] [CrossRef] [PubMed]
- Latourte, A.; Bardin, T.; Richette, P. Uric acid and cognitive decline: A double-edge sword? Curr. Opin. Rheumatol. 2018, 30, 183–187. [Google Scholar] [CrossRef] [PubMed]
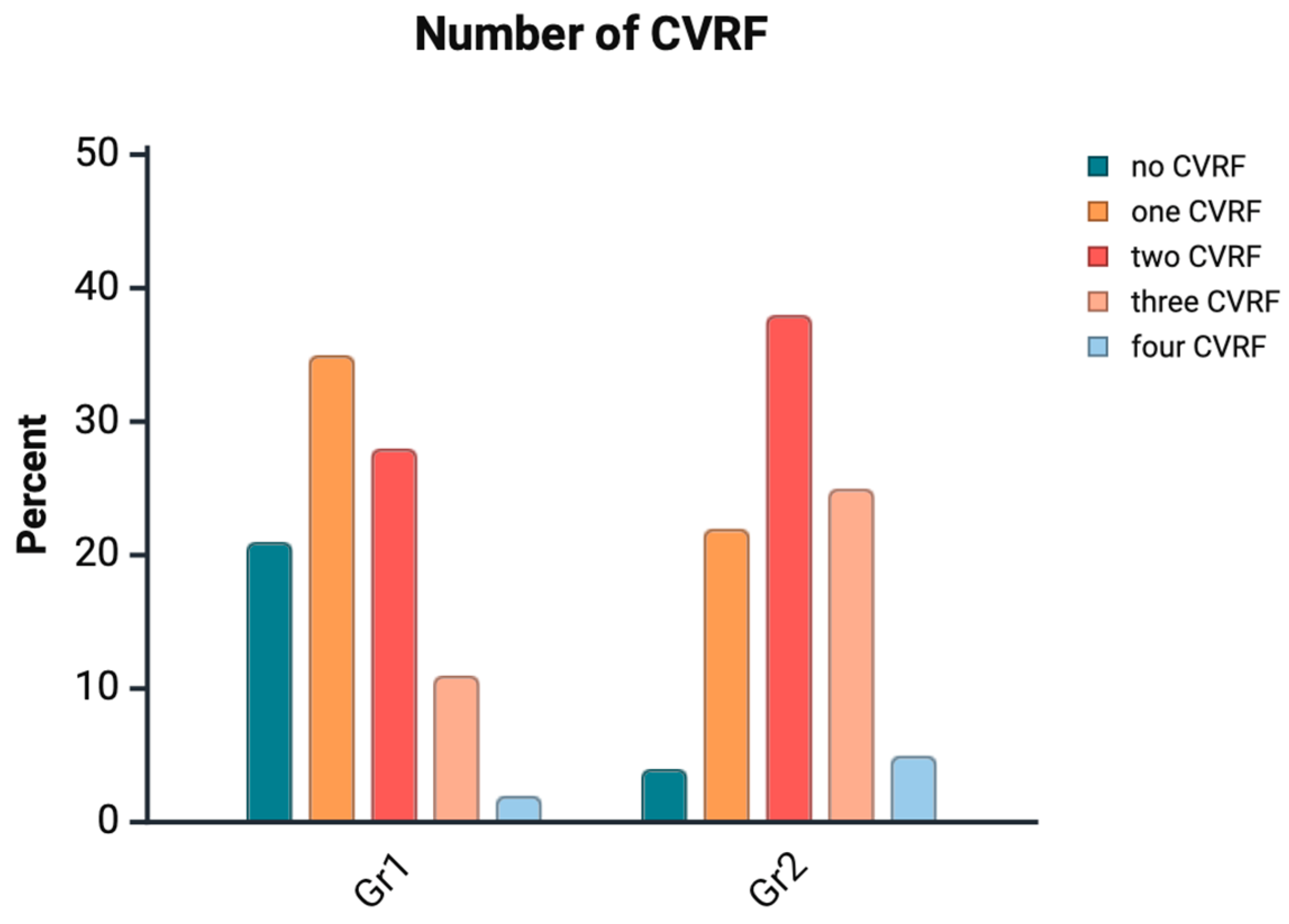
| Group 1 | Group 2 | p-Value | |
|---|---|---|---|
| Participants n (%) | 3288 (80.3) | 805 (19.7) | <0.001 |
| Age mean (SD) in years | 51.8 (13.6) | 58.0 (14.1) | <0.001 |
| Male, n (%) | 1589 (48.5) | 490 (60.9) | <0.001 |
| Female, n (%) | 1688 (51.5) | 315 (39.1) | <0.001 |
| Serum uric acid, mean (SD) in mg/dL | 4.8 (1.03) | 7.1 (1.13) | <0.001 |
| Serum uric acid level > 6.5 mg/dL, n (%) | 743 (92.2) | <0.001 | |
| Serum uric acid > 10 mg/dL, n (%) | 13 (1.6) | <0.001 | |
| Diabetes mellitus, n (%) | 146 (4.9) | 103 (12.8) | <0.001 |
| Arterial Hypertension, n (%) | 1847 (56.4) | 666 (82.7) | <0.001 |
| BMI > 30 kg/m2 n (%) | 553 (16.9) | 350 (42.5) | <0.001 |
| Tobacco use, n (%) | 467 (14.3) | 91 (11.3) | 0.116 |
| HbA1C * %, mean (SD) in % | 5.2 (0.56) | 5.6 (0.65) | <0.001 |
| LDL *, mean (SD) in mg/dL | 128.9 (35.4) | 136.9 (34.9) | <0.001 |
| HDL *, mean (SD) in mg/dL | 63 (18.7) | 52.6 (15.6) | <0.001 |
| Lp(a) *, mean (SD) in nmol/L | 42.1 (20.3) | 43.4 (16.4) | <0.001 |
| eGFR *, mean (SD) in mL/min | 102.7 (24.5) | 82.1 (26.4) | <0.001 |
| Stroke, n (%) | 54 (1.64) | 28 (3.4) | 0.002 |
| CAD *, n (%) | 96 (2.9) | 75 (9.3) | <0.001 |
| MI *, n (%) | 38 (1.15) | 40 (4.9) | <0.001 |
| HF *, n (%) | 56 (1.7) | 36 (4.4) | <0.001 |
| AF *, n (%) | 94 (2.8) | 55 (6.8) | <0.001 |
| PAD *, n (%) | 154 (4.7) | 59 (7.3) | 0.003 |
| CKD *, n (%) | 63 (1.92) | 114 (14.1) | <0.001 |
| Regular physical activity, n (%) | 1630 (43.1) | 275 (33.5) | <0.001 |
| Moderate physical activity, n (%) | 1081 (28.6) | 244 (29.6) | <0.001 |
| Sparse physical activity, n (%) | 1071 (28.3) | 301 (36.7) | <0.001 |
| High fruit intake, n (%) | 2221 (58.7) | 460 (56.1) | <0.001 |
| Moderate fruit intake, n (%) | 934 (24.7) | 205 (25.0) | 0.104 |
| Low fruit intake, n (%) | 579 (15.3) | 142 (17.3) | 0.27 |
| High meat intake, n (%) | 900 (13.3) | 186 (22.7) | 0.016 |
| Moderate meat intake, n (%) | 2159 (65.0) | 494 (61.3) | 0.024 |
| Low meat intake, n (%) | 686 (20.9) | 129 (16.0) | 0.002 |
| DemTect baseline, mean (SD) in points | 16.3 (2.2) | 15.5 (2.8) | |
| DemTect follow-up, mean (SD) in points | 16.6 (2.2) | 16.4 (2.2) | |
| Group 1 | Group 2 | p-Value | |
|---|---|---|---|
| CAD *, n (%) | 108 (3.3) | 67 (8.4) | <0.001 |
| HF *, n (%) | 31 (0.9) | 27 (3.4) | <0.001 |
| Stroke *, n (%) | 40 (1.2) | 21 (2.6) | <0.001 |
| MI *, n (%) | 44 (1.3) | 25 (3.1) | <0.001 |
| PAD *, n (%) | 77 (2.4) | 36 (4.5) | <0.001 |
| AF *, n (%) | 67 (2.0) | 39 (4.9) | <0.001 |
| CKD *, n (%) | 62 (1.9) | 44 (5.5) | <0.001 |
| Age | Diabetes Mellitus | Hyperuricemia | |
|---|---|---|---|
| CAD *, p-Value | <0.001 | <0.001 | <0.001 |
| HF *, p-Value | <0.001 | 0.018 | 0.092 |
| Stroke *, p-Value | <0.001 | <0.001 | 0.049 |
| PAD *, p-Value | <0.001 | 0.526 | 0.115 |
| AF *, p-Value | <0.001 | 0.414 | 0.441 |
| CKD *, p-Value | <0.001 | 0.015 | 0.022 |
| MI *, p-Value | <0.001 | 0.011 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Azem, F.; Schrader, B.; Schrader, J.; Elsässer, A.; Vaske, B.; Lüders, S. Hyperuricemia as an Early Indicator of Cardiovascular Risk in the General Population. J. Clin. Med. 2025, 14, 7922. https://doi.org/10.3390/jcm14227922
Al-Azem F, Schrader B, Schrader J, Elsässer A, Vaske B, Lüders S. Hyperuricemia as an Early Indicator of Cardiovascular Risk in the General Population. Journal of Clinical Medicine. 2025; 14(22):7922. https://doi.org/10.3390/jcm14227922
Chicago/Turabian StyleAl-Azem, Fady, Bastian Schrader, Joachim Schrader, Albrecht Elsässer, Bernhard Vaske, and Stephan Lüders. 2025. "Hyperuricemia as an Early Indicator of Cardiovascular Risk in the General Population" Journal of Clinical Medicine 14, no. 22: 7922. https://doi.org/10.3390/jcm14227922
APA StyleAl-Azem, F., Schrader, B., Schrader, J., Elsässer, A., Vaske, B., & Lüders, S. (2025). Hyperuricemia as an Early Indicator of Cardiovascular Risk in the General Population. Journal of Clinical Medicine, 14(22), 7922. https://doi.org/10.3390/jcm14227922





