Sarcopenic Obesity and Sarcopenic Visceral Obesity, Calculated Using the Skeletal Muscle İndex and Visceral Fat İndex at the L3 Vertebra Level, Do Not Predict Survival Rates in Endometrial Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Patient Characteristics
- Body mass index; BMI < 25 normal Weight, BMI: 25–29 Overweight, BMI ≥ 30 Obesity.
- Sarcopenia was calculated as skeletal muscle area divided by height squared (cm2/m2) at L3 vertebral level. Since there was no standardized cut-off value, the cut-off value was accepted as the median value. Calculated measurements below the median value were considered as sarcopenia.
- VFI was calculated as the median value due to the lack of standardized cut-off values for VFI. The cohort was divided into low and high VFI.
- Low SMI = sarcopenia,
- High BMI + Low SMI = sarcopenic obesity,
- High VFI + Low SMI = sarcopenic visceral obesity [16].
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EC | Endometrial Cancer |
| SMI | Skeletal muscle index |
| VFI | Visceral fat index |
| L3 | Lumbar 3 vertebral level |
| GLOBOCAN | Global Cancer Statistics 2020 |
| CT | Computed tomography |
| MRI | Magnetic resonance imaging |
| mOS | Median overall survival |
| pMMR | Proficient mismatch repair gene |
| ECOG | Eastern Cooperative Oncology Group |
| MMR | Miss Match Repair gene |
| dMMR | Deficient mismatch repair gene |
| PTEN | Loss of phosphatase and tensin homolog gene |
| ER | Estrogen Receptor |
| PR | Progesterone Receptor |
| LVI | Lymphovascular invasion |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Epidemiology of Endometrial Cancer Consortium (E2C2). Available online: https://epi.grants.cancer.gov/eecc/ (accessed on 10 October 2019).
- Soliman, P.T.; Oh, J.C.; Schmeler, K.M.; Sun, C.C.; Slomovitz, B.M.; Gershenson, D.M.; Burke, T.W.; Lu, K.H. Risk factors for young premenopausal women with endometrial cancer. Obstet. Gynecol. 2005, 105, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Schmeler, K.M.; Soliman, P.T.; Sun, C.C.; Slomovitz, B.M.; Gershenson, D.M.; Lu, K.H. Endometrial cancer in young, normal-weight women. Gynecol. Oncol. 2005, 99, 388–392. [Google Scholar] [CrossRef]
- Lachance, J.A.; Everett, E.N.; Greer, B.; Mandel, L.; Swisher, E.; Tamimi, H.; Goff, B. The effect of age on clinical/pathologic features, surgical morbidity, and outcome in patients with endometrial cancer. Gynecol. Oncol. 2006, 101, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Smrz, S.A.; Calo, C.; Fisher, J.L.; Salani, R. An ecological evaluation of the increasing incidence of endometrial cancer and the obesity epidemic. Am. J. Obstet. Gynecol. 2021, 224, 506.e1–506.e8. [Google Scholar] [CrossRef]
- Cote, M.L.; Ruterbusch, J.J.; Olson, S.H.; Lu, K.; Ali-Fehmi, R. The Growing Burden of Endometrial Cancer: A Major Racial Disparity Affecting Black Women. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1407–1415. [Google Scholar] [CrossRef]
- Berek, J.S.; Matias-Guiu, X.; Creutzberg, C.; Fotopoulou, C.; Gaffney, D.; Kehoe, S.; Lindemann, K.; Mutch, D.; Concin, N.; Endometrial Cancer Staging Subcommittee; et al. FIGO staging of endometrial cancer: 2023. Int. J. Gynaecol. Obstet. 2023, 162, 383–394, Erratum in Int. J. Gynaecol. Obstet. 2024, 166, 1374. [Google Scholar] [CrossRef]
- Zeleniuch-Jacquotte, A.; Akhmedkhanov, A.; Kato, I.; Koenig, K.L.; Shore, R.E.; Kim, M.Y.; Levitz, M.; Mittal, K.R.; Raju, U.; Banerjee, S.; et al. Postmenopausal endogenous estrogens and risk of endometrial cancer: Results of a prospective study. Br. J. Cancer 2001, 84, 975–981. [Google Scholar] [CrossRef]
- Hacker, N.F.; Friedlander, M.L. Uterine Cancer. In Berek & Hacker’s Gynecologic Oncology, 7th ed.; Berek, J.S., Hacker, N.F., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2020; pp. 371–428. [Google Scholar]
- Siiteri, P.K. Adipose tissue as a source of hormones. Am. J. Clin. Nutr. 1987, 45, 277–282. [Google Scholar] [CrossRef]
- Cao, Z.; Zheng, X.; Yang, H.; Li, S.; Xu, F.; Yang, X.; Wang, Y. Association of obesity status and metabolic syndrome with site-specific cancers: A population-based cohort study. Br. J. Cancer 2020, 123, 1336–1344. [Google Scholar] [CrossRef]
- Li, L.; Li, W.; Xu, D.; He, H.; Yang, W.; Guo, H.; Liu, X.; Ji, W.; Song, C.; Xu, H.; et al. Association Between Visceral Fat Area and Cancer Prognosis: A Population-Based Multicenter Prospective Study. Am. J. Clin. Nutr. 2023, 118, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Tisdale, M.J. Cachexia in cancer patients. Nat. Rev. Cancer 2002, 2, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.N.; Yang, S.J.; Yoo, H.J.; Lim, K.I.; Kang, H.J.; Song, W.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; et al. Prevalence of sarcopenia and sarcopenic obesity in Korean adults: The Korean sarcopenic obesity study. Int. J. Obes. 2009, 33, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Feng, W.; Huang, M.; Zhao, X. Severe loss of visceral fat and skeletal muscle after chemotherapy predicts poor prognosis in metastatic gastric cancer patients without gastrectomy. J. Cancer 2020, 11, 3310–3317. [Google Scholar] [CrossRef]
- Bosello, O.; Vanzo, A. Obesity paradox and aging. Eat. Weight Disord. 2021, 26, 27–35. [Google Scholar] [CrossRef]
- Fader, A.N.; Arriba, L.N.; Frasure, H.E.; von Gruenigen, V.E. Endometrial cancer and obesity: Epidemiology biomarkers, prevention and survivorship. Gynecol. Oncol. 2009, 114, 121–127. [Google Scholar] [CrossRef]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef]
- Abu-Abid, S.; Szold, A.; Klausner, J. Obesity and cancer. J. Med. 2002, 33, 73–86. [Google Scholar]
- Schouten, L.J.; Goldbohm, R.A.; van den Brandt, P.A. Anthropometry, physical activity, and endometrial cancer risk: Results from the Netherlands Cohort Study. J. Natl. Cancer Inst. 2004, 96, 1635–1638. [Google Scholar] [CrossRef]
- Moore, S.C.; Gierach, G.L.; Schatzkin, A.; Matthews, C.E. Physical activity, sedentary behaviours, and the prevention of endometrial cancer. Br. J. Cancer 2010, 103, 933–938. [Google Scholar] [CrossRef]
- Zhou, B.; Yang, L.; Sun, Q.; Cong, R.; Gu, H.; Tang, N.; Zhu, H.; Wang, B. Cigarette smoking and the risk of endometrial cancer: A meta-analysis. Am. J. Med. 2008, 121, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Schmid, D.; Behrens, G.; Keimling, M.; Jochem, C.; Ricci, C.; Leitzmann, M. A systematic review and meta-analysis of physical activity and endometrial cancer risk. Eur. J. Epidemiol. 2015, 30, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, R.N.; Waters, D.L.; Gallagher, D.; Morley, J.E.; Garry, P.J. Predictors of skeletal muscle mass in elderly men and women. Mech. Ageing Dev. 1999, 107, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Donini, L.M.; Pinto, A.; Giusti, A.M.; Lenzi, A.; Poggiogalle, E. Obesity or BMI Paradox? Beneath the Tip of the Iceberg. Front. Nutr. 2020, 7, 53. [Google Scholar] [CrossRef]
- Preston, S.H.; Stokes, A. Obesity paradox: Conditioning on disease enhances biases in estimating the mortality risks of obesity. Epidemiology 2014, 25, 454–461. [Google Scholar] [CrossRef]
- Gonzalez, M.C.; Correia, M.I.T.D.; Heymsfield, S.B. A requiem for BMI in the clinical setting. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 314–321. [Google Scholar] [CrossRef]
- Caan, B.J.; Meyerhardt, J.A.; Kroenke, C.H. Explaining the Obesity Paradox: The Association between Body Composition and Colorectal Cancer Survival (C-SCANS Study). Cancer Epidemiol. Biomark. Prev. 2017, 26, 1008–1015. [Google Scholar] [CrossRef]
- Anker, S.D.; Negassa, A.; Coats, A.J. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin-converting-enzyme inhibitors: An observational study. Lancet 2003, 361, 1077–1083. [Google Scholar] [CrossRef]
- Lennon, H.; Sperrin, M.; Badrick, E.; Renehan, A.G. The Obesity Paradox in Cancer: A Review. Curr. Oncol. Rep. 2016, 18, 56. [Google Scholar] [CrossRef]
- Demark-Wahnefried, W.; Platz, E.A.; Ligibel, J.A.; Blair, C.K.; Courneya, K.S.; Meyerhardt, J.A. The role of obesity in cancer survival and recurrence. Cancer Epidemiol. Biomark. Prev. 2012, 21, 1244–1259. [Google Scholar] [CrossRef]
- Hakimi, A.A.; Furberg, H.; Zabor, E.C.; Jacobsen, A.; Schultz, N.; Ciriello, G. An epidemiologic and genomic investigation into the obesity paradox in renal cell carcinoma. J. Natl. Cancer Inst. 2013, 105, 1862–1870. [Google Scholar] [CrossRef] [PubMed]
- Peixoto da Silva, S.; Santos, J.M.O.; Costa e Silva, M.P.; Gil da Costa, R.M.; Medeiros, R. Cancer cachexia and its pathophysiology: Links with sarcopenia, anorexia and asthenia. J. Cachexia Sarcopenia Muscle 2020, 11, 619–635. [Google Scholar] [CrossRef] [PubMed]
- Marasco, G.; Serenari, M.; Renzulli, M.; Alemanni, L.V.; Rossini, B.; Pettinari, I.; Dajti, E.; Ravaioli, F.; Golfieri, R.; Cescon, M.; et al. Clinical impact of sarcopenia assessment in patients with hepatocellular carcinoma undergoing treatments. J. Gastroenterol. 2020, 55, 927–943. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Lee, Y.H.; Park, S.K.; Cho, H.; Ahn, K.J. Association between obesity and local control of advanced rectal cancer after combined surgery and radiotherapy. Radiat. Oncol. J. 2016, 34, 113–120. [Google Scholar] [CrossRef]
- Meyerhardt, J.A.; Tepper, J.E.; Niedzwiecki, D.; Hollis, D.R.; McCollum, A.D.; Brady, D.; O’Connell, M.J.; Mayer, R.J.; Cummings, B.; Willett, C.; et al. Impact of body mass index on outcomes and treatment-related toxicity in patients with stage II and III rectal cancer: Findings from Intergroup Trial 0114. J. Clin. Oncol. 2004, 22, 648–657. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Gelber, R.P.; Gaziano, J.M.; Orav, E.J.; Manson, J.E.; Buring, J.E.; Kurth, T. Measures of obesity and cardiovascular risk among men and women. J. Am. Coll. Cardiol. 2008, 52, 605–615. [Google Scholar] [CrossRef]
- Donkers, H.; Fasmer, K.E.; Mcgrane, J.; Pijnenborg, J.M.A.; Bekkers, R.; Haldorsen, I.S.; Galaal, K. The role of sarcopenic obesity in high-grade endometrial cancer. Int. J. Gynaecol. Obstet. 2021, 154, 248–255. [Google Scholar] [CrossRef]
- Seebacher, V.; Rockall, A.; Nobbenhuis, M.; Sohaib, S.A.; Knogler, T.; Alvarez, R.M.; Kolomainen, D.; Shepherd, J.H.; Shaw, C.; Barton, D.P. The impact of nutritional risk factors and sarcopenia on survival in patients treated with pelvic exenteration for recurrent gynaecological malignancy: A retrospective cohort study. Arch. Gynecol. Obstet. 2022, 305, 1343–1352. [Google Scholar] [CrossRef]
- Martin, L.; Birdsell, L.; Macdonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor.; independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef]
- Blauwhoff-Buskermolen, S.; Versteeg, K.S.; de van der Schueren, M.A.; den Braver, N.R.; Berkhof, J.; Langius, J.A.; Verheul, H.M. Loss of Muscle Mass During Chemotherapy Is Predictive for Poor Survival of Patients with Metastatic Colorectal Cancer. J. Clin. Oncol. 2016, 34, 1339–1344. [Google Scholar] [CrossRef]
- Shachar, S.S.; Williams, G.R.; Muss, H.B.; Nishijima, T.F. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur. J. Cancer 2016, 57, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.P.; Bader, N.; Basting, J.; Vanenkevort, E.; Koppenhaver, N.; Patel, A.; Gupta, M.; Lagerman, B.; Wojtowicz, M. Are Muscle and Fat Loss Predictive of Clinical Events in Pancreatic Cancer? The Importance of Precision Metrics. J. Pain Symptom Manag. 2025, 69, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Sibarani, C.; Salima, S.; Suardi, D.; Winarno, G.; Adrianto, N.; Mangkuliguna, G. The Impact of Lymphadenectomy on Survival Outcomes in Preoperative Early Stage Endometrial Cancer with Intermediate to High Risk: A Systematic Review and Meta-analysis. Indian J. Gynecol. Oncol. 2025, 23, 114. [Google Scholar] [CrossRef]
- Yi, F.; Jian, C.; Lin, Y.; Yingtao, L.; Yao, L.; Rong, L.; Xingfa, C. Minimally invasive versus open surgery in uterine serous carcinoma: Impact on recurrence and survival in a multicenter cohort. Front. Oncol. 2025, 15, 1665803. [Google Scholar] [CrossRef]
- Jiao, S.; Wei, L.; Zou, L.; Wang, T.; Hu, K.; Zhang, F.; Hou, X. Prognostic values of tumor size and location in early stage endometrial cancer patients who received radiotherapy. J. Gynecol. Oncol. 2024, 35, e84. [Google Scholar] [CrossRef]
- Miao, D.; Ellenson, L.H.; Fader, A.N. Uterine serous carcinoma. In Diagnosis and Treatment of Rare Gynecologic Cancers; Elsevier: Amsterdam, The Netherlands, 2023; pp. 197–212. [Google Scholar] [CrossRef]
- Bogani, G.; Ray-Coquard, I.; Concin, N.; Ngoi, N.Y.L.; Morice, P.; Enomoto, T.; Takehara, K.; Denys, H.; Nout, R.A.; Lorusso, D.; et al. Uterine serous carcinoma. Gynecol. Oncol. 2020, 162, 226–234. [Google Scholar] [CrossRef]
- Yalcin, Y.; Kosan, B.; Yalcin, S.; Abay, M.; Ozerkan, K. The Impact of Lymphovascular Space Invasion on Recurrence and Survival in FIGO Stage I Node-Negative Endometrioid Endometrial Cancer. J. Clin. Med. 2025, 14, 6535. [Google Scholar] [CrossRef]
| Variables | Numbers (%) | |
|---|---|---|
| Sarcopenia | − | 122 (51.69) |
| + | 114 (48.31) | |
| Visceral Fat Index (VFI) | Low | 117 (49.58) |
| High | 119 (50.42) | |
| Obesity | − | 93 (39.41) |
| + | 143 (60.59) | |
| Sarcopenic Obesity | − | 157 (66.53) |
| + | 79 (33.47) | |
| Sarcopenic Visceral Obesity | − | 182 (77.12) |
| + | 54 (22.88) | |
| Age at Diagnosis | <65 Years | 148 (62.71) |
| ≥65 Years | 88 (37.29) | |
| Age at Diagnosis (median ± SS) | 61.06 ± 10.18 | 61.15 (53.95–68.81) |
| Body Mass Index (median ± SS) | 31.55 ± 6.38 | 31.25 (27.56–35.16) |
| History of Smoking | absence | 234 (99.57) |
| <43 Packet/year | 1 (0.43) | |
| Eastern Cooperative Oncology Group (ECOG) Performance Status | 0 | 183 (77.87) |
| 1 | 48 (20.43) | |
| 2 | 2 (0.85) | |
| 4 | 2 (0.85) | |
| Histopathology | Endometrioid Carcinoma | 194 (82.55) |
| Serous Carcinoma | 30 (12.77) | |
| Mixed | 4 (1.7) | |
| Clear Cell Carcinoma | 3 (1.28) | |
| Undifferentiated Carcinoma | 2 (0.85) | |
| High Grade Mucinous Carcinoma | 2 (0.85) | |
| Endometrioid Carcinoma | Grade 1 | 38 (17.27) |
| Grade 2 | 100 (45.45) | |
| Grade 3 | 82 (37.27) | |
| Stage | Stage 1 | 127 (54.04) |
| Stage 2 | 20 (8.51) | |
| Stage 3 | 44 (18.72) | |
| Stage 4 | 44 (18.72) | |
| Miss Match Repair gene (MMR) | Proficient mismatch repair (pMMR) gene | 113 (47.88) |
| Deficient mismatch repair (dMMR) gene | 24 (10.17) | |
| unknown | 99 (41.95) | |
| Loss of phosphatase and tensin homolog (PTEN) gene | (−) negative | 21 (8.9) |
| (+) positive | 63 (26.69) | |
| unknown | 152 (64.41) | |
| Estrogen Receptor (ER) | unknown | 63 (26.69) |
| Positive | 173 (73.31) | |
| Progesterone Receptor (PR) | Positive | 236 (100) |
| HER2 Score | 0 | 151 (63.98) |
| (+) weak positive | 13 (5.51) | |
| (++) moderate positive | 8 (3.39) | |
| (++++) strong positive | 12 (5.08) | |
| unknown | 52 (22.03) | |
| P53 Mutation | Positive | 146 (61.86) |
| unknown | 90 (38.14) | |
| Lymphovascular invasion (LVI) | − | 146 (62.13) |
| + | 89 (37.87) | |
| Primary tumor localization | Uterine corpus | 175 (74.47) |
| Lower Uterine Segment | 60 (25.53) | |
| Metastasis | − | 138 (75.41) |
| + | 45 (24.59) | |
| Time to Metastasis | Denova metastasis | 49 (51.04) |
| Metachronous metastasis | 47 (48.96) | |
| Location of metastasis | Lung | 23 (24.21) |
| Bone | 11 (11.58) | |
| Liver | 14 (14.74) | |
| Adrenal | 2 (2.11) | |
| Peritoneum | 33 (34.74) | |
| Distant lymph node | 12 (12.63) | |
| Primer Surgery | − | 13 (5.53) |
| + | 222 (94.47) | |
| Pelvic Radiotherapy | − | 99 (42.13) |
| + | 136 (57.87) | |
| Brachytherapy | − | 198 (84.26) |
| + | 37 (15.74) | |
| Adjuvant Therapy | − | 129 (55.13) |
| + | 105 (44.87) | |
| Adjuvant treatment protocol | Carboplatin + Paclitaxel | 81 (77.14) |
| others | 24 (22.86) | |
| Local Treatment | − | 225 (95.74) |
| Metastazektomi | 4 (1.7) | |
| Akciğer Radyoterapi | 1 (0.43) | |
| Kemik Radyoterapi | 5 (2.13) | |
| First Line Treatment | Cisplatin + Gemcitabine | 11 (12.09) |
| Carboplatin + Paclitaxel | 65 (71.43) | |
| Cisplatin + Gemcitabine +Bevacizumab | 3 (3.3) | |
| none | 7 (7.69) | |
| others | 5 (5.49) | |
| Progression | − | 143 (60.59) |
| + | 93 (39.41) | |
| Living Situation | Alive | 154 (65.25) |
| Exitus | 82 (34.75) |
| Variables | Sarcopenia | p | Visceral Fat Index (VFI) | p | |||
|---|---|---|---|---|---|---|---|
| Normal | Sarcopenic | Low | High | ||||
| N (%) | N (%) | N (%) | N (%) | ||||
| Age at Diagnosis | <65 Years | 82 (67.21) | 66 (57.89) | 0.139 | 75 (64.1) | 73(61.34) | 0.661 |
| ≥65 Years | 40 (32.79) | 48 (42.11) | 42 (35.9) | 46 (38.66) | |||
| Obesity | no obesity | 58 (47.54) | 35 (30.7) | 0.008 * | 50 (42.74) | 43 (36.13) | 0.299 |
| Obesity | 64 (52.46) | 79 (69.3) | 67 (57.26) | 76 (63.87) | |||
| Eastern Cooperative Oncology Group (ECOG) Performance Status | 0 | 99 (81.15) | 84 (73.68) | 0.101 | 91(77.78) | 92(77.31) | 0.919 |
| 1 | 19 (15.57) | 29 (25.44) | 23(19.66) | 25(21.01) | |||
| 2 | 4 (3.28) | 1 (0.88) | 3(2.56) | 2(1.68) | |||
| Histopathology | Endometrioid Carcinoma | 102(83.61) | 92 (81.42) | 0.161 | 99(84.62) | 95(80.51) | 0.220 |
| Serous Carcinoma | 12 (9.84) | 18 (15.93) | 11 (9.4) | 19 (16.1) | |||
| Others | 8 (6.56) | 3 (2.65) | 7 (5.98) | 4 (3.39) | |||
| Endometrioid Carcinoma | Grade 1 | 17 (15.04) | 21 (19.63) | 0.417 | 20 (18.02) | 18 (16.51) | 0.642 |
| Grade 2 | 56 (49.56) | 44 (41.12) | 47 (42.34) | 53 (48.62) | |||
| Grade 3 | 40 (35.4) | 42 (39.25) | 44 (39.64) | 38 (34.86) | |||
| Stage | Stage 1 | 66 (54.1) | 61 (53.98) | 0.417 | 56 (47.86) | 71 (60.17) | 0.184 |
| Stage 2 | 7 (5.74) | 13 (11.5) | 12 (10.26) | 8 (6.78) | |||
| Stage 3 | 25 (20.49) | 19 (16.81) | 27 (23.08) | 17 (14.41) | |||
| Stage 4 | 24 (19.67) | 20 (17.7) | 22 (18.8) | 22 (18.64) | |||
| MMR (Miss match repair gen) | Proficient mismatch repair (pMMR) gene | 49 (40.16) | 64 (56.14) | 0.042 * | 54 (46.15) | 59 (49.58) | 0.505 |
| Deficient mismatch repair (dMMR) gene | 13 (10.66) | 11 (9.65) | 10 (8.55) | 14 (11.76) | |||
| unknown | 60 (49.18) | 39 (34.21) | 53 (45.3) | 46 (38.66) | |||
| Loss of phosphatase and tensin homolog (PTEN) gene | − | 11 (9.02) | 10 (8.77) | 0.253 | 9 (7.69) | 12 (10.08) | 0.447 |
| + | 27 (22.13) | 36 (31.58) | 28 (23.93) | 35 (29.41) | |||
| unknown | 84 (68.85) | 68 (59.65) | 80 (68.38) | 72 (60.5) | |||
| Estrogen Receptor (ER) | unknown | 29 (23.77) | 34 (29.82) | 0.293 | 34 (29.06) | 29 (24.37) | 0.415 |
| + | 93 (76.23) | 80 (70.18) | 83 (70.94) | 90 (75.63) | |||
| P53 Mutation | + | 84 (68.85) | 62 (54.39) | 0.022 * | 67 (57.26) | 79 (66.39) | 0.149 |
| unknown | 38 (31.15) | 52 (45.61) | 50 (42.74) | 40 (33.61) | |||
| Lymphovascular invasion (LVI) | − | 75 (61.48) | 71 (62.83) | 0.830 | 68 (58.12) | 78 (66.1) | 0.207 |
| + | 47 (38.52) | 42 (37.17) | 49 (41.88) | 40 (33.9) | |||
| Primary tumor localization | Uterine corpus | 91 (74.59) | 84 (74.34) | 0.964 | 83 (70.94) | 92 (77.97) | 0.217 |
| Lower Uterine Segment | 31 (25.41) | 29 (25.66) | 34 (29.06) | 26 (22.03) | |||
| Metastasis | − | 71 (74.74) | 67 (76.14) | 0.826 | 67 (73.63) | 71 (77.17) | 0.577 |
| + | 24 (25.26) | 21 (23.86) | 24 (26.37) | 21 (22.83) | |||
| Surgery | − | 8 (6.56) | 5 (4.42) | 0.475 | 7 (5.98) | 6 (5.08) | 0.763 |
| + | 114(93.44) | 108 (95.58) | 110(94.02) | 112(94.92) | |||
| Pelvic Radiotherapy | − | 55 (45.08) | 44 (38.94) | 0.341 | 45 (38.46) | 54 (45.76) | 0.257 |
| + | 67 (54.92) | 69 (61.06) | 72 (61.54) | 64 (54.24) | |||
| Brachytherapy | − | 100(81.97) | 98 (86.73) | 0.317 | 96 (82.05) | 102(86.44) | 0.356 |
| + | 22(18.03) | 15 (13.27) | 21 (17.95) | 16 (13.56) | |||
| Adjuvant Therapy | − | 69(56.56) | 60 (53.57) | 0.646 | 58 (49.57) | 71 (60.68) | 0.088 |
| + | 53(43.44) | 52 (46.43) | 59 (50.43) | 46 (39.32) | |||
| Progression | − | 69(56.56) | 74(64.91) | 0.189 | 73(62.39) | 70(58.82) | 0.575 |
| + | 53(43.44) | 40(35.09) | 44(37.61) | 49 (41.18) | |||
| Variables | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | ||
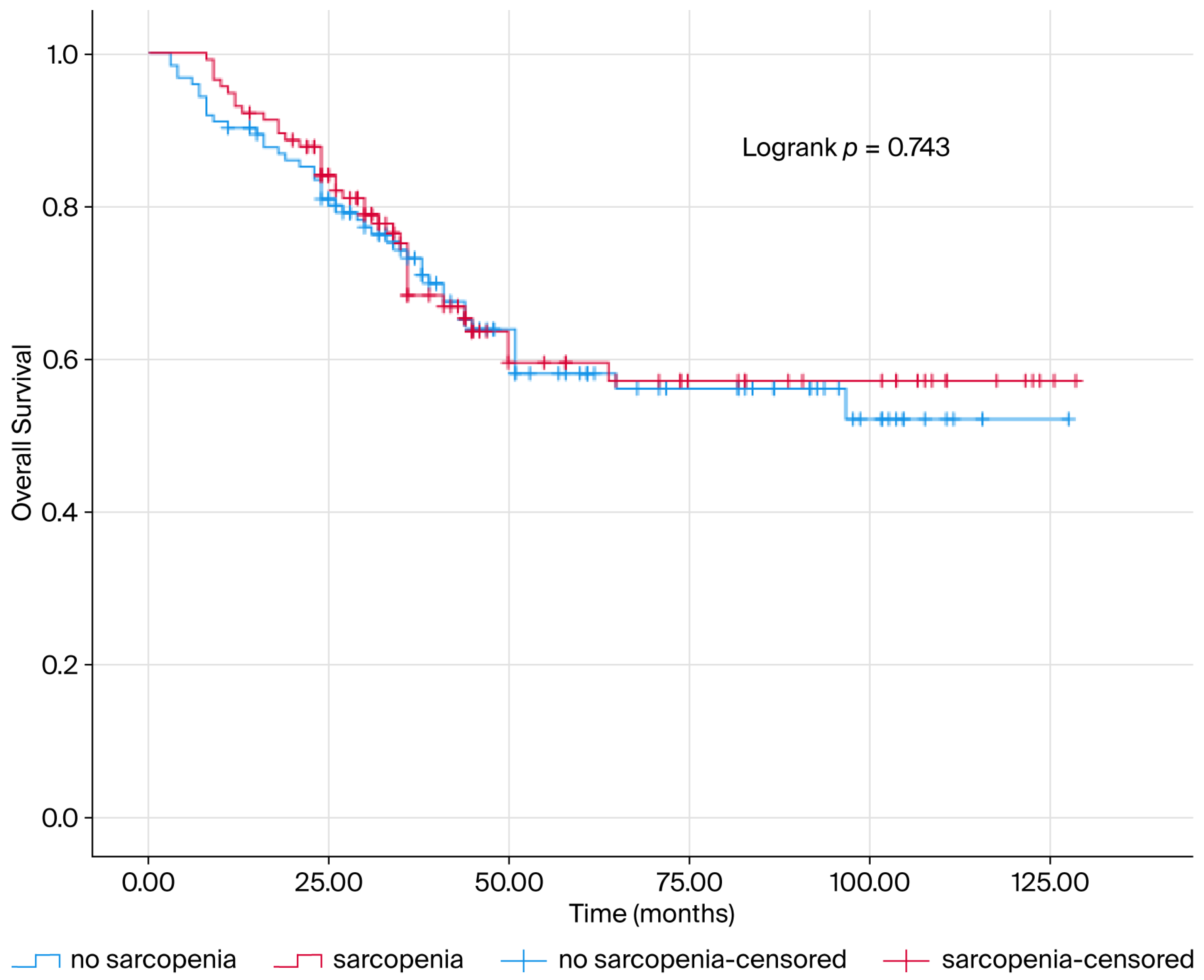
| Sarcopenia | No sarcopenia | 1 (reference) | |||
| Sarcopenia | 1.075 (0.695–1.662) | 0.745 | |||
| Visceral fat index (VFI) | Low | 1 (reference) | |||
| High | 1.219 (0.790–1.881) | 0.371 | |||
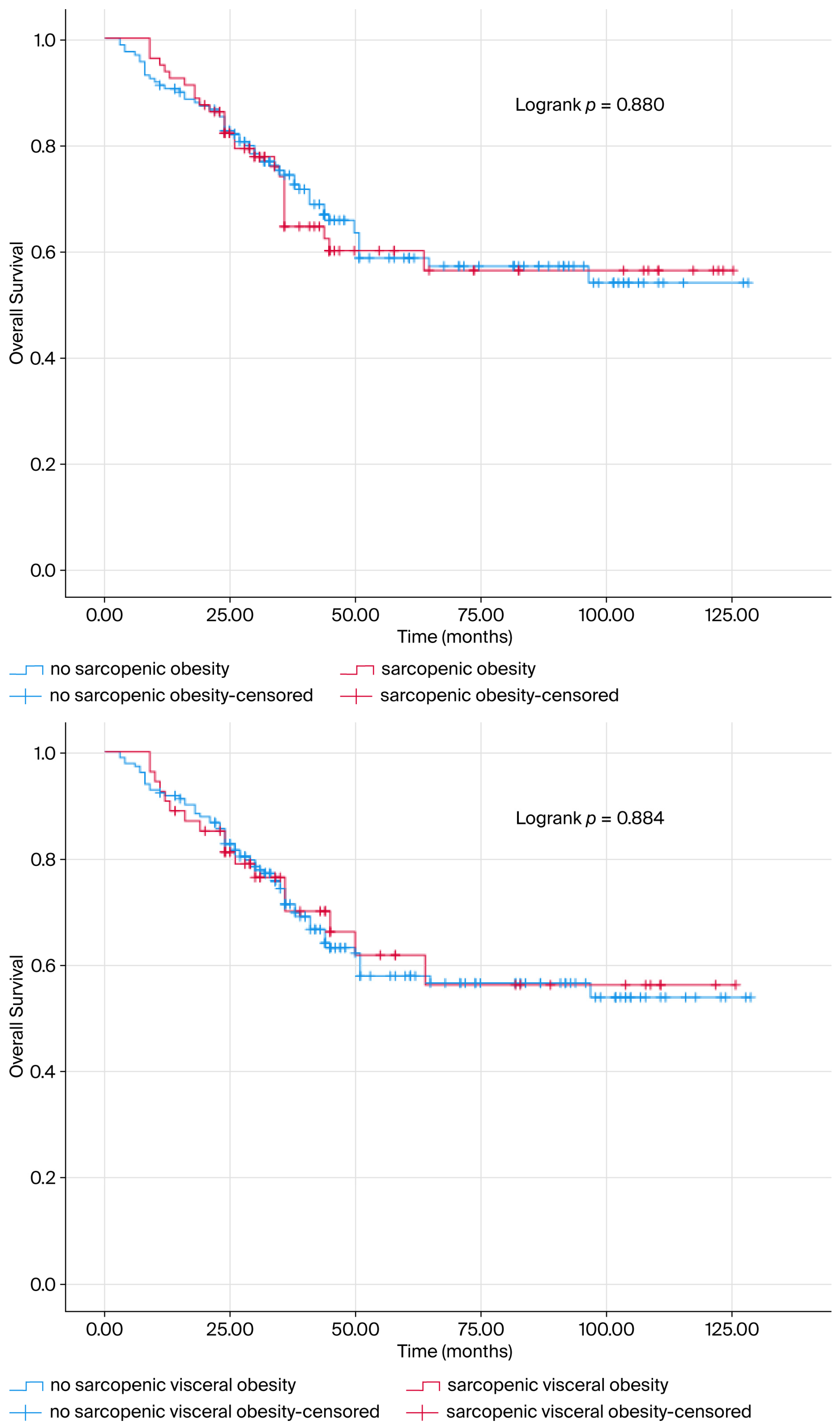
| Sarcopenic obesity | − | 1 (reference) | |||
| + | 1.036 (0.653–1.643) | 0.881 | |||
| Sarcopenic visceral obesity | − | 1 (reference) | |||
| + | 0.961 (0.563–1.640) | 0.885 | |||
| Age at Diagnosis | <65 Years | 1 (reference) | 1 (reference) | ||
| ≥65 Years | 2.590 (1.672–4.014) | 0.001 * | 2.882 (1.459–5.692) | 0.002 * | |
| Obesity | − | 1 (reference) | |||
| Obesity | 1.046 (0.671–1.631) | 0.844 | |||
| Eastern Cooperative Oncology Group (ECOG) Performance Status | 0 | 1 (reference) | 0.173 | ||
| 1 | 1.588 (0.972–2.594) | 0.065 | |||
| 2 | 1.436 (0.350–5.887) | 0.615 | |||
| Histopathology | Endometrioid Carcinoma | 1 (reference) | 0.001 * | 1 (reference) | 0.084 |
| Serous Carcinoma | 3.129 (1.799–5.442) | 0.001 * | 2.226(0.945–5.242) | 0.067 | |
| Others | 2.343 (1.009–5.441) | 0.048 * | 3.178 (0.686–14.734) | 0.140 | |
| Endometrioid Carcinoma | Grade 1 | 1 (reference) | 0.021 * | 1 (reference) | 0.095 |
| Grade 2 | 1.396 (0.636–3.062) | 0.406 | 1.685 (0.575–4.940) | 0.342 | |
| Grade 3 | 2.438 (1.130–5.259) | 0.023 * | 0.728 (0.234–2.262) | 0.583 | |
| Stage | Stage 1 | 1 (reference) | 0.001 * | 1 (reference) | 0.519 |
| Stage 2 | 3.183 (1.413–7.170) | 0.005 * | 0.904 (0.250–3.274) | 0.878 | |
| Stage 3 | 2.728 (1.500–4.962) | 0.001 * | 1.421 (0.600–3.364) | 0.424 | |
| Stage 4 | 8.886 (5.086–15.528) | 0.001 * | 1.932 (0.698–5.343) | 0.205 | |
| Miss match repair gene (MMR) | Proficient mismatch repair (pMMR) gene | 0.836 (0.526–1.330) | 0.743 | ||
| Deficient mismatch repair (dMMR) gene | 0.868 (0.389–1.937) | 0.450 | |||
| Unknown | 1 (reference) | 0.743 | |||
| Loss of phosphatase and tensin homolog (PTEN) gene | − | 0.742 (0.298–1.846) | 0.521 | 0.378 (0.086–1.671) | 0.200 |
| + | 0.413 (0.204–0.833) | 0.014 * | 0.364 (0.130–1.018) | 0.054 | |
| Unknown | 1 (reference) | 0.044 * | 1 (reference) | 0.084 | |
| Estrogen Receptor (ER) | Unknown | 1 (reference) | |||
| + | 0.711 (0.450–1.122) | 0.143 | |||
| P53 Mutation | + | 0.785 (0.508–1.213) | 0.275 | ||
| Unknown | 1 (reference) | ||||
| Lymphovascular invasion (LVI) | − | 1 (reference) | 1 (reference) | ||
| + | 3.558 (2.275–5.567) | 0.001 * | 1.596 (0.732–3.479) | 0.240 | |
| Primary tumor localization | Uterine corpus | 1 (reference) | 1 (reference) | ||
| Lower Uterine Segment | 2.438 (1.565–3.797) | 0.001 * | 1.799 (0.881–3.672) | 0.107 | |
| Metastasis | − | 1 (reference) | 1 (reference) | ||
| + | 7.555 (4.043–14.119) | 0.001 * | 4.620 (2.128–10.030) | 0.001 * | |
| Surgery | − | 1 (reference) | 1 (reference) | ||
| + | 0.117 (0.061–0.224) | 0.001 * | 0.198 (0.063–0.621) | 0.005 * | |
| Pelvic Radiotherapy | − | 1 (reference) | |||
| + | 0.989 (0.637–1.535) | 0.962 | |||
| Brachytherapy | − | 1 (reference) | |||
| + | 0.589 (0.295–1.777) | 0.134 | |||
| Adjuvant Therapy | − | 1 (reference) | |||
| + | 1.036 (0.672–1.598) | 0.873 | |||
| Age at Diagnosis | 1.082 (1.055–1.110) | 0.001 * | |||
| Body Mass Index (BMI) | 1.002 (0.963–1.042) | 0.932 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özdemir, M.; Doğu, G.G.; Taşköylü, B.Y.; Arslan, M.; Kurnaz, B.; Demiray, A.G.; Yaren, A.; Değirmencioğlu, S.; Karakaya, Y.A. Sarcopenic Obesity and Sarcopenic Visceral Obesity, Calculated Using the Skeletal Muscle İndex and Visceral Fat İndex at the L3 Vertebra Level, Do Not Predict Survival Rates in Endometrial Cancer Patients. J. Clin. Med. 2025, 14, 7915. https://doi.org/10.3390/jcm14227915
Özdemir M, Doğu GG, Taşköylü BY, Arslan M, Kurnaz B, Demiray AG, Yaren A, Değirmencioğlu S, Karakaya YA. Sarcopenic Obesity and Sarcopenic Visceral Obesity, Calculated Using the Skeletal Muscle İndex and Visceral Fat İndex at the L3 Vertebra Level, Do Not Predict Survival Rates in Endometrial Cancer Patients. Journal of Clinical Medicine. 2025; 14(22):7915. https://doi.org/10.3390/jcm14227915
Chicago/Turabian StyleÖzdemir, Melek, Gamze Gököz Doğu, Burcu Yapar Taşköylü, Muhammet Arslan, Burak Kurnaz, Atike Gökçen Demiray, Arzu Yaren, Serkan Değirmencioğlu, and Yeliz Arman Karakaya. 2025. "Sarcopenic Obesity and Sarcopenic Visceral Obesity, Calculated Using the Skeletal Muscle İndex and Visceral Fat İndex at the L3 Vertebra Level, Do Not Predict Survival Rates in Endometrial Cancer Patients" Journal of Clinical Medicine 14, no. 22: 7915. https://doi.org/10.3390/jcm14227915
APA StyleÖzdemir, M., Doğu, G. G., Taşköylü, B. Y., Arslan, M., Kurnaz, B., Demiray, A. G., Yaren, A., Değirmencioğlu, S., & Karakaya, Y. A. (2025). Sarcopenic Obesity and Sarcopenic Visceral Obesity, Calculated Using the Skeletal Muscle İndex and Visceral Fat İndex at the L3 Vertebra Level, Do Not Predict Survival Rates in Endometrial Cancer Patients. Journal of Clinical Medicine, 14(22), 7915. https://doi.org/10.3390/jcm14227915






