Gut–Liver Axis, Microbiota, Bile Acids, and Immune Response in Pathogenesis of Primary Sclerosing Cholangitis: An Overview
Abstract
1. Introduction
2. Leaky Gut Hypothesis
| Population/Model | Study Design | Methodology | Findings | Study |
|---|---|---|---|---|
| 166 patients with PSC and 100 healthy controls | Cross-sectional cohort study | Measurement of serum biomarkers of bacterial translocation (zonulin, intestinal fatty acid-binding protein, soluble CD14, lipopolysaccharide, and LPS-binding protein) | PSC patients showed elevated soluble CD14 and LPS-binding protein compared with controls. High levels were independently associated with reduced liver transplantation-free survival. | Dhillon et al. [34] |
| 26 PSC patients with recurrence after liver transplantation (rPSC), 87 PSC patients without recurrence (non-rPSC), and 113 post-transplant controls with alcohol-related cirrhosis | Cross-sectional study | Measurement of serological markers of intestinal barrier function (Reg3a, iFABP, zonulin, calprotectin) and generalized linear modeling to assess associations with PSC recurrence | Elevated Reg3a associated with PSC diagnosis; lower Reg3a linked to non-recurrence. rPSC incidence correlated with higher fecal calprotectin and serum zonulin levels. Suggests that impaired intestinal permeability contributes to rPSC pathophysiology. | Hlavaty et al. [35] |
| PSC patients and gnotobiotic mice colonized with PSC-derived microbiota | Experimental mechanistic study (human–animal translation) | Microbiota analysis, bacterial culture from mesenteric lymph nodes, and bacterial–organoid co-culture; functional assessment of epithelial barrier integrity and TH17 immune response; antibiotic intervention | Identified Klebsiella pneumoniae as a key pathobiont disrupting the epithelial barrier and inducing TH17-mediated hepatobiliary injury. Antibiotic treatment reduced inflammation. | Nakamoto et al. [36] |
| 67 PSC patients (pediatric and adult; 67% with IBD, 20% with cirrhosis), 153 healthy controls, and 172 ulcerative colitis controls | Observational cohort study | Measurement of serum antibodies (AAA IgA/IgG, AGA IgA/IgG), I-FABP, LPS-binding protein, and antimicrobial antibodies (EndoCAb, anti-OMP Plus IgA) by ELISA | AAA IgA positivity identified PSC patients with worse prognosis and higher enterocyte damage (elevated I-FABP). Strongly associated with enhanced mucosal immune response to microbial antigens, indicating gut–liver axis involvement. | Tornai et al. [38] |
| Rats with colitis induced by rectal administration of N-formyl L-methionine L-leucine L-tyrosine derived from E. coli | Experimental animal study | Histological and electron microscopy analysis of bile ducts and hepatocytes | Portal inflammation and small bile duct injury resembling early PSC. Lymphocytes adhered directly to biliary epithelium. | Yamada et al. [40] |
3. Gut Lymphocyte Homing in Liver Immunity
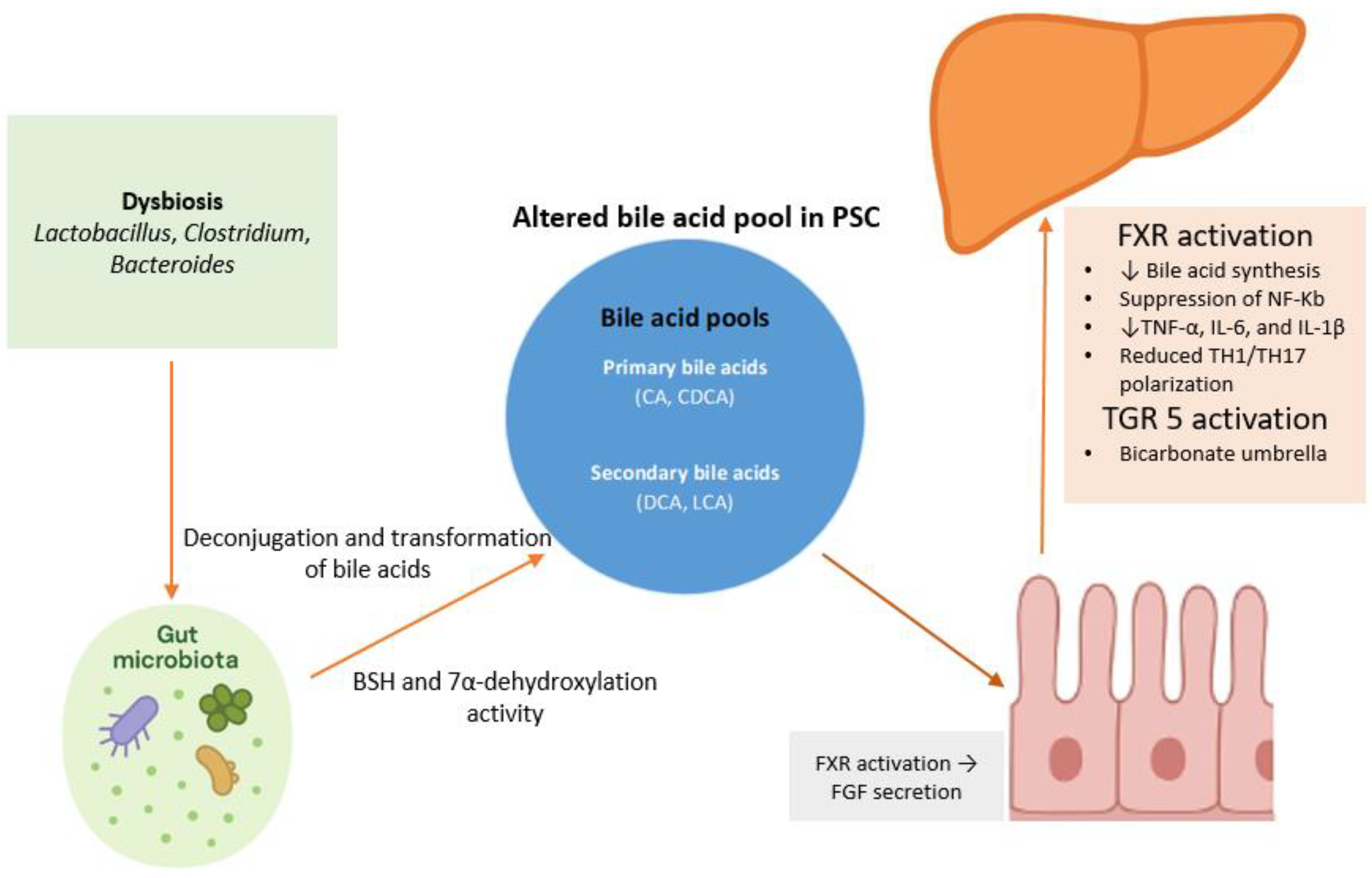
4. The Role of Bile Acids in Gut–Liver Crosstalk
5. Microbiota Modification
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dyson, J.K.; Beuers, U.; Jones, D.E.J.; Lohse, A.W.; Hudson, M. Primary sclerosing cholangitis. Lancet 2018, 391, 2547–2559. [Google Scholar] [CrossRef] [PubMed]
- Dave, M.; Elmunzer, B.J.; Dwamena, B.A.; Higgins, P.D. Primary sclerosing cholangitis: Meta-analysis of diagnostic performance of MR cholangiopancreatography. Radiology 2010, 256, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Bowlus, C.L.; Arrive, L.; Bergquist, A.; Deneau, M.; Forman, L.; Ilyas, S.I.; Lunsford, K.E.; Martinez, M.; Sapisochin, G.; Shroff, R.; et al. AASLD practice guidance on primary sclerosing cholangitis and cholangiocarcinoma. Hepatology. 2023, 77, 659–702. [Google Scholar] [CrossRef]
- Angulo, P.; Maor-Kendler, Y.; Lindor, K.D. Small-duct primary sclerosing cholangitis: A long-term follow-up study. Hepatology 2002, 35, 1494–1500. [Google Scholar] [CrossRef]
- Bjornsson, E.; Olsson, R.; Bergquist, A.; Lindgren, S.; Braden, B.; Chapman, R.W.; Boberg, K.M.; Angulo, P. The natural history of small-duct primary sclerosing cholangitis. Gastroenterology 2008, 134, 975–980. [Google Scholar] [CrossRef]
- Broome, U.; Olsson, R.; Loof, L.; Bodemar, G.; Hultcrantz, R.; Danielsson, A.; Prytz, H.; Sandberg-Gertzen, H.; Wallerstedt, S.; Lindberg, G. Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut 1996, 38, 610–615. [Google Scholar] [CrossRef]
- Deneau, M.; Jensen, M.K.; Holmen, J.; Williams, M.S.; Book, L.S.; Guthery, S.L. Primary sclerosing cholangitis, autoimmune hepatitis, and overlap in Utah children: Epidemiology and natural history. Hepatology 2013, 58, 1392–1400. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on sclerosing cholangitis. J. Hepatol. 2022, 77, 761–806. [Google Scholar] [CrossRef]
- Carbone, M.; Della Penna, A.; Mazzarelli, C.; De Martin, E.; Villard, C.; Bergquist, A.; Line, P.D.; Neuberger, J.M.; Al-Shakhshir, S.; Trivedi, P.J.; et al. Liver Transplantation for Primary Sclerosing Cholangitis (PSC) With or Without Inflammatory Bowel Disease (IBD)—A European Society of Organ Transplantation (ESOT) Consensus Statement. Transpl. Int. 2023, 36, 11729. [Google Scholar] [CrossRef]
- Manns, M.P.; Bergquist, A.; Karlsen, T.H.; Levy, C.; Muir, A.J.; Ponsioen, C.; Trauner, M.; Wong, G.; Younossi, Z.M. Primary sclerosing cholangitis. Nat. Rev. Dis. Primers 2025, 11, 17. [Google Scholar] [CrossRef]
- Barberio, B.; Massimi, D.; Cazzagon, N.; Zingone, F.; Ford, A.C.; Savarino, E.V. Prevalence of Primary Sclerosing Cholangitis in Patients With Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. Gastroenterology 2021, 161, 1865–1877. [Google Scholar] [CrossRef] [PubMed]
- Miard, C.; Desfourneaux, V.; Dewitte, M.; Houssel-Debry, P.; Bendavid, C.; Harnoy, Y.; Siproudhis, L.; Bouguen, G. P217 Usefulness of systematic liver biopsy during a surgery for inflammatory bowel disease for the diagnosis of primary sclerosing cholangitis. J. Crohn’s Colitis 2018, 12, S209–S210. [Google Scholar] [CrossRef]
- van Munster, K.N.; Bergquist, A.; Ponsioen, C.Y. Inflammatory bowel disease and primary sclerosing cholangitis: One disease or two? J. Hepatol. 2024, 80, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.H.; Jiang, X.L. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease: A meta-analysis of 16 observational studies. Eur. J. Gastroenterol. Hepatol. 2016, 28, 383–390. [Google Scholar] [CrossRef]
- Vera, A.; Moledina, S.; Gunson, B.; Hubscher, S.; Mirza, D.; Olliff, S.; Neuberger, J. Risk factors for recurrence of primary sclerosing cholangitis of liver allograft. Lancet 2002, 360, 1943–1944. [Google Scholar] [CrossRef]
- Alabraba, E.; Nightingale, P.; Gunson, B.; Hubscher, S.; Olliff, S.; Mirza, D.; Neuberger, J. A re-evaluation of the risk factors for the recurrence of primary sclerosing cholangitis in liver allografts. Liver Transplant. 2009, 15, 330–340. [Google Scholar] [CrossRef]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef]
- Hov, J.R.; Karlsen, T.H. The microbiota and the gut-liver axis in primary sclerosing cholangitis. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 135–154. [Google Scholar] [CrossRef]
- Blesl, A.; Stadlbauer, V. The Gut-Liver Axis in Cholestatic Liver Diseases. Nutrients 2021, 13, 1018. [Google Scholar] [CrossRef]
- Kobayashi, T.; Iwaki, M.; Nakajima, A.; Nogami, A.; Yoneda, M. Current Research on the Pathogenesis of NAFLD/NASH and the Gut-Liver Axis: Gut Microbiota, Dysbiosis, and Leaky-Gut Syndrome. Int. J. Mol. Sci. 2022, 23, 11689. [Google Scholar] [CrossRef]
- Camilleri, M. Leaky gut: Mechanisms, measurement and clinical implications in humans. Gut 2019, 68, 1516–1526. [Google Scholar] [CrossRef]
- Quigley, E.M. Leaky gut—Concept or clinical entity? Curr. Opin. Gastroenterol. 2016, 32, 74–79. [Google Scholar] [CrossRef]
- Odenwald, M.A.; Turner, J.R. The intestinal epithelial barrier: A therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Dean, G.; Hanauer, S.; Levitsky, J. The Role of the Intestine in the Pathogenesis of Primary Sclerosing Cholangitis: Evidence and Therapeutic Implications. Hepatology 2020, 72, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Bozward, A.G.; Ronca, V.; Osei-Bordom, D.; Oo, Y.H. Gut-Liver Immune Traffic: Deciphering Immune-Pathogenesis to Underpin Translational Therapy. Front. Immunol. 2021, 12, 711217. [Google Scholar] [CrossRef] [PubMed]
- Provine, N.M.; Klenerman, P. MAIT Cells in Health and Disease. Annu. Rev. Immunol. 2020, 38, 203–228. [Google Scholar] [CrossRef]
- Kjer-Nielsen, L.; Patel, O.; Corbett, A.J.; Le Nours, J.; Meehan, B.; Liu, L.; Bhati, M.; Chen, Z.; Kostenko, L.; Reantragoon, R.; et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 2012, 491, 717–723. [Google Scholar] [CrossRef]
- Jeffery, H.C.; Hunter, S.; Humphreys, E.H.; Bhogal, R.; Wawman, R.E.; Birtwistle, J.; Atif, M.; Bagnal, C.J.; Blanco, G.R.; Richardson, N.; et al. Bidirectional Cross-Talk between Biliary Epithelium and Th17 Cells Promotes Local Th17 Expansion and Bile Duct Proliferation in Biliary Liver Diseases. J. Immunol. 2019, 203, 1151–1159. [Google Scholar] [CrossRef]
- Dorner, H.; Stolzer, I.; Mattner, J.; Kaminski, S.; Leistl, S.; Edrich, L.M.; Schwendner, R.; Hobauer, J.; Sebald, A.; Leikam, S.; et al. Gut Pathobiont-Derived Outer Membrane Vesicles Drive Liver Inflammation and Fibrosis in Primary Sclerosing Cholangitis-Associated Inflammatory Bowel Disease. Gastroenterology 2024, 167, 1183–1197.e16. [Google Scholar] [CrossRef]
- Losa, M.; Schwarzfischer, M.; Emmenegger, M.; Spalinger, M.R.; Rogler, G.; Scharl, M. Unraveling the Converging Roles of ASC-Dependent Inflammasomes, Interleukin-1 Superfamily Members, Serum Amyloid A, and Non-Sterile Inflammation in Disease Pathology and Fibrosis in Inflammatory Bowel Disease and Primary Sclerosing Cholangitis. Int. J. Mol. Sci. 2025, 26, 8042. [Google Scholar] [CrossRef]
- Seki, E.; Schnabl, B. Role of innate immunity and the microbiota in liver fibrosis: Crosstalk between the liver and gut. J. Physiol. 2012, 590, 447–458. [Google Scholar] [CrossRef]
- von Seth, E.; Zimmer, C.L.; Reuterwall-Hansson, M.; Barakat, A.; Arnelo, U.; Bergquist, A.; Ivarsson, M.A.; Björkström, N.K. Primary sclerosing cholangitis leads to dysfunction and loss of MAIT cells. Eur. J. Immunol. 2018, 48, 1997–2004. [Google Scholar] [CrossRef]
- Wang, W.; Weng, J.; Zhang, H.; Wu, M.; Zhou, T.; Jiang, Y.; Wu, X.; Ye, C.; Weng, X. Dysregulation and impaired anti-bacterial potential of mucosal-associated invariant T cells in autoimmune liver diseases. Int. Immunopharmacol. 2024, 142 Pt B, 113175. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, A.K.; Kummen, M.; Troseid, M.; Akra, S.; Liaskou, E.; Moum, B.; Vesterhus, M.; Karlsen, T.H.; Seljeflot, I.; Hov, J.R. Circulating markers of gut barrier function associated with disease severity in primary sclerosing cholangitis. Liver Int. 2019, 39, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Hlavaty, M.; Brezina, J.; Osadcha, T.; Fabian, O.; Vajsova, A.; Drastich, P.; Cahova, M.; Bajer, L. Serological Markers of Intestinal Barrier Function and Inflammation as Potential Predictors of Recurrent Primary Sclerosing Cholangitis. Clin. Exp. Gastroenterol. 2025, 18, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, N.; Sasaki, N.; Aoki, R.; Miyamoto, K.; Suda, W.; Teratani, T.; Suzuki, T.; Koda, Y.; Chu, P.-S.; Taniki, N.; et al. Gut pathobionts underlie intestinal barrier dysfunction and liver T helper 17 cell immune response in primary sclerosing cholangitis. Nat. Microbiol. 2019, 4, 492–503. [Google Scholar] [CrossRef]
- Ichikawa, M.; Nakamoto, N.; Kredo-Russo, S.; Weinstock, E.; Weiner, I.N.; Khabra, E.; Ben-Ishai, N.; Inbar, D.; Kowalsman, N.; Mordoch, R.; et al. Bacteriophage therapy against pathological Klebsiella pneumoniae ameliorates the course of primary sclerosing cholangitis. Nat. Commun. 2023, 14, 3261. [Google Scholar] [CrossRef]
- Tornai, T.; Palyu, E.; Vitalis, Z.; Tornai, I.; Tornai, D.; Antal-Szalmas, P.; Norman, G.L.; Shums, Z.; Veres, G.; Dezsofi, A.; et al. Gut barrier failure biomarkers are associated with poor disease outcome in patients with primary sclerosing cholangitis. World J. Gastroenterol. 2017, 23, 5412–5421. [Google Scholar] [CrossRef]
- Giordano, D.M.; Pinto, C.; Maroni, L.; Benedetti, A.; Marzioni, M. Inflammation and the Gut-Liver Axis in the Pathophysiology of Cholangiopathies. Int. J. Mol. Sci. 2018, 19, 3003. [Google Scholar] [CrossRef]
- Yamada, S.; Ishii, M.; Liang, L.S.; Yamamoto, T.; Toyota, T. Small duct cholangitis induced by N-formyl L-methionine L-leucine L-tyrosine in rats. J. Gastroenterol. 1994, 29, 631–636. [Google Scholar] [CrossRef]
- Yamada, S.; Ishii, M.; Kisara, N.; Nagatomi, R.; Toyota, T. Macrophages are essential for lymphocyte infiltration in formyl peptide-induced cholangitis in rat liver. Liver 1999, 19, 253–258. [Google Scholar] [CrossRef]
- Schrumpf, E.; Kummen, M.; Valestrand, L.; Greiner, T.U.; Holm, K.; Arulampalam, V.; Reims, H.M.; Baines, J.; Bäckhed, F.; Karlsen, T.H.; et al. The gut microbiota contributes to a mouse model of spontaneous bile duct inflammation. J. Hepatol. 2017, 66, 382–389. [Google Scholar] [CrossRef] [PubMed]
- von Andrian, U.H.; Mempel, T.R. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 2003, 3, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Iwata, M.; Hirakiyama, A.; Eshima, Y.; Kagechika, H.; Kato, C.; Song, S.Y. Retinoic acid imprints gut-homing specificity on T cells. Immunity 2004, 21, 527–538. [Google Scholar] [CrossRef]
- Hernandez-Ruiz, M.; Zlotnik, A. Mucosal Chemokines. J. Interferon Cytokine Res. 2017, 37, 62–70. [Google Scholar] [CrossRef]
- Papadakis, K.A.; Prehn, J.; Nelson, V.; Cheng, L.; Binder, S.W.; Ponath, P.D.; Andrew, D.P.; Targan, S.R. The role of thymus-expressed chemokine and its receptor CCR9 on lymphocytes in the regional specialization of the mucosal immune system. J. Immunol. 2000, 165, 5069–5076. [Google Scholar] [CrossRef]
- de Krijger, M.; Wildenberg, M.E.; de Jonge, W.J.; Ponsioen, C.Y. Return to sender: Lymphocyte trafficking mechanisms as contributors to primary sclerosing cholangitis. J. Hepatol. 2019, 71, 603–615. [Google Scholar] [CrossRef]
- Liaskou, E.; Hirschfield, G.M.; Gershwin, M.E. Mechanisms of tissue injury in autoimmune liver diseases. Semin. Immunopathol. 2014, 36, 553–568. [Google Scholar] [CrossRef]
- Fickert, P. Bad memories from the gut may cause nightmares for the bile ducts. J. Hepatol. 2017, 66, 5–7. [Google Scholar] [CrossRef]
- Shuai, Z.; Leung, M.W.; He, X.; Zhang, W.; Yang, G.; Leung, P.S.; Gershwin, M.E. Adaptive immunity in the liver. Cell. Mol. Immunol. 2016, 13, 354–368. [Google Scholar] [CrossRef]
- Seidel, D.; Eickmeier, I.; Kuhl, A.A.; Hamann, A.; Loddenkemper, C.; Schott, E. CD8 T cells primed in the gut-associated lymphoid tissue induce immune-mediated cholangitis in mice. Hepatology 2014, 59, 601–611. [Google Scholar] [CrossRef]
- Eksteen, B.; Grant, A.J.; Miles, A.; Curbishley, S.M.; Lalor, P.F.; Hubscher, S.G.; Briskin, M.; Salmon, M.; Adams, D.H. Hepatic endothelial CCL25 mediates the recruitment of CCR9+ gut-homing lymphocytes to the liver in primary sclerosing cholangitis. J. Exp. Med. 2004, 200, 1511–1517. [Google Scholar] [CrossRef]
- Henriksen, E.K.; Jorgensen, K.K.; Kaveh, F.; Holm, K.; Hamm, D.; Olweus, J.; Melum, E.; Chung, B.K.; Eide, T.J.; Lundin, K.E.; et al. Gut and liver T-cells of common clonal origin in primary sclerosing cholangitis-inflammatory bowel disease. J. Hepatol. 2017, 66, 116–122. [Google Scholar] [CrossRef]
- de Krijger, M.; Visseren, T.; Wildenberg, M.E.; Hooijer, G.K.J.; Verstegen, M.M.A.; van der Laan, L.J.W.; de Jonge, W.J.; Verheij, J.; Ponsioen, C.Y. Characterization of gut-homing molecules in non-endstage livers of patients with primary sclerosing cholangitis and inflammatory bowel disease. J. Transl. Autoimmun. 2020, 3, 100054. [Google Scholar] [CrossRef]
- Graham, J.J.; Mukherjee, S.; Yuksel, M.; Sanabria Mateos, R.; Si, T.; Huang, Z.; Huang, X.; Abu Arqoub, H.; Patel, V.; McPhail, M.; et al. Aberrant hepatic trafficking of gut-derived T cells is not specific to primary sclerosing cholangitis. Hepatology 2022, 75, 518–530. [Google Scholar] [CrossRef]
- Li, B.; Selmi, C.; Tang, R.; Gershwin, M.E.; Ma, X. The microbiome and autoimmunity: A paradigm from the gut-liver axis. Cell. Mol. Immunol. 2018, 15, 595–609. [Google Scholar] [CrossRef]
- Chung, B.K.; Henriksen, E.K.K.; Jorgensen, K.K.; Karlsen, T.H.; Hirschfield, G.M.; Liaskou, E. Gut and Liver B Cells of Common Clonal Origin in Primary Sclerosing Cholangitis-Inflammatory Bowel Disease. Hepatol. Commun. 2018, 2, 956–967. [Google Scholar] [CrossRef]
- Su, X.; Gao, Y.; Yang, R. Gut microbiota derived bile acid metabolites maintain the homeostasis of gut and systemic immunity. Front. Immunol. 2023, 14, 1127743. [Google Scholar] [CrossRef]
- Ticho, A.L.; Malhotra, P.; Dudeja, P.K.; Gill, R.K.; Alrefai, W.A. Intestinal Absorption of Bile Acids in Health and Disease. Compr. Physiol. 2019, 10, 21–56. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Zhang, K.; Guo, J.; Xu, L. Bile acid-mediated gut-liver axis crosstalk: The role of nuclear receptor signaling in dynamic regulation of inflammatory networks. Front. Immunol. 2025, 16, 1595486. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Nie, R.; Shen, C. The role of bile acids in regulating glucose and lipid metabolism. Endocr. J. 2023, 70, 359–374. [Google Scholar] [CrossRef] [PubMed]
- Pathak, P.; Liu, H.; Boehme, S.; Xie, C.; Krausz, K.W.; Gonzalez, F.; Chiang, J.Y. Farnesoid X receptor induces Takeda G-protein receptor 5 cross-talk to regulate bile acid synthesis and hepatic metabolism. J. Biol. Chem. 2017, 292, 11055–11069. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.L.; Ferrell, J.M. Discovery of farnesoid X receptor and its role in bile acid metabolism. Mol. Cell. Endocrinol. 2022, 548, 111618. [Google Scholar] [CrossRef]
- Fang, Y.; Hegazy, L.; Finck, B.N.; Elgendy, B. Recent Advances in the Medicinal Chemistry of Farnesoid X Receptor. J. Med. Chem. 2021, 64, 17545–17571. [Google Scholar] [CrossRef]
- Sun, L.; Cai, J.; Gonzalez, F.J. The role of farnesoid X receptor in metabolic diseases, and gastrointestinal and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 335–347. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Bonfrate, L.; Baj, J.; Khalil, M.; Garruti, G.; Stellaard, F.; Wang, H.H.; Wang, D.Q.-H.; Portincasa, P. Recent Advances in the Digestive, Metabolic and Therapeutic Effects of Farnesoid X Receptor and Fibroblast Growth Factor 19: From Cholesterol to Bile Acid Signaling. Nutrients 2022, 14, 4950. [Google Scholar] [CrossRef]
- Wang, Y.D.; Chen, W.D.; Wang, M.; Yu, D.; Forman, B.M.; Huang, W. Farnesoid X receptor antagonizes nuclear factor kappaB in hepatic inflammatory response. Hepatology 2008, 48, 1632–1643. [Google Scholar] [CrossRef]
- Vavassori, P.; Mencarelli, A.; Renga, B.; Distrutti, E.; Fiorucci, S. The bile acid receptor FXR is a modulator of intestinal innate immunity. J. Immunol. 2009, 183, 6251–6261. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Ratziu, V.; Loomba, R.; Rinella, M.; Anstee, Q.M.; Goodman, Z.; Bedossa, P.; Geier, A.; Beckebaum, S.; Newsome, P.N.; et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: Interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2019, 394, 2184–2196. [Google Scholar] [CrossRef]
- Nevens, F.; Andreone, P.; Mazzella, G.; Strasser, S.I.; Bowlus, C.; Invernizzi, P.; Drenth, J.P.; Pockros, P.J.; Regula, J.; Beuers, U.; et al. A Placebo-Controlled Trial of Obeticholic Acid in Primary Biliary Cholangitis. N. Engl. J. Med. 2016, 375, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Malik, A.; Yang Vom Hofe, A.; Matuschek, L.; Mullen, M.; Lages, C.S.; Kudira, R.; Singh, R.; Zhang, W.; Setchell, K.D.; et al. Farnesoid X receptor antagonizes macrophage-dependent licensing of effector T lymphocytes and progression of sclerosing cholangitis. Sci. Transl. Med. 2022, 14, eabi4354. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.D.; Sroda, N.; Scharnagl, H.; Gupta, R.; Minto, W.; Stojakovic, T.; Liles, J.T.; Budas, G.; Hollenback, D.; Trauner, M. Non-steroidal FXR agonist cilofexor improves cholestatic liver injury in the Mdr2(-/-) mouse model of sclerosing cholangitis. JHEP Rep. 2023, 5, 100874. [Google Scholar] [CrossRef] [PubMed]
- Milkiewicz, M.; Klak, M.; Kempinska-Podhorodecka, A.; Wiechowska-Kozlowska, A.; Urasinska, E.; Blatkiewicz, M.; Wunsch, E.; Elias, E.; Milkiewicz, P. Impaired Hepatic Adaptation to Chronic Cholestasis induced by Primary Sclerosing Cholangitis. Sci. Rep. 2016, 6, 39573. [Google Scholar] [CrossRef]
- Keitel, V.; Reich, M.; Haussinger, D. TGR5: Pathogenetic role and/or therapeutic target in fibrosing cholangitis? Clin. Rev. Allergy Immunol. 2015, 48, 218–225. [Google Scholar] [CrossRef]
- Sun, D.; Xie, C.; Zhao, Y.; Liao, J.; Li, S.; Zhang, Y.; Wang, D.; Hua, K.; Gu, Y.; Du, J.; et al. The gut microbiota-bile acid axis in cholestatic liver disease. Mol. Med. 2024, 30, 104. [Google Scholar] [CrossRef]
- Reich, M.; Spomer, L.; Klindt, C.; Fuchs, K.; Stindt, J.; Deutschmann, K.; Höhne, J.; Liaskou, E.; Hov, J.R.; Karlsen, T.H.; et al. Downregulation of TGR5 (GPBAR1) in biliary epithelial cells contributes to the pathogenesis of sclerosing cholangitis. J. Hepatol. 2021, 75, 634–646. [Google Scholar] [CrossRef]
- Yoneno, K.; Hisamatsu, T.; Shimamura, K.; Kamada, N.; Ichikawa, R.; Kitazume, M.T.; Mori, M.; Uo, M.; Namikawa, Y.; Matsuoka, K.; et al. TGR5 signalling inhibits the production of pro-inflammatory cytokines by in vitro differentiated inflammatory and intestinal macrophages in Crohn’s disease. Immunology 2013, 139, 19–29. [Google Scholar] [CrossRef]
- Hov, J.R.; Keitel, V.; Laerdahl, J.K.; Spomer, L.; Ellinghaus, E.; ElSharawy, A.; Melum, E.; Boberg, K.M.; Manke, T.; Balschun, T.; et al. Mutational characterization of the bile acid receptor TGR5 in primary sclerosing cholangitis. PLoS ONE 2010, 5, e12403. [Google Scholar] [CrossRef]
- Li, Y.; Tang, R.; Leung, P.S.C.; Gershwin, M.E.; Ma, X. Bile acids and intestinal microbiota in autoimmune cholestatic liver diseases. Autoimmun. Rev. 2017, 16, 885–896. [Google Scholar] [CrossRef]
- Oleszycka, E.; O’Brien, E.C.; Freeley, M.; Lavelle, E.C.; Long, A. Bile acids induce IL-1alpha and drive NLRP3 inflammasome-independent production of IL-1beta in murine dendritic cells. Front. Immunol. 2023, 14, 1285357. [Google Scholar] [CrossRef]
- Kubota, H.; Ishizawa, M.; Kodama, M.; Nagase, Y.; Kato, S.; Makishima, M.; Sakurai, K. Vitamin D Receptor Mediates Attenuating Effect of Lithocholic Acid on Dextran Sulfate Sodium Induced Colitis in Mice. Int. J. Mol. Sci. 2023, 24, 3517. [Google Scholar] [CrossRef]
- Maccauro, V.; Fianchi, F.; Gasbarrini, A.; Ponziani, F.R. Gut Microbiota in Primary Sclerosing Cholangitis: From Prognostic Role to Therapeutic Implications. Dig. Dis. 2024, 42, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, B.; Li, Y.; Wei, Y.; Huang, B.; Liang, J.; You, Z.; Li, Y.; Qian, Q.; Wang, R.; et al. Altered faecal microbiome and metabolome in IgG4-related sclerosing cholangitis and primary sclerosing cholangitis. Gut 2022, 71, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Ozdirik, B.; Muller, T.; Wree, A.; Tacke, F.; Sigal, M. The Role of Microbiota in Primary Sclerosing Cholangitis and Related Biliary Malignancies. Int. J. Mol. Sci. 2021, 22, 6975. [Google Scholar] [CrossRef] [PubMed]
- Sabino, J.; Vieira-Silva, S.; Machiels, K.; Joossens, M.; Falony, G.; Ballet, V.; Ferrante, M.; Van Assche, G.; Van der Merwe, S.; Vermeire, S.; et al. Primary sclerosing cholangitis is characterised by intestinal dysbiosis independent from IBD. Gut 2016, 65, 1681–1689. [Google Scholar] [CrossRef]
- Ruhlemann, M.; Liwinski, T.; Heinsen, F.A.; Bang, C.; Zenouzi, R.; Kummen, M.; Thingholm, L.; Tempel, M.; Lieb, W.; Karlsen, T.; et al. Consistent alterations in faecal microbiomes of patients with primary sclerosing cholangitis independent of associated colitis. Aliment. Pharmacol. Ther. 2019, 50, 580–589. [Google Scholar] [CrossRef]
- Hole, M.J.; Jorgensen, K.K.; Holm, K.; Braadland, P.R.; Meyer-Myklestad, M.H.; Medhus, A.W.; Reikvam, D.H.; Götz, A.; Grzyb, K.; Boberg, K.M.; et al. A shared mucosal gut microbiota signature in primary sclerosing cholangitis before and after liver transplantation. Hepatology 2023, 77, 715–728. [Google Scholar] [CrossRef]
- Kummen, M.; Thingholm, L.B.; Ruhlemann, M.C.; Holm, K.; Hansen, S.H.; Moitinho-Silva, L.; Liwinski, T.; Zenouzi, R.; Storm-Larsen, C.; Midttun, Ø.; et al. Altered Gut Microbial Metabolism of Essential Nutrients in Primary Sclerosing Cholangitis. Gastroenterology 2021, 160, 1784–1798.e0. [Google Scholar] [CrossRef]
- Bajer, L.; Kverka, M.; Kostovcik, M.; Macinga, P.; Dvorak, J.; Stehlikova, Z.; Brezina, J.; Wohl, P.; Spicak, J.; Drastich, P. Distinct gut microbiota profiles in patients with primary sclerosing cholangitis and ulcerative colitis. World J. Gastroenterol. 2017, 23, 4548–4558. [Google Scholar] [CrossRef]
- Ozdirik, B.; Schnabl, B. Microbial Players in Primary Sclerosing Cholangitis: Current Evidence and Concepts. Cell. Mol. Gastroenterol. Hepatol. 2024, 17, 423–438. [Google Scholar] [CrossRef]
- De Cruz, P.; Kang, S.; Wagner, J.; Buckley, M.; Sim, W.H.; Prideaux, L.; Lockett, T.; McSweeney, C.; Morrison, M.; Kirkwood, C.D.; et al. Association between specific mucosa-associated microbiota in Crohn’s disease at the time of resection and subsequent disease recurrence: A pilot study. J. Gastroenterol. Hepatol. 2015, 30, 268–278. [Google Scholar] [CrossRef]
- Tang, R.; Wei, Y.; Li, Y.; Chen, W.; Chen, H.; Wang, Q.; Yang, F.; Miao, Q.; Xiao, X.; Zhang, H.; et al. Gut microbial profile is altered in primary biliary cholangitis and partially restored after UDCA therapy. Gut 2018, 67, 534–541. [Google Scholar] [CrossRef]
- Xin, Z.; Wang, Z.; Chu, M. Insights Into Intestinal Flora in Metabolic Dysfunction-Associated Steatotic Liver Disease. FASEB J. 2025, 39, e70932. [Google Scholar] [CrossRef]
- Liwinski, T.; Casar, C.; Ruehlemann, M.C.; Bang, C.; Sebode, M.; Hohenester, S.; Denk, G.; Lieb, W.; Lohse, A.W.; Franke, A.; et al. A disease-specific decline of the relative abundance of Bifidobacterium in patients with autoimmune hepatitis. Aliment. Pharmacol. Ther. 2020, 51, 1417–1428. [Google Scholar] [CrossRef]
- Quraishi, M.N.; Acharjee, A.; Beggs, A.D.; Horniblow, R.; Tselepis, C.; Gkoutos, G.; Ghosh, S.; E Rossiter, A.; Loman, N.; van Schaik, W.; et al. A Pilot Integrative Analysis of Colonic Gene Expression, Gut Microbiota, and Immune Infiltration in Primary Sclerosing Cholangitis-Inflammatory Bowel Disease: Association of Disease with Bile Acid Pathways. J. Crohns Colitis. 2020, 14, 935–947. [Google Scholar] [CrossRef]
- Gasaly, N.; Hermoso, M.A.; Gotteland, M. Butyrate and the Fine-Tuning of Colonic Homeostasis: Implication for Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 3061. [Google Scholar] [CrossRef]
- Wang, R.X.; Lee, J.S.; Campbell, E.L.; Colgan, S.P. Microbiota-derived butyrate dynamically regulates intestinal homeostasis through regulation of actin-associated protein synaptopodin. Proc. Natl. Acad. Sci. USA 2020, 117, 11648–11657. [Google Scholar] [CrossRef]
- Muratori, P.; Muratori, L.; Guidi, M.; Maccariello, S.; Pappas, G.; Ferrari, R.; Gionchetti, P.; Campieri, M.; Bianchi, F.B. Anti-Saccharomyces cerevisiae antibodies (ASCA) and autoimmune liver diseases. Clin. Exp. Immunol. 2003, 132, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, G.; Gotthardt, D.; Kloters-Plachky, P.; Kulaksiz, H.; Rost, D.; Stiehl, A. Influence of dominant bile duct stenoses and biliary infections on outcome in primary sclerosing cholangitis. J. Hepatol. 2009, 51, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Lemoinne, S.; Kemgang, A.; Ben Belkacem, K.; Straube, M.; Jegou, S.; Corpechot, C.; Network, S.-A.I.; Chazouillères, O.; Housset, C.; Sokol, H. Fungi participate in the dysbiosis of gut microbiota in patients with primary sclerosing cholangitis. Gut 2020, 69, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Ruhlemann, M.C.; Solovjeva, M.E.L.; Zenouzi, R.; Liwinski, T.; Kummen, M.; Lieb, W.; Hov, J.R.; Schramm, C.; Franke, A.; Bang, C. Gut mycobiome of primary sclerosing cholangitis patients is characterised by an increase of Trichocladium griseum and Candida species. Gut 2020, 69, 1890–1892. [Google Scholar] [CrossRef]
- Zeng, S.; Rosati, E.; Saggau, C.; Messner, B.; Chu, H.; Duan, Y.; Hartmann, P.; Wang, Y.; Ma, S.; Huang, W.J.M.; et al. Candida albicans-specific Th17 cell-mediated response contributes to alcohol-associated liver disease. Cell Host Microbe 2023, 31, 389–404.E7. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Schnabl, B. Gut mycobiome alterations and implications for liver diseases. PLoS Pathog. 2024, 20, e1012377. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, P.; Schnabl, B. Fungal infections and the fungal microbiome in hepatobiliary disorders. J. Hepatol. 2023, 78, 836–851. [Google Scholar] [CrossRef] [PubMed]
- Meacci, D.; Bruni, A.; Cocquio, A.; Dell’Anna, G.; Mandarino, F.V.; Marasco, G.; Cecinato, P.; Barbara, G.; Zagari, R.M. Microbial Landscapes of the Gut-Biliary Axis: Implications for Benign and Malignant Biliary Tract Diseases. Microorganisms 2025, 13, 1980. [Google Scholar] [CrossRef]
- Ali, A.H.; Juran, B.D.; Schlicht, E.M.; Bianchi, J.K.; McCauley, B.M.; Atkinson, E.J.; Lazaridis, K.N. The PSC scientific community resource: An asset for multi-omics interrogation of primary sclerosing cholangitis. BMC Gastroenterol. 2021, 21, 353. [Google Scholar] [CrossRef]
- Shah, A.; Macdonald, G.A.; Morrison, M.; Holtmann, G. Targeting the Gut Microbiome as a Treatment for Primary Sclerosing Cholangitis: A Conceptional Framework. Am. J. Gastroenterol. 2020, 115, 814–822. [Google Scholar] [CrossRef]
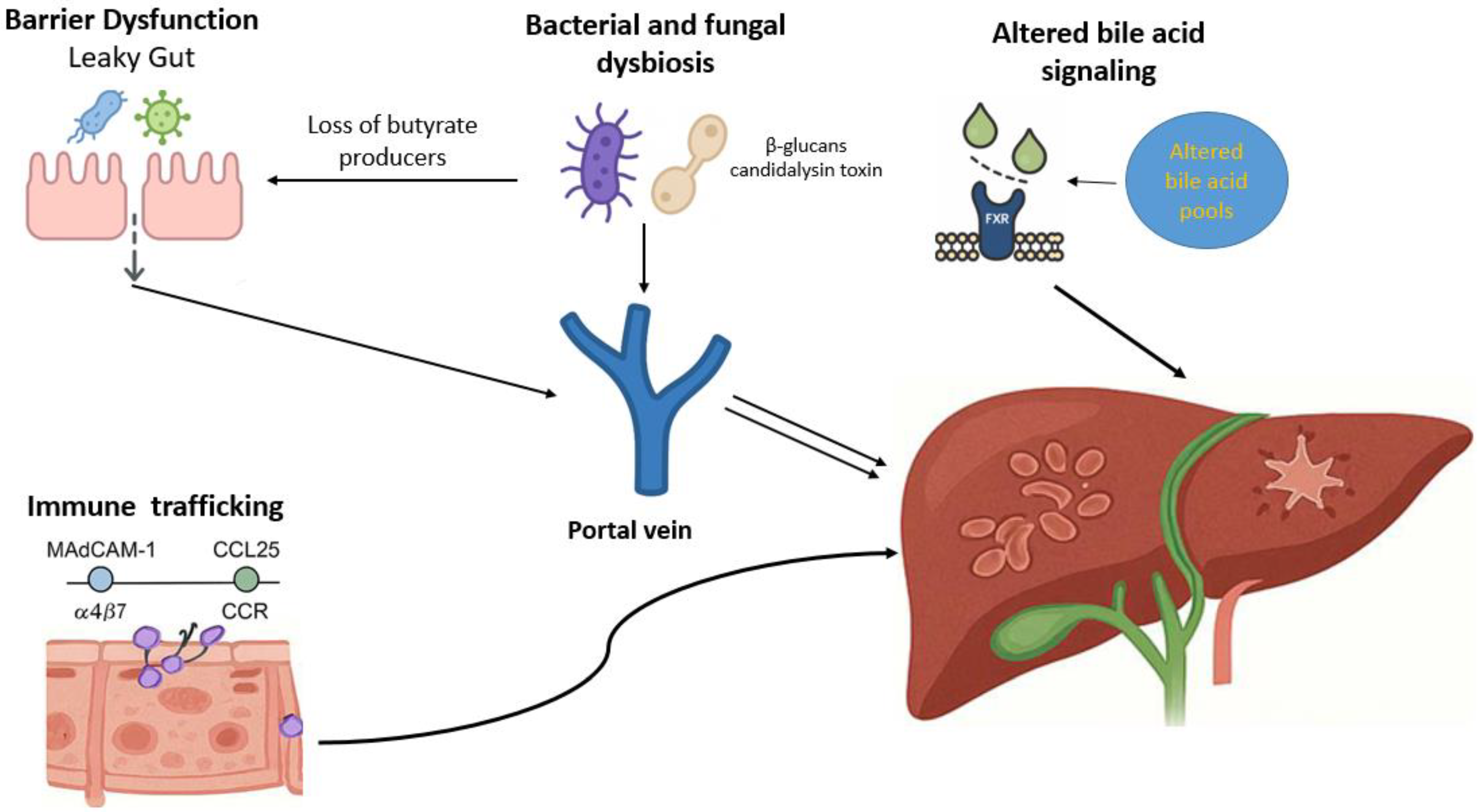
| Pathogenic Mechanism | Molecular and Cellular Components | Pathophysiological Consequences | Evidence |
|---|---|---|---|
| Intestinal barrier dysfunction |
| Increased intestinal permeability allows microbial products to reach the liver, activating Kupffer and sinusoidal cells and driving inflammation and fibrosis | [29,32,33,35,36] |
| Aberrant lymphocyte trafficking |
| Gut-primed lymphocytes and B cells migrate to the liver via mucosal adhesion pathways, sustaining chronic biliary inflammation and immune-mediated injury | [51,52,55,56,57] |
| Bile acid dysregulation |
| Disturbed bile acid signaling and altered composition impair epithelial integrity and modulate hepatic inflammation and fibrosis | [71,76,78,81] |
| Bacterial dysbiosis |
| Dysbiosis disturbs gut–liver homeostasis by weakening epithelial barrier function, altering bile acid metabolism, and enhancing mucosal and hepatic immune activation | [85,86,89,90,91] |
| Fungal dysbiosis |
| Fungal components trigger Th17 and Kupffer cell activation, promoting inflammation and fibrosis | [100,101,102,103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fousekis, F.S.; Mpakogiannis, K.; Lianos, G.D.; Antonelli, E.; Bassotti, G.; Katsanos, K.H. Gut–Liver Axis, Microbiota, Bile Acids, and Immune Response in Pathogenesis of Primary Sclerosing Cholangitis: An Overview. J. Clin. Med. 2025, 14, 7817. https://doi.org/10.3390/jcm14217817
Fousekis FS, Mpakogiannis K, Lianos GD, Antonelli E, Bassotti G, Katsanos KH. Gut–Liver Axis, Microbiota, Bile Acids, and Immune Response in Pathogenesis of Primary Sclerosing Cholangitis: An Overview. Journal of Clinical Medicine. 2025; 14(21):7817. https://doi.org/10.3390/jcm14217817
Chicago/Turabian StyleFousekis, Fotios S., Konstantinos Mpakogiannis, Georgios D. Lianos, Elisabetta Antonelli, Gabrio Bassotti, and Konstantinos H. Katsanos. 2025. "Gut–Liver Axis, Microbiota, Bile Acids, and Immune Response in Pathogenesis of Primary Sclerosing Cholangitis: An Overview" Journal of Clinical Medicine 14, no. 21: 7817. https://doi.org/10.3390/jcm14217817
APA StyleFousekis, F. S., Mpakogiannis, K., Lianos, G. D., Antonelli, E., Bassotti, G., & Katsanos, K. H. (2025). Gut–Liver Axis, Microbiota, Bile Acids, and Immune Response in Pathogenesis of Primary Sclerosing Cholangitis: An Overview. Journal of Clinical Medicine, 14(21), 7817. https://doi.org/10.3390/jcm14217817







