Misleading Lesions in Gynecological Malignancies: A Case Report of Desmoid Tumor During Pregnancy and a Narrative Review of the Literature
Abstract
1. Introduction
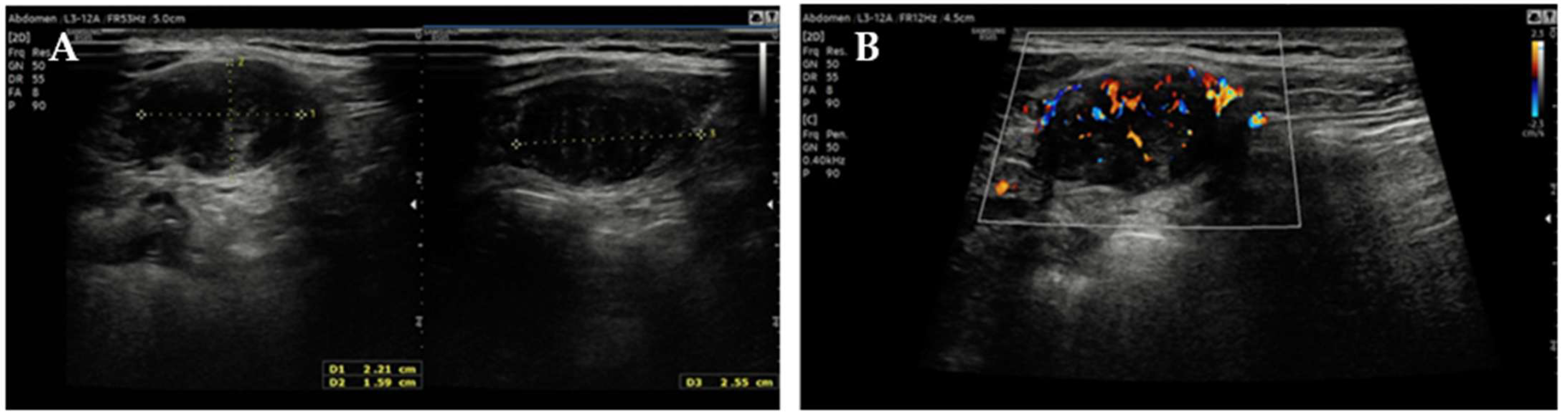
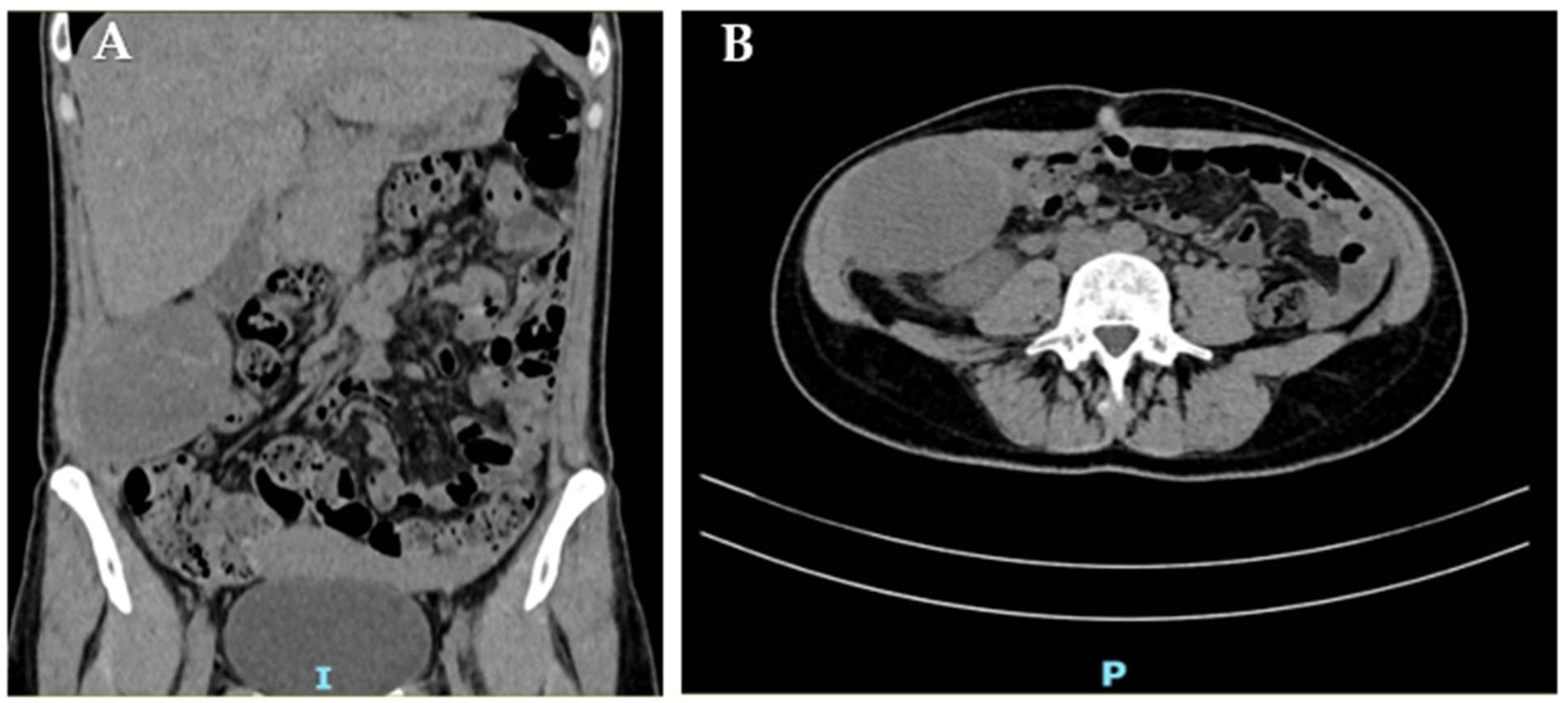
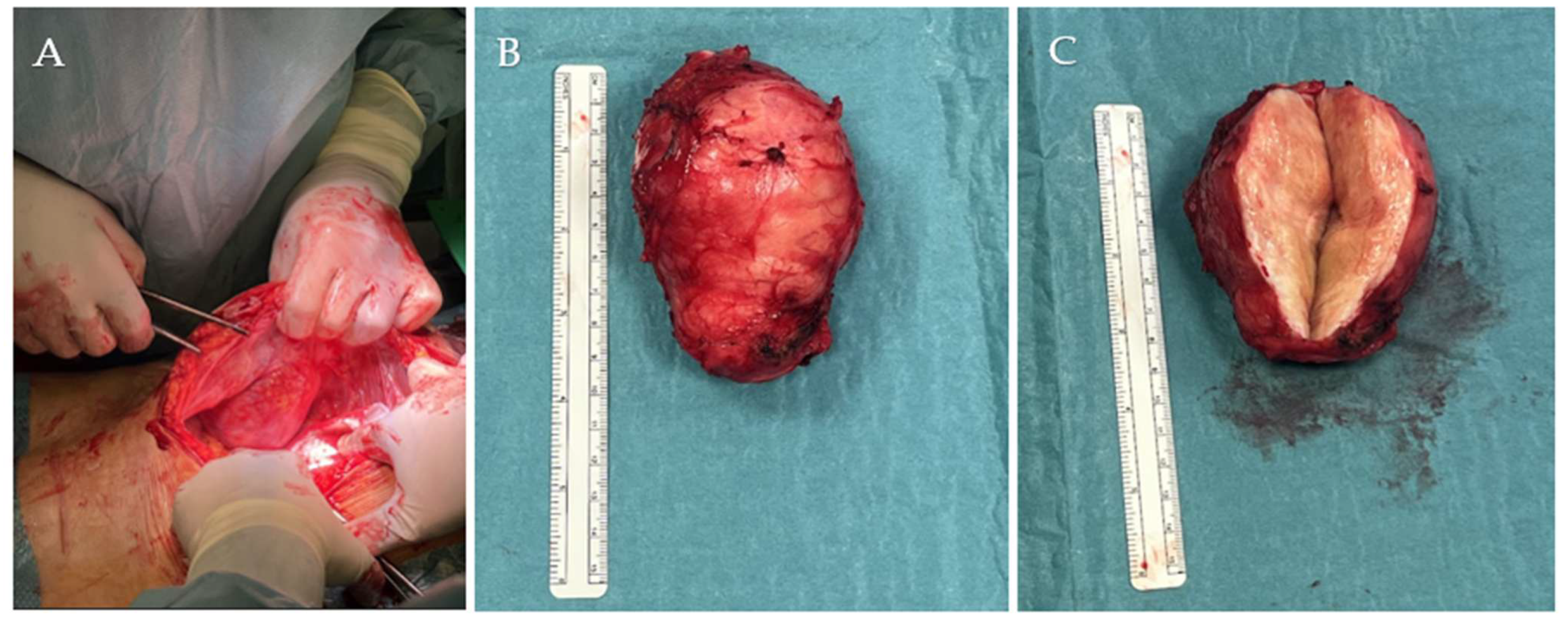
2. Case Report
3. Materials and Methods
4. Results
4.1. Epidemiology
4.2. Etiology
4.3. Risk Factors
4.4. Histological and Immunohistochemical Factors
4.5. Diagnosis
4.6. Differential Diagnosis
4.7. Treatment
4.8. Natural History and Risk of Recurrence
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APC | adenomatous polyposis coli |
| CIN3 | cervical intraepithelial neoplasia 3 |
| CT | computed tomography |
| C-section | cesarean section |
| DF | desmoid fibromatosis |
| DT | desmoid tumor |
| ER | estrogen receptor |
| ESMO | European Society for Medical Oncology |
| F | female |
| FAP | familial adenomatous polyposis |
| IFN-γ | interferon gamma |
| IVF | in vitro fertilization |
| LEF1 | lymphoid enhancer–binding factor 1 |
| M | male |
| MRI | magnetic resonance imaging |
| NCCN | National Comprehensive Cancer Network |
| NSAIDs | nonsteroidal anti-inflammatory drugs |
| PD-1 | programmed cell death protein 1 |
| PD-L1 | programmed death-ligand 1 |
| SOX10 | SRY-box transcription factor 10 |
| TKIs | tyrosine kinase inhibitors |
| US | ultrasound |
| USL | uterosacral ligament |
| WHO | World Health Organization |
References
- Desmoid Tumor Working Group. The management of desmoid tumours: A joint global consensus-based guideline approach for adult and pediatric patients. Eur. J. Cancer 2020, 127, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cordon-Cardo, C.; Gerald, W.L.; Rosai, J. Desmoid fibromatosis is a clonal process. Hum. Pathol. 1996, 27, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Jo, V.Y.; Fletcher, C.D. WHO classification of soft tissue tumours: An update based on the 2013 (4th) edition. Pathology 2014, 46, 95–104. [Google Scholar] [CrossRef]
- Panel, N.; Coindre, J.M.; Bonvalot, S.; Italiano, A.; Neuville, A.; Le Cesne, A.; Terrier, P.; Ray-Coquard, I.; Ranchere-Vince, D.; Robin, Y.M.; et al. Management of desmoid tumours: A nationwide survey of labelled reference centre networks in France. Eur. J. Cancer 2016, 58, 90–96. [Google Scholar] [CrossRef]
- Anneberg, M.; Svane, H.M.L.; Fryzek, J.; Nicholson, G.; White, J.B.; Edris, B.; Smith, L.M.; Hooda, N.; Petersen, M.M.; Baad-Hansen, T.; et al. The epidemiology of desmoid tumors in Denmark. Cancer Epidemiol. 2022, 77, 102114. [Google Scholar] [CrossRef]
- Kasper, B.; Baumgarten, C.; Garcia, J.; Bonvalot, S.; Haas, R.; Haller, F.; Hohenberger, P.; Penel, N.; Messiou, C.; van der Graaf, W.T.; et al. Desmoid Working Group. An update on the management of sporadic desmoid-type fibromatosis: A European Consensus Initiative between Sarcoma PAtients EuroNet (SPAEN) and European Organization for Research and Treatment of Cancer (EORTC)/Soft Tissue and Bone Sarcoma Group (STBSG). Ann. Oncol. 2017, 28, 2399–2408. [Google Scholar] [CrossRef]
- Charifa, A.; Jamil, R.T.; Sathe, N.C.; Zhang, X. Gardner Syndrome; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Sakorafas, G.H.; Nissotakis, C.; Peros, G. Abdominal desmoid tumors. Surg. Oncol. 2007, 16, 131–142. [Google Scholar] [CrossRef]
- Lim, C.L.; Walker, M.J.; Mehta, R.R.; Das Gupta, T.K. Estrogen and antiestrogen binding sites in desmoid tumors. Eur. J. Cancer Clin. Oncol. 1986, 22, 583–587. [Google Scholar] [CrossRef]
- Arima, K.; Komohara, Y.; Uchihara, T.; Yamashita, K.; Uemura, S.; Hanada, N.; Baba, H. A Case of Mesenteric Desmoid Tumor Causing Bowel Obstruction After Laparoscopic Surgery. Anticancer. Res. 2022, 42, 381–384. [Google Scholar] [CrossRef]
- Debaudringhien, M.; Blay, J.Y.; Bimbai, A.M.; Bonvalot, S.; Italiano, A.; Rousset-Jablonski, C.; Corradini, N.; Piperno-Neumann, S.; Chevreau, C.; Kurtz, J.E.; et al. Association between recent pregnancy or hormonal contraceptive exposure and outcome of desmoid-type fibromatosis. ESMO Open 2022, 7, 100578. [Google Scholar] [CrossRef]
- Zubor, P.; Henriksen, C.M.; Økstad, M.E.; Cerskuviene, E.; Visnovsky, J.; Kajo, K.; Valkov, A.; Lind, K.O. Desmoid Fibromatosis of the Anterior Abdominal Wall in Pregnancy: A Case Report and Review of the Literature. Diseases 2024, 12, 27. [Google Scholar] [CrossRef]
- Simonetti, I.; Bruno, F.; Fusco, R.; Cutolo, C.; Setola, S.V.; Patrone, R.; Masciocchi, C.; Palumbo, P.; Arrigoni, F.; Picone, C.; et al. Multimodality Imaging Assessment of Desmoid Tumors: The Great Mime in the Era of Multidisciplinary Teams. J. Pers. Med. 2022, 12, 1153. [Google Scholar] [CrossRef]
- Vural, B.; Vural, F.; Müezzinoglu, B. An Abdominal Wall Desmoid Tumour Mimicking Cesarean Scar Endometriomas: A Case Report and Review of the Literature. J. Clin. Diagn. Res. 2015, 9, 14–16. [Google Scholar] [CrossRef]
- Leon, M.G.; Moussa, H.N.; Movahedian, M.; Viteri, O.A.; Longo, M.; Sibai, B.M. A Rapidly Growing Abdominal Mass: Desmoid Tumor in Pregnancy. AJP Rep. 2015, 5, 14–17. [Google Scholar] [CrossRef]
- Fujita, M.; Yamamoto, M.; Kaizaki, Y.; Kato, M.; Tsuchida, T. Treatment of a Desmoid Tumor That Enlarged During Pregnancy: A Case Report and Literature Review. Kurume Med. J. 2023, 69, 99–102. [Google Scholar] [CrossRef]
- Shields, C.J.; Winter, D.C.; Kirwan, W.O.; Redmond, H.P. Desmoid tumours. Eur. J. Surg. Oncol. 2001, 27, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Devata, S.; Chugh, R. Desmoid tumors: A comprehensive review of the evolving biology, unpredictable behavior, and myriad of management options. Hematol. Oncol. Clin. N. Am. 2013, 27, 989–1005. [Google Scholar] [CrossRef] [PubMed]
- Reitamo, J.J.; Häyry, P.; Nykyri, E.; Saxén, E. The desmoid tumor. I. Incidence, sex-, age- and anatomical distribution in the Finnish population. Am. J. Clin. Pathol. 1982, 77, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Salas, S.; Dufresne, A.; Bui, B.; Blay, J.Y.; Terrier, P.; Ranchere-Vince, D.; Bonvalot, S.; Stoeckle, E.; Guillou, L.; Le Cesne, A.; et al. Prognostic factors influencing progression-free survival determined from a series of sporadic desmoid tumors: A wait-and-see policy according to tumor presentation. J. Clin. Oncol. 2011, 29, 3553–3558. [Google Scholar] [CrossRef]
- Latchford, A.R.; Sturt, N.J.; Neale, K.; Rogers, P.A.; Phillips, R.K. A 10-year review of surgery for desmoid disease associated with familial adenomatous polyposis. Br. J. Surg. 2006, 93, 1258–1264. [Google Scholar] [CrossRef]
- de Bree, E.; Keus, R.; Melissas, J.; Tsiftsis, D.; van Coevorden, F. Desmoid tumors: Need for an individualized approach. Expert. Rev. Anticancer. Ther. 2009, 9, 525–535. [Google Scholar] [CrossRef]
- de Bree, E.; Dimitriadis, E.; Giannikaki, E.; Chryssou, E.G.; Melissas, J. A giant pregnancy-associated intra-abdominal desmoid tumour: Not necessarily a contraindication for subsequent pregnancy. World J. Surg. Oncol. 2013, 11, 277. [Google Scholar] [CrossRef]
- Lopez, R.; Kemalyan, N.; Moseley, H.S.; Dennis, D.; Vetto, R.M. Problems in diagnosis and management of desmoid tumors. Am. J. Surg. 1990, 159, 450–453. [Google Scholar] [CrossRef]
- Ormonde, M.; Argyropoulou, D.; Lourenço, C.; Bastos, J.; Quintas, A. Pelvic Desmoid Tumor: A Rare Case with Difficult Diagnosis and Treatment. Case Rep. Obstet. Gynecol. 2022, 2022, 7653246. [Google Scholar] [CrossRef] [PubMed]
- Ganeshan, D.; Amini, B.; Nikolaidis, P.; Assing, M.; Vikram, R. Current Update on Desmoid Fibromatosis. J. Comput. Assist. Tomogr. 2019, 43, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Kotiligam, D.; Lazar, A.J.; Pollock, R.E.; Lev, D. Desmoid tumor: A disease opportune for molecular insights. Histol. Histopathol. 2008, 23, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.A.; Petscavage, J.M.; Brian, P.L.; Logie, C.I.; Montini, K.M.; Murphey, M.D. Imaging features of superficial and deep fibromatoses in the adult population. Sarcoma 2012, 2012, 215810. [Google Scholar] [CrossRef]
- Braschi-Amirfarzan, M.; Keraliya, A.R.; Krajewski, K.M.; Tirumani, S.H.; Shinagare, A.B.; Hornick, J.L.; Baldini, E.H.; George, S.; Ramaiya, N.H.; Jagannathan, J.P. Role of Imaging in Management of Desmoid-type Fibromatosis: A Primer for Radiologists. Radiographics 2016, 36, 767–782. [Google Scholar] [CrossRef]
- Quinn, S.F.; Erickson, S.J.; Dee, P.M.; Walling, A.; Hackbarth, D.A.; Knudson, G.J.; Moseley, H.S. MR imaging in fibromatosis: Results in 26 patients with pathologic correlation. AJR Am. J. Roentgenol. 1991, 156, 539–542. [Google Scholar] [CrossRef]
- von Mehren, M.; Benjamin, R.S.; Bui, M.M.; Casper, E.S.; Conrad, E.U., 3rd; De Laney, T.F.; Ganjoo, K.N.; George, S.; Gonzalez, R.; Heslin, M.J.; et al. Soft tissue sarcoma, version 2.2012: Featured updates to the NCCN guidelines. J. Natl. Compr. Canc. Netw. 2012, 10, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Leithner, A.; Gapp, M.; Radl, R.; Pascher, A.; Krippl, P.; Leithner, K.; Windhager, R.; Beham, A. Immunohistochemical analysis of desmoid tumours. J. Clin. Pathol. 2005, 58, 1152–1156. [Google Scholar] [CrossRef]
- Toulmonde, M.; Pulido, M.; Ray-Coquard, I.; Andre, T.; Isambert, N.; Chevreau, C.; Penel, N.; Bompas, E.; Saada, E.; Bertucci, F.; et al. Pazopanib or methotrexate-vinblastine combination chemotherapy in adult patients with progressive desmoid tumours (DESMOPAZ): A non-comparative, randomised, open-label, multicentre, phase 2 study. Lancet Oncol. 2019, 20, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Church, J.M.; McGannon, E. Prior pregnancy ameliorates the course of intra-abdominal desmoid tumors in patients with familial adenomatous polyposis. Dis. Colon. Rectum. 2000, 43, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Coppola, S.; Cannell, A.J.; Colombo, C.; Bertagnolli, M.M.; George, S.; Le Cesne, A.; Gladdy, R.A.; Casali, P.G.; Swallow, C.J.; et al. Desmoid-type fibromatosis and pregnancy: A multi-institutional analysis of recurrence and obstetric risk. Ann. Surg. 2014, 259, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.K.; Neale, K.F.; Landgrebe, J.C.; Phillips, R.K. Desmoid tumours complicating familial adenomatous polyposis. Br. J. Surg. 1999, 86, 1185–1189. [Google Scholar] [CrossRef]
- Heiskanen, I.; Järvinen, H.J. Occurrence of desmoid tumours in familial adenomatous polyposis and results of treatment. Int. J. Color. Dis. 1996, 11, 157–162. [Google Scholar] [CrossRef]
- Ballo, M.T.; Zagars, G.K.; Pollack, A.; Pisters, P.W.; Pollack, R.A. Desmoid tumor: Prognostic factors and outcome after surgery, radiation therapy, or combined surgery and radiation therapy. J. Clin. Oncol. 1999, 17, 158–167. [Google Scholar] [CrossRef]
- Johner, A.; Tiwari, P.; Zetler, P.; Wiseman, S.M. Abdominal wall desmoid tumors associated with pregnancy: Current concepts. Expert. Rev. Anticancer. Ther. 2009, 9, 1675–1682. [Google Scholar] [CrossRef]
- Hanna, D.; Magarakis, M.; Twaddell, W.S.; Alexander, H.R.; Kesmodel, S.B. Rapid progression of a pregnancy-associated intra-abdominal desmoid tumor in the post-partum period: A case report. Int. J. Surg. Case Rep. 2016, 29, 30–33. [Google Scholar] [CrossRef]
- Mohd Sulaiman, N.; Mohd Dali, F.; Mohd Hussain, M.S.B.; Ramli, R. Abdominal wall desmoid tumour in pregnancy. BMJ Case Rep. 2022, 15, 249966. [Google Scholar] [CrossRef]
- Mulik, V.; Griffiths, A.N.; Beattie, R.B. Desmoid tumours with familial adenomatous polyposis in pregnancy. J. Obstet. Gynaecol. 2003, 23, 307–308. [Google Scholar] [CrossRef]
- Robinson, W.A.; McMillan, C.; Kendall, A.; Pearlman, N. Desmoid tumors in pregnant and postpartum women. Cancers 2012, 4, 184–192. [Google Scholar] [CrossRef]
- Carneiro, C.; Hurtubis, C.; Singh, M.; Robinson, W. Desmoid tumors of the right rectus abdominus muscle in postpartum women. Arch. Gynecol. Obstet. 2009, 279, 869–873. [Google Scholar] [CrossRef]
- Michopoulou, A.; Germanos, S.; Kanakopoulos, D.; Milonas, A.; Orfanos, N.; Spyratou, C.; Markidis, P. Management of a large abdominal wall desmoid tumor during pregnancy. Case report. Ann. Ital. Chir. 2010, 81, 153–156. [Google Scholar] [PubMed]
- Zhou, H.; Lu, H.; Wang, L.; Xie, L.; Wu, M.; Li, J.; Abdallahi, N.; Lin, Z. Abdominal wall desmoid tumor during pregnancy: Case report and literature review. Clin. Exp. Obstet. Gynecol. 2015, 42, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Viriyaroj, V.; Yingsakmongkol, N.; Pasukdee, P.; Rermluk, N. A large abdominal desmoid tumor associated with pregnancy. J. Med. Assoc. Thai. 2009, 92, 72–75. [Google Scholar] [PubMed]
- Gurluler, E.; Gures, N.; Citil, I.; Kemik, O.; Berber, I.; Sumer, A.; Gurkan, A. Desmoid tumor in puerperium period: A case report. Clin. Med. Insights Case Rep. 2014, 7, 29–32. [Google Scholar] [CrossRef]
- Way, J.C.; Culham, B.A. Desmoid tumour. The risk of recurrent or new disease with subsequent pregnancy: A case report. Can. J. Surg. 1999, 42, 51–54. [Google Scholar] [PubMed]
- Hashimoto, K.; Nishimura, S.; Shinyashiki, Y.; Ito, T.; Kakinoki, R.; Akagi, M. Clinicopathological assessment of PD-1/PD-L1 immune checkpoint expression in desmoid tumors. Eur. J. Histochem. 2023, 67, 3688. [Google Scholar] [CrossRef]
- Le Roc’h, A.; Montaigne, K.; Leblond, P.; Subtil, D.; Boukerrou, M. Desmoid tumour of the rectus abdominis muscle during pregnancy. J. Obstet. Gynaecol. 2009, 29, 668–669. [Google Scholar] [CrossRef]
- Durkin, A.J.; Korkolis, D.P.; Al-Saif, O.; Zervos, E.E. Full-term gestation and transvaginal delivery after wide resection of an abdominal desmoid tumor during pregnancy. J. Surg. Oncol. 2005, 89, 86–90. [Google Scholar] [CrossRef] [PubMed]
- De Cian, F.; Delay, E.; Rudigoz, R.C.; Ranchère, D.; Rivoire, M. Desmoid tumor arising in a cesarean section scar during pregnancy: Monitoring and management. Gynecol. Oncol. 1999, 75, 145–148. [Google Scholar] [CrossRef]
- Camiel, M.R.; Solish, G.I. Desmoid tumor during pregnancy. Am. J. Obstet. Gynecol. 1982, 144, 988–989. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Patra, S.R.; Nishi; Arya, A.P. Aggressive Fibromatosis in the Abdominal Wall: A Rare Case of Intramuscular Desmoid Tumor. Indian J. Surg. Oncol. 2024, 15, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Awwad, J.; Hammoud, N.; Farra, C.; Fares, F.; Abi Saad, G.; Ghazeeri, G. Abdominal Wall Desmoid during Pregnancy: Diagnostic Challenges. Case Rep. Obstet. Gynecol. 2013, 2013, 350894. [Google Scholar] [CrossRef]
- Njoku, O.C.; Umezurike, C.C. Giant desmoid tumour mimicking recurrent uterine myoma in a nulliparous young Nigerian: A case report. J. Med. Case Rep. 2022, 16, 319. [Google Scholar] [CrossRef]
- Al-Jefout, M.; Walid, A.; Esam, A.; Amin, A.; Nather, H.; Sultan, N.; Maysa, K. Abdominal wall desmoid tumor mimicking a subserosal uterine leiomyoma. Int. J. Gen. Med. 2011, 4, 443–446. [Google Scholar] [CrossRef]
- Krentel, H.; Tchartchian, G.; De Wilde, R.L. Desmoid tumor of the anterior abdominal wall in female patients: Comparison with endometriosis. Case Rep. Med. 2012, 2012, 725498. [Google Scholar] [CrossRef]
- Yarmish, G.; Sala, E.; Goldman, D.A.; Lakhman, Y.; Soslow, R.A.; Hricak, H.; Gardner, G.J.; Vargas, H.A. Abdominal wall endometriosis: Differentiation from other masses using CT features. Abdom. Radiol. 2017, 42, 1517–1523. [Google Scholar] [CrossRef]
- Granese, R.; Cucinella, G.; Barresi, V.; Navarra, G.; Candiani, M.; Triolo, O. Isolated endometriosis on the rectus abdominis muscle in women without a history of abdominal surgery: A rare and intriguing finding. J. Minim. Invasive Gynecol. 2009, 16, 798–801. [Google Scholar] [CrossRef]
- Testa, S.; Bui, N.Q.; Charville, G.W.; Avedian, R.S.; Steffner, R.; Ghanouni, P.; Mohler, D.G.; Ganjoo, K.N. Management of Patients with Newly Diagnosed Desmoid Tumors in a First-Line Setting. Cancers 2022, 14, 3907. [Google Scholar] [CrossRef] [PubMed]
- Cates, J.M.M. Pregnancy does not increase the local recurrence rate after surgical resection of desmoid-type fibromatosis. Int. J. Clin. Oncol. 2015, 20, 617–622. [Google Scholar] [CrossRef]
- Jin, L.; Tan, Y.; Su, Z.; Huang, S.; Pokhrel, S.; Shi, H.; Chen, Y. Gardner syndrome with giant abdominal desmoid tumor during pregnancy: A case report. BMC Surg. 2020, 20, 282. [Google Scholar] [CrossRef]
- Barbier, O.; Anract, P.; Pluot, E.; Larouserie, F.; Sailhan, F.; Babinet, A.; Tomeno, B. Primary or recurring extra-abdominal desmoid fibromatosis: Assessment of treatment by observation only. Orthop. Traumatol. Surg. Res. 2010, 96, 884–889. [Google Scholar] [CrossRef]
- Guo, H.P.; Zhang, H.; Li, Y.; Pan, X.H.; Sun, C.L.; Zhang, J.J. Desmoid tumors of rectus abdominis: A case report and literature review. Medicine 2024, 103, 39089. [Google Scholar] [CrossRef]
- Ferrari, F.; Valenti, G.; Forte, S.; Ardighieri, L.; Iraci Sareri, M.; Barra, F.; Sartori, E.; Odicino, F. Clear cell degeneration associated with endometriosis of abdominal wall after cesarean section: A case report and systematic review of literature. J. Obstet. Gynaecol. Res. 2021, 47, 1243–1252. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonetti Palermo, E.; Ferrari, F.; Dell’Avalle, C.; Nodari, I.; Ongarini, E.P.; Ghini, I.; Giannini, A.; Soleymani majd, H.; Ciravolo, G.; Odicino, F. Misleading Lesions in Gynecological Malignancies: A Case Report of Desmoid Tumor During Pregnancy and a Narrative Review of the Literature. J. Clin. Med. 2025, 14, 7815. https://doi.org/10.3390/jcm14217815
Bonetti Palermo E, Ferrari F, Dell’Avalle C, Nodari I, Ongarini EP, Ghini I, Giannini A, Soleymani majd H, Ciravolo G, Odicino F. Misleading Lesions in Gynecological Malignancies: A Case Report of Desmoid Tumor During Pregnancy and a Narrative Review of the Literature. Journal of Clinical Medicine. 2025; 14(21):7815. https://doi.org/10.3390/jcm14217815
Chicago/Turabian StyleBonetti Palermo, Emma, Federico Ferrari, Cecilia Dell’Avalle, Ilaria Nodari, Emma Paola Ongarini, Iacopo Ghini, Andrea Giannini, Hooman Soleymani majd, Giuseppe Ciravolo, and Franco Odicino. 2025. "Misleading Lesions in Gynecological Malignancies: A Case Report of Desmoid Tumor During Pregnancy and a Narrative Review of the Literature" Journal of Clinical Medicine 14, no. 21: 7815. https://doi.org/10.3390/jcm14217815
APA StyleBonetti Palermo, E., Ferrari, F., Dell’Avalle, C., Nodari, I., Ongarini, E. P., Ghini, I., Giannini, A., Soleymani majd, H., Ciravolo, G., & Odicino, F. (2025). Misleading Lesions in Gynecological Malignancies: A Case Report of Desmoid Tumor During Pregnancy and a Narrative Review of the Literature. Journal of Clinical Medicine, 14(21), 7815. https://doi.org/10.3390/jcm14217815








