Erythroferrone, Hepcidin, and Erythropoietin in Chronic Kidney Disease: Associations with Hemoglobin and Renal Function
Abstract
1. Introduction
2. Materials and Methods
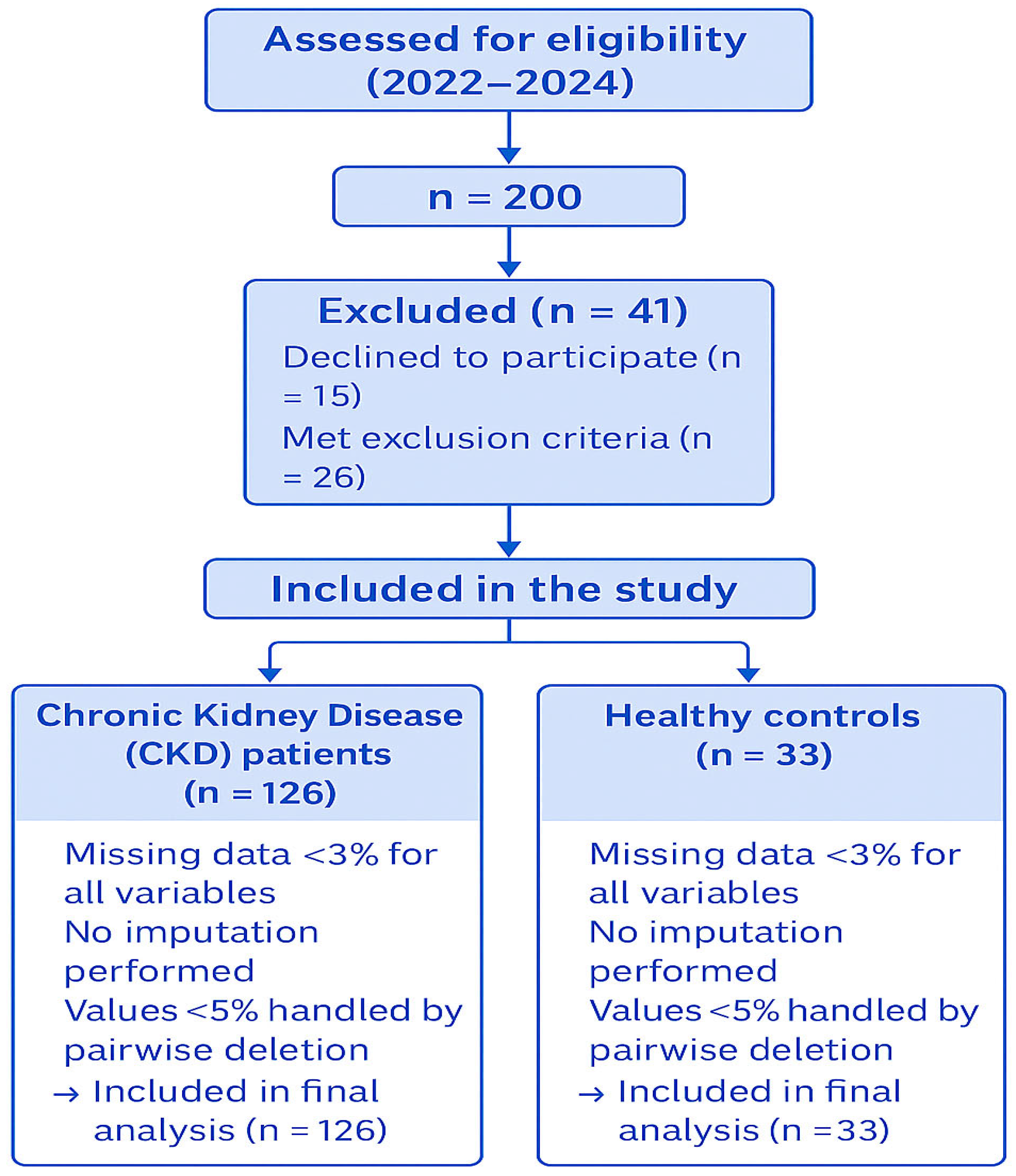
2.1. Study Design and Population
2.2. Inclusion and Exclusion Criteria
2.3. Clinical and Laboratory Assessments
2.4. Ethical Considerations
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CKD | Chronic Kidney Disease |
| ERFE | Erythroferrone |
| EPO | Erythropoietin |
| TSAT | Transferrin Saturation |
| eGFR | Estimated Glomerular Filtration Rate |
| BUN | Blood Urea Nitrogen |
| Hb | Hemoglobin |
| Hct | Hematocrit |
| RDW | Red Cell Distribution Width |
| NLR | Neutrophil-to-Lymphocyte Ratio |
| WBC | White Blood Cell |
References
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Guo, S.; Liu, Y.; Zhou, Y.; Liu, Y.; Zheng, X.; Yu, X.; Shuai, P. Global, regional, and national burden of chronic kidney disease and its underlying etiologies from 1990 to 2021: A systematic analysis for the Global Burden of Disease Study 2021. BMC Public Health 2025, 25, 636. [Google Scholar] [CrossRef]
- Lim, Y.J.; Sidor, N.A.; Tonial, N.C. Uremic Toxins in the Progression of Chronic Kidney Disease and Cardiovascular Disease: Mechanisms and Therapeutic Targets. Toxins 2021, 13, 142. [Google Scholar] [CrossRef]
- Lu, Y.; Meng, L.; Wang, X.; Zhang, Y.; Zhang, C.; Zhang, M. The Non-Traditional Cardiovascular Culprits in Chronic Kidney Disease: Mineral Imbalance and Uremic Toxin Accumulation. Int. J. Mol. Sci. 2025, 26, 7938. [Google Scholar] [CrossRef] [PubMed]
- Badura, K.; Janc, J. Anemia of Chronic Kidney Disease-A Narrative Review of Its Pathophysiology, Diagnosis, and Management. Biomedicines 2024, 12, 1191. [Google Scholar] [CrossRef]
- Portolés, J.; Martín, L.; Broseta, J.J.; Cases, A. Anemia in Chronic Kidney Disease: From Pathophysiology and Current Treatments, to Future Agents. Front. Med. 2021, 8, 642296. [Google Scholar] [CrossRef]
- Buliga-Finis, O.N.; Ouatu, A.; Tanase, D.M.; Gosav, E.M.; Seritean Isac, P.N.; Richter, P.; Rezus, C. Managing anemia: Point of convergence for heart failure and chronic kidney disease? Life 2023, 13, 1311. [Google Scholar] [CrossRef] [PubMed]
- Poggiali, E.; De Amicis, M.M.; Motta, I. Anemia of chronic disease: A unique defect of iron recycling for many different chronic diseases. Eur. J. Intern. Med. 2014, 25, 12–17. [Google Scholar] [CrossRef]
- Nemeth, E.; Ganz, T. Hepcidin-ferroportin interaction controls systemic iron homeostasis. Int. J. Mol. Sci. 2021, 22, 6493. [Google Scholar] [CrossRef]
- Ganz, T.; Nemeth, E. Iron Balance and the Role of Hepcidin in Chronic Kidney Disease. Semin. Nephrol. 2016, 36, 87–93. [Google Scholar] [CrossRef]
- Srole, D.N.; Ganz, T. Erythroferrone structure, function, and physiology: Iron homeostasis and beyond. J. Cell. Physiol. 2021, 236, 4888–4901. [Google Scholar] [CrossRef]
- Coffey, R.; Ganz, T. Erythroferrone: An Erythroid Regulator of Hepcidin and Iron Metabolism. HemaSphere 2018, 2, e35. [Google Scholar] [CrossRef]
- Wojtaszek, E.; Glogowski, T.; Malyszko, J. Iron and Chronic Kidney Disease: Still a Challenge. Front. Med. 2020, 7, 565135. [Google Scholar] [CrossRef]
- Spoto, B.; Kakkar, R.; Lo, L.; Devalaraja, M.; Pizzini, P.; Torino, C.; Leonardis, D.; Cutrupi, S.; Tripepi, G.; Mallamaci, F. Serum erythroferrone levels associate with mortality and cardiovascular events in hemodialysis and in CKD patients: A two cohorts study. J. Clin. Med. 2019, 8, 523. [Google Scholar] [CrossRef] [PubMed]
- Quintana-Castanedo, L.; Maseda, R. Interplay between iron metabolism, inflammation, and EPO-ERFE-hepcidin axis in RDEB-associated chronic anemia. Blood Adv. 2025, 9, 2321–2335. [Google Scholar] [CrossRef] [PubMed]
- Babar, S.; Saboor, M. Erythroferrone in focus: Emerging perspectives in iron metabolism and hematopathologies. Blood Sci. 2024, 6, e00198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, P.; Yan, X. Mechanism-Based Pharmacokinetic/Pharmacodynamic Modeling of Erythroferrone in Anemic Rats with Chronic Kidney Disease and Chemotherapy-Induced Anemia: An Early Biomarker for Hemoglobin Response and rHuEPO Hyporesponsiveness. ACS Pharmacol. Transl. Sci. 2025, 8, 189–202. [Google Scholar] [CrossRef]
- Arezes, J.; Foy, N.; McHugh, K.; Quinkert, D.; Benard, S.; Sawant, A.; Frost, J.N.; Armitage, A.E.; Pasricha, S.-R.; Lim, P.J.; et al. Antibodies against the erythroferrone N-terminal domain prevent hepcidin suppression and ameliorate murine thalassemia. Blood 2020, 135, 547–557. [Google Scholar] [CrossRef]
- Głogowski, T.; Wojtaszek, E.; Malyszko, J. Iron status and anemia control are related to peritoneal membrane properties in peritoneally dialyzed patients. Front. Med. 2023, 10, 1148094. [Google Scholar] [CrossRef]
- Xu, P.; Wong, R.S.M.; Krzyzanski, W.; Yan, X. Dynamics of Erythroferrone Response to Erythropoietin in Rats. Front. Pharmacol. 2022, 13, 876573. [Google Scholar] [CrossRef]
- Pirotte, M.; Fillet, M.; Seidel, L.; Jaspers, A.; Baron, F. Erythroferrone and hepcidin as mediators between erythropoiesis and iron metabolism during allogeneic hematopoietic stem cell transplant. Am. J. Hematol. 2021, 96, 1275–1286. [Google Scholar] [CrossRef]
- Delaney, K.M.; Guillet, R. Umbilical Cord Erythroferrone Is Inversely Associated with Hepcidin, but Does Not Capture the Most Variability in Iron Status of Neonates Born to Teens Carrying Singletons and Women Carrying Multiples. J. Nutr. 2021, 151, 2590–2600. [Google Scholar] [CrossRef]
- Amaral, T.L.M.; Amaral, C.d.A.; Vasconcellos, M.T.L.d.; Monteiro, G.T.R. Prevalence and factors associated to chronic kidney disease in older adults. Rev. De Saude Publica 2019, 53, 44. [Google Scholar] [CrossRef] [PubMed]
- Alfano, G.; Perrone, R.; Fontana, F. Rethinking Chronic Kidney Disease in the Aging Population. Life 2022, 12, 1724. [Google Scholar] [CrossRef]
- Vosters, T.G.; Kingma, F.M.; Stel, V.S.; Jager, K.J.; van Ittersum, F.J.; van den Born, B.-J.H.; Vogt, L.; van Valkengoed, I.G. The association and contribution of gender-related characteristics to prevalent chronic kidney disease in women and men in a multi-ethnic population-The HELIUS study. BMC Public Health 2025, 25, 853. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.H.; Wang, A.Y.-M. Vitamin B12 and chronic kidney disease. Vitam. Horm. 2022, 119, 325–353. [Google Scholar]
- Finkelstein, F.O.; Story, K.; Firanek, C.; Mendelssohn, D.; Barre, P.; Takano, T.; Soroka, S.; Mujais, S. Health-related quality of life and hemoglobin levels in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Khan, W.M.; Ayub, M.; Humayun, M.; Haroon, M. Ferritin Is a Marker of Inflammation rather than Iron Deficiency in Overweight and Obese People. J. Obes. 2016, 2016, 1937320. [Google Scholar] [CrossRef]
- Cappellini, M.D.; Comin-Colet, J.; de Francisco, A.; Dignass, A.; Doehner, W.; Lam, C.S.; Macdougall, I.C.; Rogler, G.; Camaschella, C.; Kadir, R.; et al. Iron deficiency across chronic inflammatory conditions: International expert opinion on definition, diagnosis, and management. Am. J. Hematol. 2017, 92, 1068–1078. [Google Scholar] [CrossRef]
- Pasare, M.-A.; Prepeliuc, C.S.; Grigoriu, M.G.; Miftode, I.-L.; Miftode, E.G. Biomarkers as Beacons: Illuminating Sepsis-Associated Hepato-Renal Injury. Int. J. Mol. Sci. 2025, 26, 4825. [Google Scholar] [CrossRef]
- Olivera, J.; Zhang, V.; Nemeth, E. Erythroferrone exacerbates iron overload and ineffective extramedullary erythropoiesis in a mouse model of β-thalassemia. Blood Adv. 2023, 7, 3339–3349. [Google Scholar] [CrossRef]
- Bakr, S.; Salem, K.M.; Rashed, A.M.; Tantawy, M.E.A.; Elsary, A.Y.; Shamardl, H.A.; Ezzat, E.M. Evaluation of hepcidin-25/erythroferrone ratio as a potential biomarker for iron utility and erythropoiesis responsiveness to erythropoiesis-stimulating therapy in comparison to immature erythrocyte/reticulocyte parameters in hemodialysis patients. Hematol. Transfus. Cell Ther. 2024, 46 (Suppl. 5), S214–S222. [Google Scholar] [CrossRef]
- Panjeta, M.; Tahirović, I.; Sofić, E.; Ćorić, J.; Dervišević, A. Interpretation of Erythropoietin and Haemoglobin Levels in Patients with Various Stages of Chronic Kidney Disease. J. Med. Biochem. 2017, 36, 145–152. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, X.; Huang, X.; Wei, X.; Zhao, D.; Jiang, L.; Zhao, X.; Du, Y. Advances in Understanding the Effects of Erythropoietin on Renal Fibrosis. Front. Med. 2020, 7, 47. [Google Scholar] [CrossRef]
- Mercadal, L.; Metzger, M.; Casadevall, N.; Haymann, J.P.; Karras, A.; Boffa, J.J.; Flamant, M.; Vrtovsnik, F.; Stengel, B.; Froissart, M. Timing and determinants of erythropoietin deficiency in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2012, 7, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, T.; Abe, M.; Kobayashi, H. Iron metabolism and inflammatory mediators in patients with renal dysfunction. Int. J. Mol. Sci. 2024, 25, 3745. [Google Scholar] [CrossRef] [PubMed]
- Hanudel, M.R.; Rappaport, M.; Gabayan, V.; Jung, G.; Salusky, I.B.; Nemeth, E.; Ganz, T.; Zaritsky, J. Increased serum hepcidin contributes to the anemia of chronic kidney disease in a murine model. Haematologica 2017, 102, e85–e88. [Google Scholar] [CrossRef] [PubMed]
- Czaya, B.; Olivera, J.D.; Zhang, M.; Lundin, A.; Castro, C.D.; Jung, G.; Hanudel, M.R.; Nemeth, E.; Ganz, T. Transgenic augmentation of erythroferrone in mice ameliorates anemia in adenine-induced chronic kidney disease. J. Clin. Investig. 2025, 10. [Google Scholar] [CrossRef] [PubMed]
| Variable | CKD (n = 126) | Control (n = 33) | p-Value | 95% CI (Mean Difference) |
|---|---|---|---|---|
| Age (years, mean ± SD) | 61.2 ± 14.8 | 33.4 ± 10.7 | <0.001 | 23.8–32.1 |
| Female, n (%) | 61 (48.4) | 21 (63.6) | 0.18 | — |
| CKD duration (years, median [IQR]) | 6 (3–10) | — | — | — |
| Vitamin B12 (pg/mL, median [IQR]) | 415 (210–720) | 380 (230–640) | 0.041 | — |
| EPO (mIU/mL, median [IQR]) | 13.1 (4.1–47.6] | 12.6 (4.3–31.3] | 0.68 | — |
| Parameter | Control (n = 33) | Stage 2 (n = 30) | Stage 3 (n = 35) | Stage 4 (n = 31) | Stage 5 (n = 30) | p-Value | p-Trend |
|---|---|---|---|---|---|---|---|
| eGFR (mL/min/1.73 m2) | 115 (92–136) | 73 (64–86) | 48 (36–56) | 26 (18–33) | 12 (7–17) | <0.001 | <0.001 |
| BUN (mg/dL) | 11 (7–18) | 19 (11–25) | 27 (19–45) | 41 (25–80) | 66 (39–112) | <0.001 | <0.001 |
| Creatinine (mg/dL) | 0.8 (0.6–1.1) | 1.1 (0.8–1.5) | 1.7 (1.2–2.4) | 2.9 (1.9–4.5) | 5.2 (3.0–9.4) | <0.001 | <0.001 |
| Hemoglobin (g/dL) | 13.4 (12.5–14.8) | 13.0 (11.9–14.3) | 12.2 (10.8–13.7) | 11.3 (9.8–12.6) | 10.7 (9.5–11.9) | <0.001 | <0.001 |
| TSAT (%) | 22 (15–42) | 25 (14–48) | 27 (17–52) | 24 (14–47) | 23 (13–42) | 0.59 | 0.33 |
| Ferritin (ng/mL) | 105 (40–240) | 152 (70–320) | 215 (90–460) | 268 (110–590) | 318 (150–750) | 0.002 | 0.006 |
| Hepcidin (ng/mL) | 18 (12–24) | 30 (20–41) | 37 (25–60) | 43 (28–75) | 47 (30–88) | 0.09 | 0.047 |
| ERFE (ng/mL) | 2.8 (1.1–6.3) | 6.2 (3.5–9.8) | 13.7 (6.9–25.4) | 20.8 (10.3–31.7) | 32.6 (20.1–46.3) | 0.11 | 0.031 |
| Parameters | r (Partial) | 95% CI | p-Value |
|---|---|---|---|
| Hemoglobin vs. ERFE | −0.402 | −0.53 to −0.26 | <0.001 |
| Hemoglobin vs. Hepcidin | −0.115 | −0.29 to 0.08 | 0.19 |
| Hemoglobin vs. eGFR | +0.421 | 0.28 to 0.54 | <0.001 |
| ERFE vs. Hepcidin | +0.517 | 0.39 to 0.62 | <0.001 |
| ERFE vs. eGFR | −0.648 | −0.73 to −0.53 | <0.001 |
| Hepcidin vs. BUN | +0.541 | 0.39 to 0.65 | <0.001 |
| Hepcidin vs. Creatinine | +0.536 | 0.38 to 0.64 | <0.001 |
| Independent Variable | β Coefficient | 95% CI | SE | p-Value | VIF |
| ERFE (ng/mL) | −0.29 | −0.41 to −0.18 | 0.07 | <0.001 | 1.42 |
| Hepcidin (ng/mL) | −0.10 | −0.21 to 0.02 | 0.06 | 0.09 | 1.57 |
| Ferritin (ng/mL) | −0.08 | −0.17 to 0.01 | 0.05 | 0.07 | 1.33 |
| eGFR (mL/min/1.73 m2) | +0.25 | 0.13 to 0.37 | 0.06 | <0.001 | 1.68 |
| EPO (mIU/mL) | −0.05 | −0.16 to 0.06 | 0.06 | 0.39 | 1.22 |
| Age (years) | −0.07 | −0.18 to 0.03 | 0.05 | 0.15 | 1.19 |
| Sex (female) | +0.03 | −0.07 to 0.12 | 0.04 | 0.52 | 1.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Öneç, K.; Altun, G.; Özdemir Aytekin, Ş.; Davran, F.; Öneç, B. Erythroferrone, Hepcidin, and Erythropoietin in Chronic Kidney Disease: Associations with Hemoglobin and Renal Function. J. Clin. Med. 2025, 14, 7789. https://doi.org/10.3390/jcm14217789
Öneç K, Altun G, Özdemir Aytekin Ş, Davran F, Öneç B. Erythroferrone, Hepcidin, and Erythropoietin in Chronic Kidney Disease: Associations with Hemoglobin and Renal Function. Journal of Clinical Medicine. 2025; 14(21):7789. https://doi.org/10.3390/jcm14217789
Chicago/Turabian StyleÖneç, Kürşad, Gülşah Altun, Şeyma Özdemir Aytekin, Fatih Davran, and Birgül Öneç. 2025. "Erythroferrone, Hepcidin, and Erythropoietin in Chronic Kidney Disease: Associations with Hemoglobin and Renal Function" Journal of Clinical Medicine 14, no. 21: 7789. https://doi.org/10.3390/jcm14217789
APA StyleÖneç, K., Altun, G., Özdemir Aytekin, Ş., Davran, F., & Öneç, B. (2025). Erythroferrone, Hepcidin, and Erythropoietin in Chronic Kidney Disease: Associations with Hemoglobin and Renal Function. Journal of Clinical Medicine, 14(21), 7789. https://doi.org/10.3390/jcm14217789





