Association Between Stem Anteversion and Femoral Rotation in Endoprosthetic Proximal Femoral Replacement: Insights from Two Different Prosthetic Designs
Abstract
1. Introduction
2. Materials and Methods
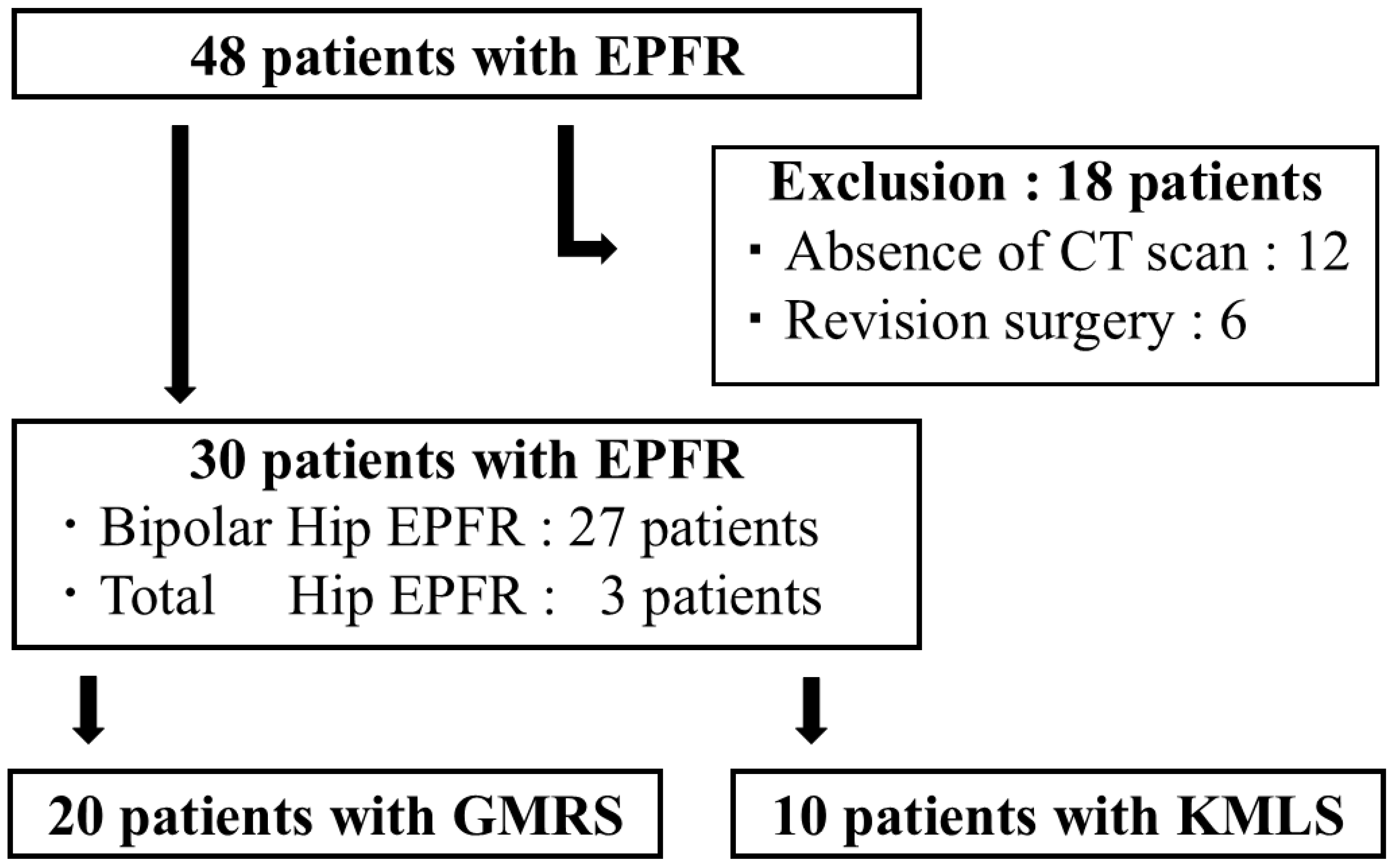
2.1. Patient Enrollment
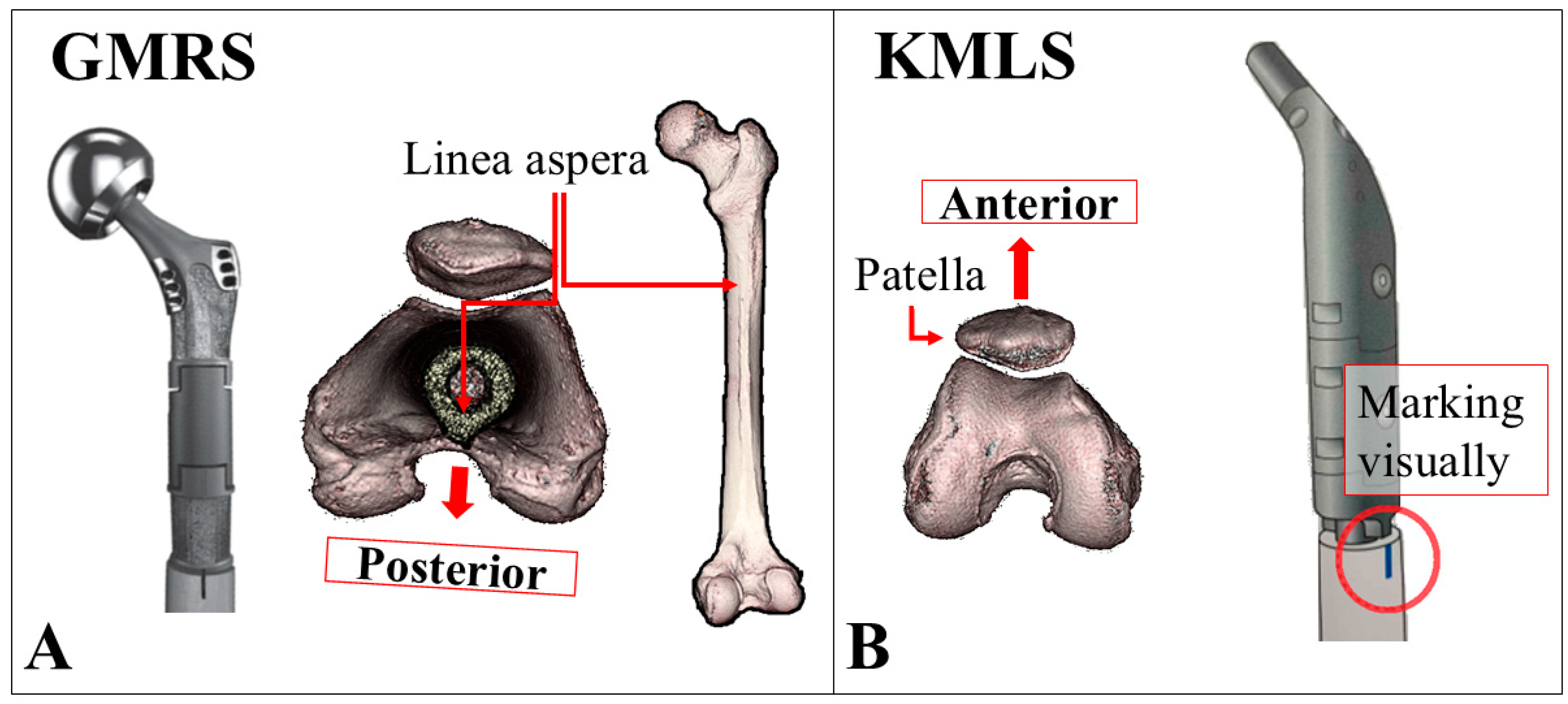
2.2. Surgical Techniques for EPFR in Our Institution
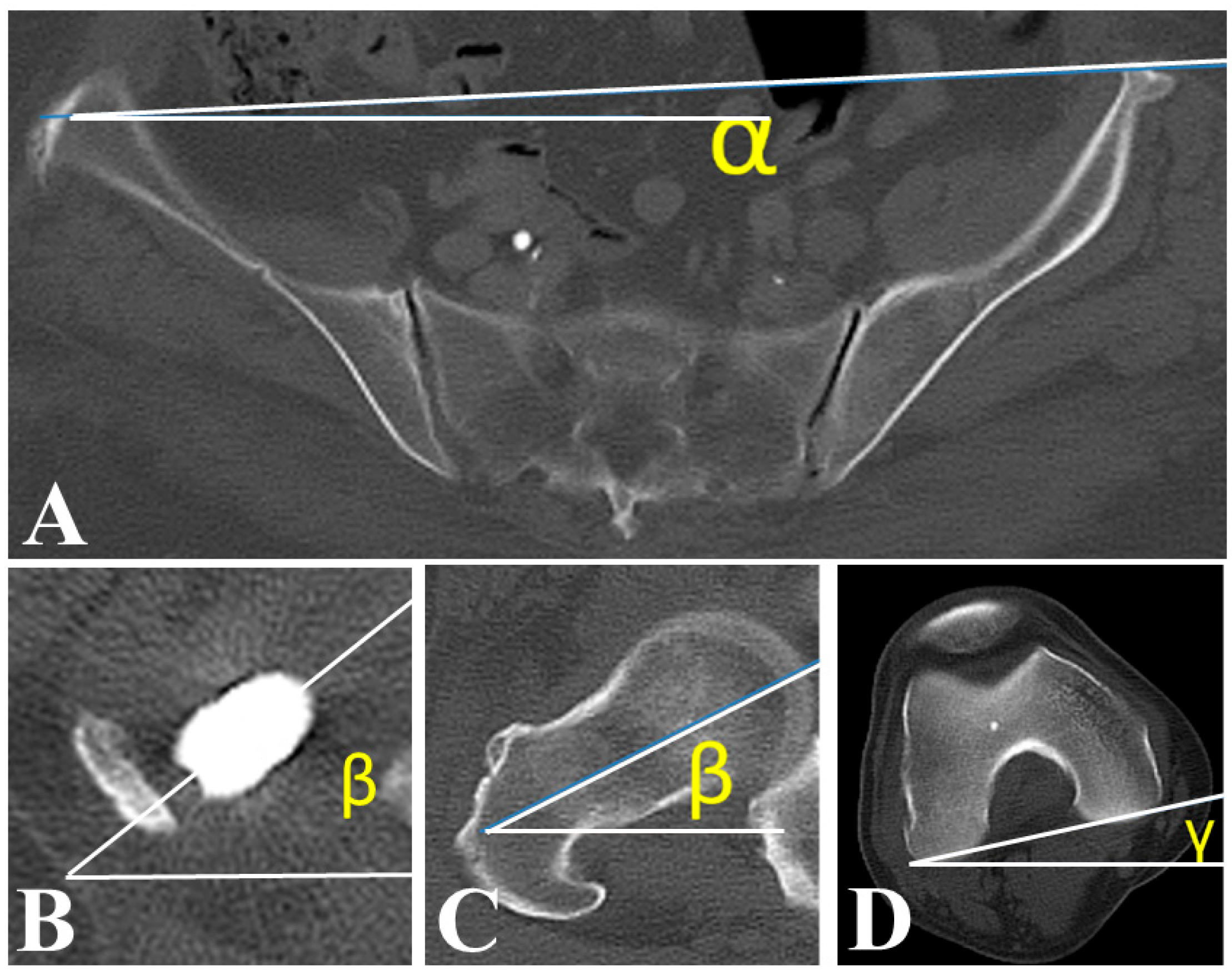
2.3. Patient Background and Postoperative Measurements
2.4. Statistical Analysis
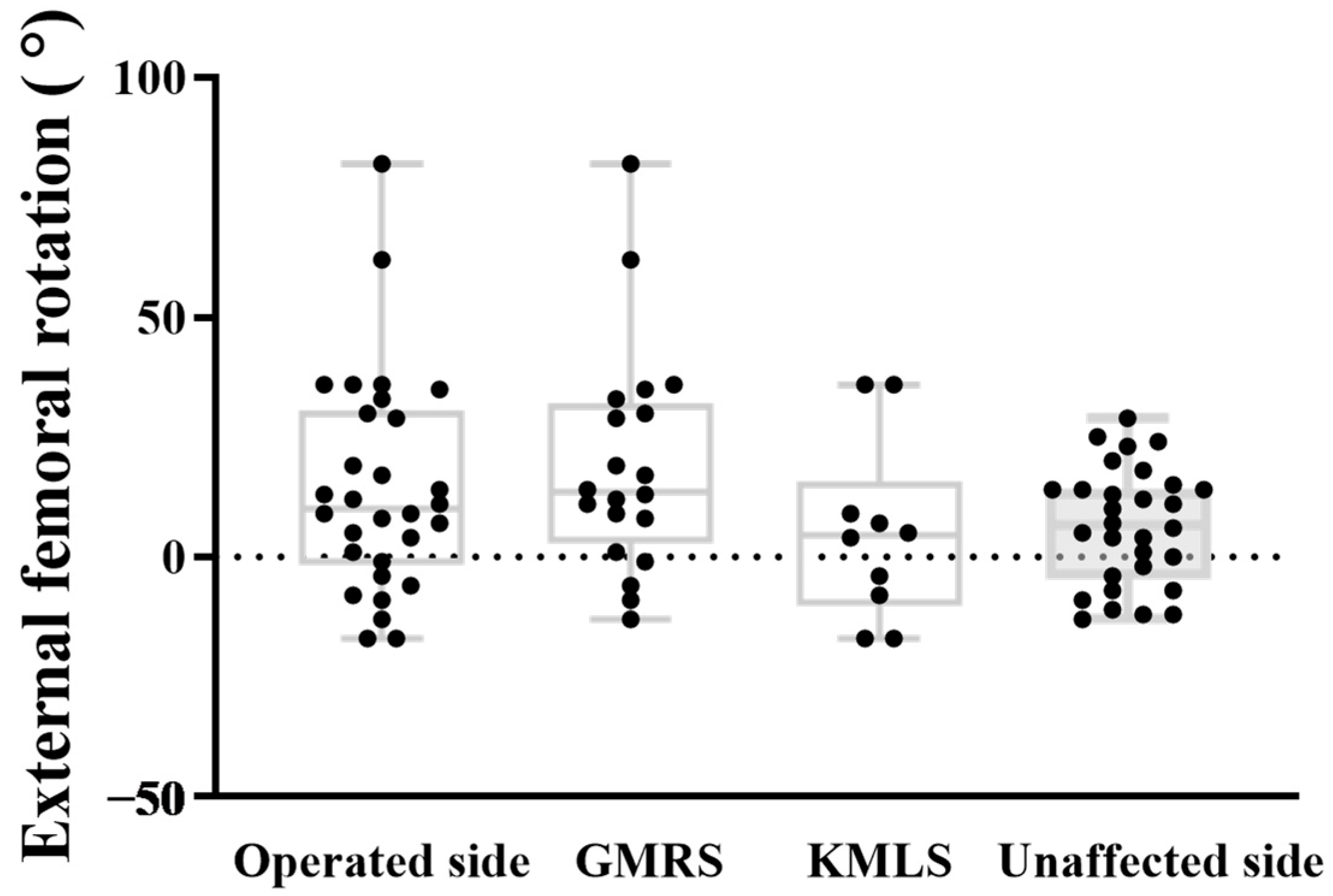
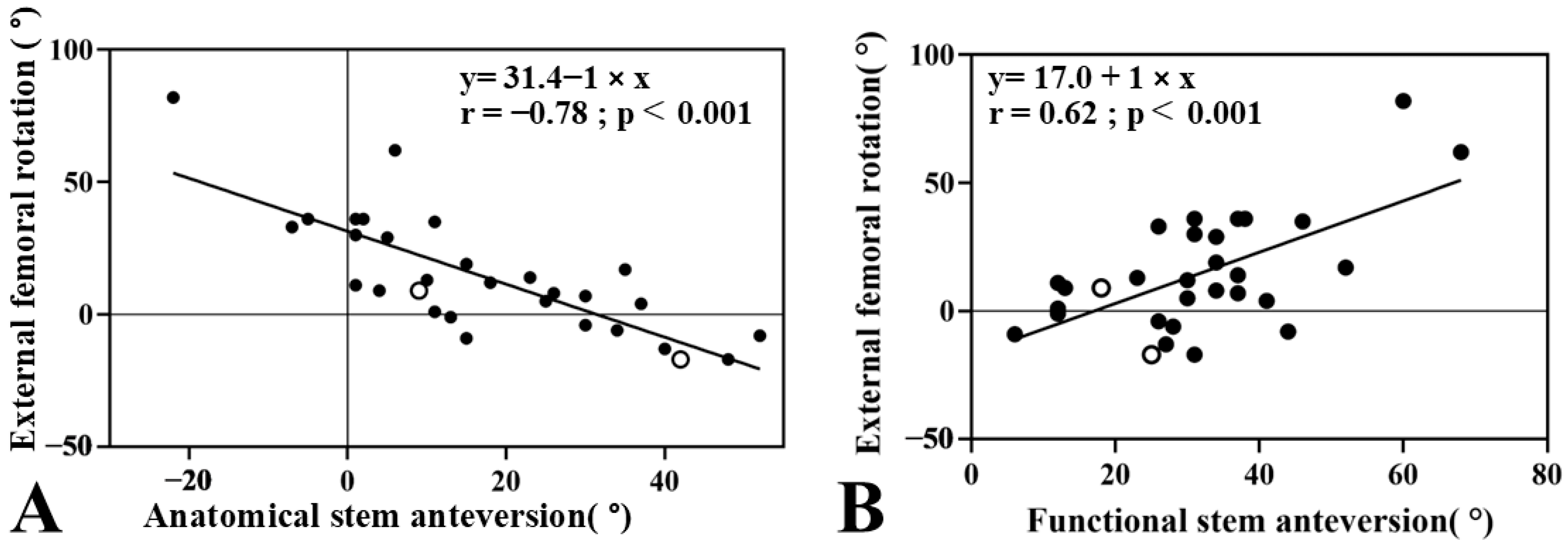
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Use of Artificial Intelligence
Acknowledgments
Conflicts of Interest
References
- Capanna, R.; Piccioli, A.; Di Martino, A.; Daolio, P.A.; Ippolito, V.; Maccauro, G.; Piana, R.; Ruggieri, P.; Gasbarrini, A.; Spinelli, M.S.; et al. Management of long bone metastases: Recommendations from the Italian Orthopaedic Society bone metastasis study group. Expert Rev. Anticancer Ther. 2014, 14, 1127–1134. [Google Scholar] [CrossRef]
- Angelini, A.; Trovarelli, G.; Berizzi, A.; Pala, E.; Breda, A.; Maraldi, M.; Ruggieri, P. Treatment of pathologic fractures of the proximal femur. Injury 2018, 49 (Suppl. 3), S77–S83. [Google Scholar] [CrossRef]
- Hage, W.D.; Aboulafia, A.J.; Aboulafia, D.M. Incidence, location, and diagnostic evaluation of metastatic bone disease. Orthop. Clin. N. Am. 2000, 31, 515–528, vii. [Google Scholar] [CrossRef]
- Jacofsky, D.J.; Haidukewych, G.J.; Zhang, H.; Sim, F.H. Complications and results of arthroplasty for salvage of failed treatment of malignant pathologic fractures of the hip. Clin. Orthop. Relat. Res. 2004, 427, 52–56. [Google Scholar] [CrossRef]
- Coleman, R.E. Metastatic bone disease: Clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 2001, 27, 165–176. [Google Scholar] [CrossRef]
- Laufer, I.; Sciubba, D.M.; Madera, M.; Bydon, A.; Witham, T.J.; Gokaslan, Z.L.; Wolinsky, J.P. Surgical management of metastatic spinal tumors. Cancer Control. 2012, 19, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Jin, P.; Liu, X.W.; Li, M.; Li, L. Cementoplasty for managing painful bone metastases outside the spine. Eur. Radiol. 2014, 24, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Morishige, M.; Muramatsu, K.; Tominaga, Y.; Hashimoto, T.; Taguchi, T. Surgical treatment of metastatic femoral fractures: Achieving an improved quality of life for cancer patients. Anticancer Res. 2015, 35, 427–432. [Google Scholar]
- Feng, H.; Wang, J.; Xu, J.; Chen, W.; Zhang, Y. The surgical management and treatment of metastatic lesions in the proximal femur: A mini review. Medicine 2016, 95, e3892. [Google Scholar] [CrossRef]
- Guzik, G. Oncological and functional results after surgical treatment of bone metastases at the proximal femur. BMC Surg. 2018, 18, 5. [Google Scholar] [CrossRef]
- Steensma, M.; Boland, P.J.; Morris, C.D.; Athanasian, E.; Healey, J.H. Endoprosthetic treatment is more durable for pathologic proximal femur fractures. Clin. Orthop. Relat. Res. 2012, 470, 920–926. [Google Scholar] [CrossRef]
- Ahlmann, E.R.; Menendez, L.R.; Kermani, C.; Gotha, H. Survivorship and clinical outcome of modular endoprosthetic reconstruction for neoplastic disease of the lower limb. J. Bone Jt. Surg. Br. 2006, 88, 790–795. [Google Scholar] [CrossRef]
- Bernthal, N.M.; Schwartz, A.J.; Oakes, D.A.; Kabo, J.M.; Eckardt, J.J. How long do endoprosthetic reconstructions for proximal femoral tumors last? Clin. Orthop. Relat. Res. 2010, 468, 2867–2874. [Google Scholar] [CrossRef]
- Potter, B.K.; Chow, V.E.; Adams, S.C.; Letson, G.D.; Temple, H.T. Endoprosthetic proximal femur replacement: Metastatic versus primary tumors. Surg. Oncol. 2009, 18, 343–349. [Google Scholar] [CrossRef]
- Farid, Y.; Lin, P.P.; Lewis, V.O.; Yasko, A.W. Endoprosthetic and allograft-prosthetic composite reconstruction of the proximal femur for bone neoplasms. Clin. Orthop. Relat. Res. 2006, 442, 223–229. [Google Scholar] [CrossRef]
- Ogilvie, C.M.; Wunder, J.S.; Ferguson, P.C.; Griffin, A.M.; Bell, R.S. Functional outcome of endoprosthetic proximal femoral replacement. Clin. Orthop. Relat. Res. 2004, 426, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Lackman, R.D.; Torbert, J.T.; Finstein, J.L.; Ogilvie, C.M.; Fox, E.J. Inaccuracies in the assessment of femoral anteversion in proximal femoral replacement prostheses. J. Arthroplast. 2008, 23, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Choe, H.; Kobayashi, N.; Kobayashi, D.; Watanabe, S.; Abe, K.; Tezuka, T.; Kawabata, Y.; Takeyama, M.; Inaba, Y. Postoperative excessive external femoral rotation in revision total hip arthroplasty is associated with muscle weakness in iliopsoas and gluteus medius and risk for hip dislocation. J. Orthop. Surg. Res. 2021, 16, 582. [Google Scholar] [CrossRef]
- Tezuka, T.; Inaba, Y.; Kobayashi, N.; Choe, H.; Higashihira, S.; Saito, T. The influence of patient factors on femoral rotation after total hip arthroplasty. BMC Musculoskelet. Disord. 2018, 19, 189. [Google Scholar] [CrossRef]
- Enneking, W.F.; Dunham, W.; Gebhardt, M.C.; Malawar, M.; Pritchard, D.J. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin. Orthop. Relat. Res. 1993, 286, 241–246. [Google Scholar] [CrossRef]
- Sbaraglia, M.; Bellan, E.; Dei Tos, A.P. The 2020 WHO Classification of Soft Tissue Tumours: News and perspectives. Pathologica 2021, 113, 70–84. [Google Scholar] [CrossRef] [PubMed]
- De Smet, A.; Verrewaere, D.; Sys, G. Enhancing rotational placement of reconstruction prostheses of the distal femur after sarcoma resection. Med. Eng. Phys. 2020, 81, 47–57. [Google Scholar] [CrossRef]
- Kobayashi, D.; Choe, H.; Kobayashi, N.; Tezuka, T.; Ike, H.; Inaba, Y. Association of Femoral Rotation with Whole-Body Alignment in Patients Who Underwent Total Hip Arthroplasty. Arthroplast. Today 2020, 6, 532–537. [Google Scholar] [CrossRef]
- Ishida, T.; Inaba, Y.; Kobayashi, N.; Iwamoto, N.; Yukizawa, Y.; Choe, H.; Saito, T. Changes in pelvic tilt following total hip arthroplasty. J. Orthop. Sci. 2011, 16, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Inaba, Y.; Kobayashi, N.; Ishida, T.; Ike, H.; Saito, T. Postural and Chronological Change in Pelvic Tilt Five Years After Total Hip Arthroplasty in Patients with Developmental Dysplasia of the Hip: A Three-Dimensional Analysis. J. Arthroplast. 2016, 31, 317–322. [Google Scholar] [CrossRef] [PubMed]
| Demographic data | Mean ± SD or n (%) |
| Age (years) | 65.2 ± 13.5 |
| Male, n (%) | 16 (53.3) |
| BMI (kg/m2) | 21.5 ± 4.7 |
| Diagnosis (hips) | Primary: 6 (20), Secondary: 24 (80) |
| Surgical data | |
| Surgical procedure (hips) | Total hip: 3 (10), Bipolar hip: 27 (90) |
| Femoral resection length (cm) | 14.3 ± 3.9 |
| CT data | |
| Postoperative anatomical SA (°) | 17.0 ± 17.7 |
| Postoperative FR (°) | 14.4 ± 22.6 |
| GMRS (n = 20) | KMLS (n = 10) | Mean Difference (95% CI) | p-Value | |
|---|---|---|---|---|
| Demographic data | ||||
| Age (years) | 65.0 ± 13.6 | 63.4 ± 12.7 | 1.6 (−8.2 to 11.4) | 0.87 |
| Male, n (%) | 12 (60) | 4 (40) | 0.44 1 | |
| BMI (kg/m2) | 21.8 ± 5.2 | 21.0 ± 4.3 | 0.8 (−3.4 to 5.0) | 0.70 |
| Diagnosis (hips) | 1ry: 4, 2ndry: 16 | 1ry: 1, 2ndry: 9 | 0.64 1 | |
| ASA ≥ III, n (%) | 2 (10) | 1 (10) | 1.00 1 | |
| Pathological fracture, n (%) | 13 (65) | 6 (60) | 1.00 1 | |
| Radiotherapy, n (%) | 3 (15) | 3 (30) | 1.00 1 | |
| Surgical data | ||||
| Surgical procedure (hips) | Total hip: 1, Bipolar hip: 19 | Total hip: 2, Bipolar hip: 8 | 0.25 1 | |
| Femoral resection length (cm) | 13.8 ± 3.6 | 15.4 ± 4.8 | −1.6 (−4.6 to 1.4) | 0.28 |
| CT data | ||||
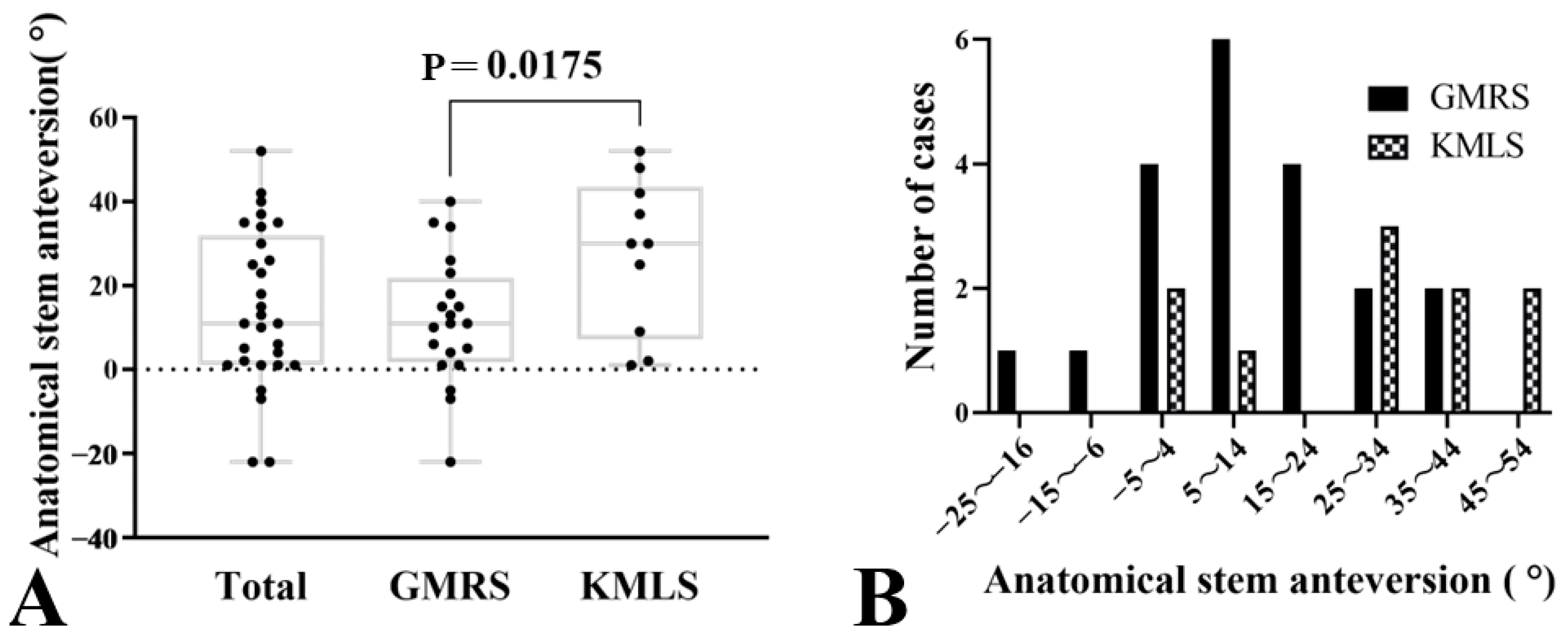
| Postoperative anatomical SA (°) | 11.7 ± 15.2 | 27.6 ± 18.4 | −15.6 (−28.8 to −3.0) | 0.02 |
| Postoperative FR (°) | 19.1 ± 23.3 | 5.1 ± 18.7 | 14.0 (−3.4 to 31.4) | 0.11 |
| Complications | ||||
| Infection, n (%) | 5 (25) | 2 (20) | 1.00 1 | |
| Dislocation, n (%) | 0 (0) | 2 (20) | 0.10 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, T.; Choe, H.; Nezu, Y.; Kawabata, Y.; Saito, K.; Takeyama, M.; Shiga, A.; Fujita, S.; Nakajima, N.; Kobayashi, N.; et al. Association Between Stem Anteversion and Femoral Rotation in Endoprosthetic Proximal Femoral Replacement: Insights from Two Different Prosthetic Designs. J. Clin. Med. 2025, 14, 7786. https://doi.org/10.3390/jcm14217786
Yoshida T, Choe H, Nezu Y, Kawabata Y, Saito K, Takeyama M, Shiga A, Fujita S, Nakajima N, Kobayashi N, et al. Association Between Stem Anteversion and Femoral Rotation in Endoprosthetic Proximal Femoral Replacement: Insights from Two Different Prosthetic Designs. Journal of Clinical Medicine. 2025; 14(21):7786. https://doi.org/10.3390/jcm14217786
Chicago/Turabian StyleYoshida, Tomotaka, Hyonmin Choe, Yutaka Nezu, Yusuke Kawabata, Keiju Saito, Masanobu Takeyama, Akira Shiga, Shintaro Fujita, Naotsugu Nakajima, Naomi Kobayashi, and et al. 2025. "Association Between Stem Anteversion and Femoral Rotation in Endoprosthetic Proximal Femoral Replacement: Insights from Two Different Prosthetic Designs" Journal of Clinical Medicine 14, no. 21: 7786. https://doi.org/10.3390/jcm14217786
APA StyleYoshida, T., Choe, H., Nezu, Y., Kawabata, Y., Saito, K., Takeyama, M., Shiga, A., Fujita, S., Nakajima, N., Kobayashi, N., Kumagai, K., Ike, H., & Inaba, Y. (2025). Association Between Stem Anteversion and Femoral Rotation in Endoprosthetic Proximal Femoral Replacement: Insights from Two Different Prosthetic Designs. Journal of Clinical Medicine, 14(21), 7786. https://doi.org/10.3390/jcm14217786






